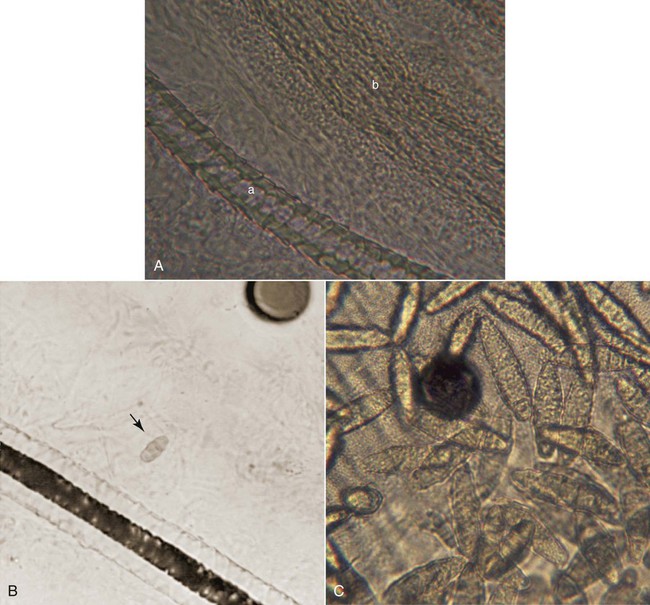
Karen A. Moriello and Douglas J. DeBoer The dermatophytoses of veterinary importance consist of fungi of the genera Microsporum, Trichophyton, and Epidermophyton. These organisms cause superficial cutaneous infections of the stratum corneum, hair shaft, and/or claw. Many dermatophytes are known to have the potential to produce several different types of spores by either sexual or asexual reproduction. The dermatophytes recovered from animal skin are known as anamorphs because they reproduce asexually by production of conidia (imperfect state). Some dermatophytes retain their ability to reproduce sexually when in contact with a strain of the opposite mating type (perfect state), leading to the formation of spores (ascospores); in these cases the sexual form is referred to as the teleomorph. For example, Microsporum canis is an anamorphic species that belongs to the telomorphic Arthroderma otae complex.149 Although there are approximately 30 species of dermatophytes, relatively few infect animals, the most common being M. canis, Microsporum persicolor, Trichophyton spp., Trichophyton erinacei, or the geophilic species Microsporum gypseum.47,143 The prevalence of infections caused by each of the three common etiologic agents (M. canis, M. gypseum, Trichophyton spp.) varies geographically. In cats, over 90% of infections are caused by M. canis worldwide. The prevalence of M. gypseum infection, which is most common in humid, tropic, and subtropic areas, varies seasonally, being more common in summer and autumn.143 Simultaneous infection of dogs with more than one dermatophyte species may occur. Of combined infections, those caused by M. gypseum and Trichophyton spp. have been the most common. Phenotypic148 and genotypic105,130 diversity within different strains of the same dermatophyte species is now widely recognized. Various DNA fingerprinting techniques have demonstrated strain variability within species such as M. canis and Trichophyton spp. and have also led to reclassification of some species.105,130,130a,135 The practical importance and clinical implications of this variation are as yet uncertain but are emerging. Results from one study indicate that one virulent genotype of M. canis may be responsible for the majority of human infections.258 The application of molecular biological techniques (e.g., sequencing the internal transcribed spacer region on rRNA genes) to these fungi is resulting in a reassessment of the taxonomy of many dermatophytes.106 For example, some infections in dogs and cats formerly thought to be Trichophyton mentagrophytes are now classified as Trichophyton spp. and as members of the T. mentagrophytes species complex.85 In this chapter, they are referred to as Trichophyton spp. Dogs and cats harbor many saprophytic molds and yeasts on their haircoats and probably on their skin as well.32a,243a The most common fungi isolated from the haircoats of clinically healthy cats are Alternaria, Aspergillus, Cladosporium, Penicillium, Rhizopus, and Trichoderma.187,188,188 From dogs, the same fungi are isolated with somewhat different frequencies.232 It has been proposed that cats are reservoirs of pathogenic dermatophytes (M. canis, M. gypseum, Microsporum vanbreuseghemii, Trichophyton verrucosum, T. mentagrophytes, Trichophyton rubrum, Epidermophyton spp.). To investigate this possibility, many populations (pet, stray, or cattery) of cats from various geographic areas have been surveyed by culture of haircoats.94,177,232,247 The prevalence of dermatophyte isolation, specifically that of M. canis, varied among the populations, depending on the geographic region, whether or not the cat was a stray or pet cat, and the presence or absence of skin disease at the time of sampling. M. canis was most commonly isolated from stray cats or those in multiple cat facilities and from warm geographic regions. In surveys of clinically healthy pet cats from the United States, Belgium, and United Kingdom, the prevalence of M. canis isolation in 172 cats was 0%, in 467 was 2.1%, and in 181 cats was 2.2%, respectively.177 In the study from the United Kingdom, all 4 cats with positive culture results were from multiple-cat households and were allowed to roam freely outside. Interestingly, in the United States study, T. rubrum was isolated from 14 cats; this organism is the human pathogen associated with tinea pedis (athlete’s foot).94 These findings highlight the importance of fomite carriage by the haircoats of clinically healthy dogs and cats of potentially pathogenic dermatophytes; however, T. rubrum infection is rare in cats. In catteries, the isolation of M. canis depends greatly on whether or not the cattery has a history of dermatophytosis. In one study of cats from catteries in temperate regions of the United States, cats without clinical dermatophytosis had negative culture results, compared to cats in clinically affected catteries, where virtually all cats had positive culture results.188 These findings were identical to those of a smaller study from a warmer region of the United States where M. canis was not isolated from the haircoats of cattery cats from facilities that were free of the disease.272 In surveys of stray and shelter cats, the prevalence of M. canis isolation was higher in shelters in warmer climates. In the United States, the isolation of M. canis from animal shelters in a subtropic climate was 17.5% compared with 4% from a temperate climate.201 Both isolations were performed at the same time of the year. In a study conducted in Belgium, the haircoats of 134 cats were cultured and M. canis was isolated from 15.7%.177 In a study from the southeast of England the prevalence in 169 cats was 2.16%.222 Results from these two studies emphasize the impact of climate and geography on the prevalence of M. canis. In a study involving stray cats (n = 40) in India, the prevalence was 42.5%.251 In Italy,247 173 stray cats without skin disease were sampled, and 47% had positive culture results for M. canis. These studies surveying the fungal flora of dogs and cats highlight several important points. First, pathogenic dermatophytic fungi (e.g., M. canis) should not be considered part of the normal fungal flora of cats or dogs. If these species were indigenous flora, they would have been isolated routinely from clinically healthy dogs and cats regardless of geographic region, lifestyle (indoor or outdoor), or domicile status (pet or stray). Second, isolating pathogens from clinically healthy animals is possible. The isolation of a known potentially pathogenic fungal organism indicates either infection (obvious or subclinical) or fomite carriage from exposure to a contaminated environment. However, if the animal is lesion free, distinguishing between these possibilities is often impossible. Finally, animals with positive culture results should be treated because dermatophytosis is an important zoonotic disease and there is evidence that in some areas of the world animal pathogens are emerging zoonoses. Data from a study on the epidemiology of dermatophyte infections in Europe have indicated that there has been a shift from Microsporum audouinii, Epidermophyton floccosum, and T. rubrum to M. canis as the most common dermatophytes causing infections in humans.257 The exact prevalence of animal dermatophytosis is unknown because the disease is not reportable, even though it is a zoonosis. In clinical practice, dermatophytosis is overdiagnosed, most likely because of its visible similarities to other skin diseases. In all studies of skin disease of dogs and cats in which fungal cultures have been performed, the prevalence of dermatophyte infections is approximately 2% of all dermatologic cases. The percentage of positive culture results from specimens submitted from animals with suspected fungal infection has ranged from less than 4% to 50%.32,143,156,261 In the United Kingdom, positive culture results were 16% of 8349 specimens from dogs or cats evaluated in veterinary clinics for suspected dermatophytosis, whereas in Turin, Italy, positive results were found in 40% of animals examined.156,261 In an animal shelter environment in the United States, 10.4% of 5644 cats over a two-year time period had positive culture results. There were 381 cats with skin lesions, and 94 (24.1%) of these had M. canis infection.276 The infectivity rates for dermatophytes just listed may reflect not only geographic variation, but also differences in the types of cases chosen for dermatophyte culture. Environmental factors, such as increased warmth and high humidity, and animal background, such as existence of debilitation or concurrent disease, extremes of age, feral behavior, or congregated housing, may increase the prevalence of infection in dogs and cats.184 Long haircoats, especially in cats, may predispose the animal to infection because the hair may become matted or protect spores from removal by grooming and increase contact with the underlying skin.184 Dermatophytes are spread between animals or to persons from animals by direct contact, by contact with infected hair and scale in the environment, or via contaminated fomites. The typical infective portion of the organism is the arthrospore, formed from the segmentation and fragmentation of fungal hyphae. These infective spores are small and can be carried on air currents and dust particles or solid fomites. Fleas from infected animals can also transmit the disease. Experimentally, Musca domestica (common housefly) was found to mechanically transmit M. canis spores with its outer body.39 The dose or quantity of infective material needed to establish spontaneous infection is unknown; under experimental conditions, at least 100 spores typically are required. Infections have been traced to contact with contaminated brushes, collars, environments, and casual contact with subclinically infected cats. Results of culture screening of grooming equipment and surfaces in veterinary clinics and grooming facilities indicated a low isolation rate of dermatophytes.6a Casual contact is of particular concern for individuals working in multiple-animal facilities because not many infected hairs are needed to transmit the infection. Transmission to a child from the contaminated interior of a used car was reported.273 After reaching the haircoat, infective spores must compete with natural host defense mechanisms to establish infection. Under optimal conditions of temperature (25° C to 37° C), infective spores can germinate within 6 hours of adherence to keratinocytes. The incubation period until lesions develop is typically 1 to 3 weeks. Arthrospores adhere very strongly to keratin. Arthrospores cannot penetrate healthy intact skin; some type of superficial trauma is needed to facilitate infection. The amount of trauma required is relatively minimal based on experimental models of infection.67 Increased hydration and maceration of the skin (e.g., within footwear) is a common predisposing factor to infection in humans. Moisture enhances the ability of dermatophytes to penetrate the skin and favors germination. In general, the normally dry condition of the skin surface and fungistatic properties of serum and sebum are natural host defense mechanisms. Normal grooming behavior of cats distributes sebum from areas of high concentration and production (chin and dorsum) to other areas of the body and may mechanically remove spores. Dermatophyte infections of dogs and cats involve the hair shaft, follicle, and surrounding stratum corneum. Infected hair shafts are fragile, and dislodged hair fragments containing infective spores are the most efficient means of transmission to other hosts. Such material may remain infectious in the environment for many months. Samples of hair stored for 18 months could yield positive culture results.268 The process by which infection occurs is complex; findings at the molecular level have been reviewed.279 The infection process has three stages: adhesion of arthroconidia to corneocytes; conidial germination; and fungal invasion of the keratin network. Little is known about the molecular aspects of these stages; however, studies using a reconstituted feline epidermis have shown that adherence to skin is time dependent, beginning within 2 hours and still increasing after 6 hours. Keratolytic proteases and fungal virulence factors were found to be important in the first stage of infection.173 Other proteolytic enzymes (keratinase, elastase, and collagenase) have been isolated from dermatophyte fungi and are likely involved in all three stages of the establishment and progression of an infection.1,33,34,82 These enzymes are produced under in vitro culture conditions and can be detected in infected skin biopsies.178 The number and quantity of enzymes produced varies from strain to strain and may, in part, explain variability in clinical findings.1 The overall immunologic status of the host influences development of infections. Various innate factors, distinct from the specific immunologic responses acquired as a consequence of infection, are involved in protection. Infections are more easily established in very young, very old, or immunocompromised animals and humans. Some factors are inherent in the defenses provided by healthy skin. Normal serum inhibits growth of dermatophytes, as do fungistatic fatty acids present in sebum. Physical occlusion leads to increased hydration of the normally dry skin surface, with subsequent maceration and easier infection. Dermal trauma facilitates development of infection. Concurrent immunosuppression, as with feline immunodeficiency virus infection, can result in an increased frequency of isolation of M. canis.150 Three different physical components of dermatophytic fungi have received the most attention for their role as antigens in the host immune response: cell wall carbohydrates, cell wall proteins, and secreted keratinases. Fungal mycelium is composed of approximately 10% protein, 20% carbohydrate (notably chitin and mannan), and 70% lipid. The immunologically active portion of the cell wall is composed of glycopeptide, with the carbohydrate portion chiefly involved in immediate-type hypersensitivity and the peptide portion important in delayed-type hypersensitivity. These glycopeptides are not fungal species–specific; abundant cross-reactivity is present across different genera of keratinophilic fungi. Most infected individuals also develop humoral and cell-mediated immunity (CMI) against fungal keratinases, proteolytic enzymes secreted at the tips of the invading hyphae. Fungal keratinases, including those from M. canis of feline origin, have been isolated and characterized chemically and immunologically.174,176 Antibodies developed against dermatophyte antigens cross-react with mycelial antigens from common saprophytic fungi such as Penicillium, Hormodendrum, and Aspergillus, and attempts to classify dermatophytes according to serologic reactivity were not successful. Though the host immune response to dermatophyte infection has been the subject of investigation for nearly a century, only relatively recently has the immunology of this disease been closely studied in companion animal species, and most information comes from studies performed in cats. Results of early animal studies indicated that CMI, rather than humoral immunity, is more important in resistance to reinfection. The majority of human patients with acquired dermatophytosis develop positive intradermal test reactions to dermatophyte antigens. Development of a delayed (48 to 72 hours) intradermal test reaction represents CMI and correlates well with both a positive lymphocyte blastogenesis test result and at least partial immunity to reinfection. Development of an immediate (15- to 30-minute) intradermal test reaction represents development of reaginic antibody-mediated hypersensitivity. Attempts have been made to relate the type of positive skin test reaction (immediate or delayed) to clinical status of the patient. As a general rule, human patients who recover from acute dermatophyte infections develop positive delayed intradermal test reactions and usually are relatively immune to further infection. In contrast, some human patients develop chronic, unrelenting dermatophytosis that persists for months or years. These patients develop strong immediate intradermal test reactions but often fail to exhibit delayed reactions even after prolonged disease. This evidence led to early speculation that a strong cell-mediated, delayed-type hypersensitivity response is responsible for elimination of the infection, whereas an immediate-type, antibody-mediated hypersensitivity response tends to inhibit or delay recovery.118 Natural infection of cats with M. canis is accompanied by both a humoral antibody response (IgG titers, and sometimes positive immediate intradermal test result) and a cell-mediated response (positive lymphocyte blastogenesis and delayed intradermal test results) against dermatophyte components, including glycoprotein extracts.66,68,68 Available data suggest that these responses are not completely Microsporum specific; that is, cross-reaction is present with Trichophyton. Delayed intradermal test reactions are often weaker in cats with active infections and in cats whose infection was aborted with antifungal treatment, suggesting that the infection must run its entire natural course for development of full immunity.194 Both intradermal test and in vitro data in cats support the concept that recovery from dermatophytosis depends on development of a strong CMI response. Genetic factors also influence the development of chronic dermatophytosis in humans, as evidenced by the higher prevalence of chronic infections among related individuals versus unrelated individuals living in the same household. Genetic factors may be important in cats as well. In one cattery study, chronic dermatophytosis was found most commonly in three facilities where the cats were genetically related.188 Cats with chronic dermatophytosis had significantly higher anti-dermatophyte antibody levels and different lymphocyte blastogenic responses. Dermatophytosis is a pleomorphic disease and cannot be diagnosed based on clinical signs alone. It is primarily a follicular disease, and the most common clinical signs include hair loss, scaling, crusting, and variable pruritus. Some patients may develop a classic ringlike lesion with central healing and fine follicular papules on the periphery. Generally, however, signs and symptoms are highly variable and depend on the degree of inflammation and hair shaft destruction (Table 56-1). Dermatophyte lesions in kittens tend to consist of areas of hair loss and scaling; erythema is variable and is often difficult to detect in dark-haired cats. Lesions are often first seen as areas of hair loss on the muzzle, face, ears, and forelegs (Fig. 56-1). Depending on the overall health of the kitten, lesions may be focal, multifocal, or generalized. Kittens with limited dermatophyte lesions that develop upper respiratory infections or gastrointestinal diseases, or both, are at increased risk for the development of generalized lesions. M. canis can cause comedone-like lesions (i.e., chin acne) in young cats. Uncommon presentations of feline dermatophytosis include an appearance clinically identical to pemphigus foliaceus, with scaling and crusting over the bridge of the nose and the face or crusting exudative paronychia, or both. Unilateral or bilateral pinnal pruritus is another underrecognized presentation of M. canis. In the cats examined by the author (KAM), infected hairs were limited to the ear margin or long hairs within the “bell” of the ear, or both. M. canis is uncommonly a cause of recurrent otitis externa.107 Rarely, diffuse alopecia with hyperpigmented patches of long hair has been observed.244 Granulomatous dermatitis, in the form of well-circumscribed, ulcerated dermal nodules, is infrequently recognized in cats (Fig. 56-2). The lesions occur on cats afflicted with more generalized typical M. canis infections. Interestingly, one report described different strains of M. canis isolated from the granulomatous lesions and from the surface infections of the same cat.178a,179 These lesions have been called mycetomas, pseudomycetomas, and Majocchi’s granulomas. This form of disease carries a poor prognosis for resolution.215a,245 Unlike cats, dogs are more likely to develop the classic foci of alopecia with follicular papules, scales and crusts, and a central area of hyperpigmentation (see Table 56-1). Dermatophytosis should be considered in any papular or pustular eruption. Facial folliculitis and furunculosis, superficially mimicking an autoimmune skin disease, can develop (Fig. 56-3). Nodular skin lesions called kerion reactions may occur on the face and legs (Fig. 56-4). These lesions appear as areas of deep pyoderma and are most often caused by M. gypseum or Trichophyton spp. infections.59a More generalized, widespread lesions should prompt a search for a potential underlying systemic cause predisposing to the infection.49 Onychomycosis may be exhibited by chronic ungual fold inflammation, with or without footpad involvement, or the claw alone may be infected, which causes claw deformity and fragility. Dermatophytic granulomas have been reported rarely in dogs.10 One of the authors (DJD) has seen several shorthaired dogs with histories of recurrent urticaria and papular eruptions caused by dermatophytosis. Although some research has focused on cellular and humoral responses to dermatophyte infections,68,75,264,266 only preliminary work has been reported on serologic diagnosis of canine dermatophytosis.98,226,226 Follow-up studies examining cross-reacting antibodies in experimental and naturally occurring feline dermatophytosis are needed because serologic detection might be a useful tool for protecting cat breeders from importing infected animals to their facilities. Additionally, investigating the appropriateness of the immune response to infection in groups of persistently infected animals may soon become possible.265 Other new developments in diagnosis include the production of polymerase chain reaction probes and immunohistochemical stains for the various species of dermatophytes.* Such techniques promise more rapid identification in clinical specimens but have yet to be developed for commercial use. Calcofluor white has been mentioned to improve the diagnostic accuracy of direct microscopic examination of hairs (see following discussion).267 Examination of the haircoat with a Wood’s lamp (ultraviolet light, 320 to 400 nm wavelength) is a quick and easy initial screen for presence of certain dermatophyte infections but clearly is not definitive. Perhaps only half of all strains of M. canis will show fluorescence, and other animal dermatophyte species will not, rendering the Wood’s lamp an insensitive test. The fluorescence results from a fungal metabolite produced only when the organism is growing on hair and not on scale or claw material. Thus, true fluorescence is bright “apple green” in color and occurs only along hair shafts, never in scale (Fig. 56-5). Debris, scale, lint, and topical medications commonly produce false fluorescence. Because of these pitfalls, suspected fluorescing hairs should always be cultured or examined microscopically, or both, to confirm presence of the infection. Compared with culture, the Wood’s lamp examination was found to have a sensitivity of approximately 50% for M. canis infection.261 Wood’s lamp examination is most useful when monitoring infection status in a cattery or multiple-animal facility where endemic infection with a fluorescing strain is involved. In examining a patient with a Wood’s lamp, make sure that the lamp has been allowed to warm up for a few minutes before the examination, and spend several minutes (with room lights out) examining the haircoat slowly and closely both to allow sufficient time for dark adaptation and to avoid missing small areas of fluorescence.191 The authors find that the larger, more powerful lamps that operate on household current are superior to smaller, battery-operated models. Mineral oil is often used to remove and suspend hairs for microscopic evaluation. However, the hair shafts are opaque, thereby reducing visualization of the fungal elements. Hair and scale may be wet mounted in 10% to 20% potassium hydroxide (KOH) overnight or heated gently in the same solution for 10 minutes for clearing of keratin and visualization of fungal elements. Mounting in chlorphenolac (50 g chloral hydrate, 25 mL liquid phenol, and 25 mL liquid lactic acid) solution accomplishes the same task in a few minutes without heating, but the solution is not commercially available and is cumbersome to prepare. Direct examinations can be performed using mineral oil; the author (KAM) recommends holding the Wood’s lamp over the glass slide to locate the glowing hairs. Even in experienced hands, direct examination is time-consuming and may be diagnostic in only a few cases; however, results of a study of animals with dermatophytosis found that it can be a reliable in-house test allowing for confirmation of infection and initiation of therapy pending fungal culture.58 In that study, use of mineral oil skin scrapings of lesions instead of hair pluckings were found to be superior. The authors (KAM and DJD) recommend both plucking and scraping of suspect lesions to maximize identification of infection in highly suspect cases. Care must be taken, because this can still lead to misinterpretation if saprophytic fungal spores are present in the specimen; debris and the complex structure of normal hair shafts can be misinterpreted as fungal elements. Dermatophytes never form macroconidia in tissue, but rather form hyphae and arthroconidia (ectothrix spores) on hair and scale. One investigation has recommended hair examination with a solution of 0.5% calcofluor white stain (Beckton Dickinson, Franklin Lakes, NJ) and Evans blue dye (1:9 solution) in equal volume of 20% KOH for superior visualization of fungal elements.267 Direct microscopic examination is most useful when fluorescing hairs are located and can be directly plucked with a forceps under a Wood’s lamp. The Wood’s lamp can also be used as a light source for the microscope to aid in visualization. In this case, the finding of ectothrix spores along the hair or mycelium, or both, growing down the center of the hair shaft (Fig. 56-6, A and B) is justification for beginning treatment while awaiting more definitive culture identification of the pathogen. Compared with normal hairs, infected ones are thicker, frayed, and indistinct with a filamentous appearance. Definitive diagnosis of dermatophytosis is made by culture, although it is neither perfectly sensitive nor always specific for the diagnosis.261 Proper specimen collection techniques, regular examination of the growing cultures, and microscopic confirmation of the fungal species are all necessary for ideal performance of this test. Culture can readily be performed as an in-office procedure employing dermatophyte test medium (DTM). DTM consists of a nutrient medium plus inhibitors of bacterial and saprophytic growth and phenol red as a pH indicator. Several variants are available, some of which claim to speed growth of the culture, but this has not been found be clinically significant and commercial medium appears to perform similarly.86,114,203a,277 DTM is sold in either vials or plates; the latter are preferable because they permit inoculation with a toothbrush. For incubation, DTM containers should be loosely covered or capped at room temperature and protected from desiccation. Placing individual cultures in a plastic self-closing bag will help prevent desiccation, cross-contamination, and mite infestation of the culture medium. Dermatophyte colonies may appear as soon as 5 to 7 days after inoculation and almost always develop within 14 days, assuming the animal has not been treated. For animals that have been treated, plates should be held for 21 days. Plates should be inspected daily for a color change of the medium to red and growth of a white to buff colored, powdery to cottony mycelium. The color change must occur at the same time the colony is first visible, never later. All fungal growth, including nonpathogens, will produce a red color change after the colony has grown for several days to a week. Dermatophyte colonies are never green, gray, brown, or black. Dysgonic varieties of M. canis (sometimes called M. canis var. distortum) may occasionally be isolated, particularly from cat colonies.231,250 These varieties grow under the surface of the agar, giving a feathery or snowflake-like appearance. They may not produce conidia or a red color change on DTM191 and can be converted to more typical forms by a diagnostic laboratory for identification.162 Plates should be kept for 21 days, especially in monitoring treated animals, because fewer viable fungal spores will be present and their growth rate is often reduced. Most often, large numbers of colonies of the dermatophyte will appear on the plate if the animal is truly infected. Table 56-2 describes the appearance of the most common isolates. The number of colonies decreases as the infection resolves spontaneously or from treatment. In some cases, only one or a few dermatophyte colonies appear. In addition to recovery, this finding may indicate either early or mild infection or poor sampling technique. This result can also be seen in uninfected cats that are living in the same environment as are infected cats; in this case, the cat’s coat has become contaminated with spores from the environment (i.e., the cat is acting as a fomite). Such innocent carrier cats are impossible to distinguish from cats with a mild infection using culture alone, and if any question arises, the suspect cat should be treated. TABLE 56-2 Comparison of Dermatophytes Isolated from Dogs or Cats One of the most common problems with in-house culturing is the lack of sporulation or growth, or both, of M. canis on DTM. It is important to note that growth will be slow in animals that are being treated. Additionally, the common recommendation is to incubate at room temperature. Increased sporulation was found when plates were incubated at higher temperatures (21° C to 23.8° C).114,277 Ambient temperature in many veterinary clinics, especially during warm-weather months when the air conditioning is being used, is well below this temperature. The current recommendation by the authors of this chapter is to incubate cultures at higher temperatures. It is also not necessary to incubate cultures in the dark, because light exposure has not been found to adversely affect sporulation.277 The diagnostic accuracy of DTM in separating pathogenic from nonpathogenic fungi is not perfect, and even an immediate red color change is not definitive for pathogenic fungi. For example, colonies of the nonpathogen Scopulariopsis can appear grossly identical to those of dermatophyte colonies and cause an immediate red color change. Therefore, suspect colonies must be examined microscopically. After 7 to 10 days of growth, most colonies will begin to produce spores, whose morphology will allow specific identification of pathogens. This is achieved by brushing a small strip of clear cellophane tape lightly over the colony surface to collect spores. The tape is placed (sticky side down) onto a drop of lactophenol cotton blue stain or new methylene blue on a microscope slide. Another drop of stain is added on top of the tape. A coverslip is applied and the slide is examined at ×100 magnification. Among the many hyphal strands will be dermatophyte macroconidia (spores), which have typical shapes according to the species (see Fig. 56-6,C; Table 56-2; and Chapter 54). If spores are not visible, the procedure is repeated 4 to 7 days later; some colonies may not sporulate until they are quite old. M. gypseum readily produces large numbers of macroconidia in comparison to M. canis. A suspect colony that fails to produce spores or is difficult to identify, as is sometimes the case in Trichophyton species, should be sent to a qualified diagnostic laboratory. Importantly, in an otherwise healthy animal, dermatophytosis is generally a self-curing disease, with full resolution after development of an appropriate CMI response. In dogs, localized lesions, and even generalized M. canis or M. gypseum infections, can resolve without treatment.70,164,164 Cats and kittens with seemingly localized disease can also self-cure, but infection can be prolonged (from at least 60 to 100 days). Positive culture results from cats do not prove that the dermatophyte is causing the skin lesions given that they can innocently harbor the organisms as a fomite, and that clinical signs are variable, mimicking other skin diseases. Treatment is recommended nevertheless because it will accelerate resolution of lesions caused by dermatophytes, thus minimizing the time course of the infection and minimizing the potential for spread to other animals or humans. Wherever possible, curing the infection in the pet, while simultaneously decontaminating the environment, is desirable. Drugs and dosage regimens are summarized in Table 56-3. TABLE 56-3 Drugs for Systemic Therapy of Dermatophytosis in Dogs and Cats B, Both dog and cat; C, cat; D, dog; PO, by mouth. aSee the Drug Formulary in the Appendix for additional information on each drug. bDose per administration at specified interval. cFollow-up brush culture results should be negative before discontinuing therapy. Two negative culture results are recommended. dTrade name: Nizoral; generic available; cats often receive 50 mg total dose daily; with side effects, every-other-day treatment is used. Not recommended for use in cats because of adverse effects: vomiting, inappetence. eTrade name: Sporanox (human). Capsules may be opened and contents divided to administer recommended doses, or use oral solution. Bioavailability of oral solution is higher than that of capsule formulation; see the Drug Formulary in the Appendix. fAfter 15 days, fungal culture is performed and cycle is repeated until negative culture result is obtained. Usually takes 1–3 cycles (15–45 days). This is not favored by the authors as it has only been evaluated in a small number of animals. gTrade name: Lamisil. Not yet evaluated in dogs. Monitor liver enzymes in cats. Can be substituted for itraconazole in various regimens. hTrade names: Grifulvin V, Fulvin U/F, Grisactin. For small kittens, usually culture negative at 8 weeks and cured at 10 weeks. iDose can be divided and given every 12 hours. Dose of 50 mg/kg daily, of microsize, has often been effective after 41–70 days of therapy. jDose is approximately two thirds that of microsize preparation. Some preparations contain polyethylene glycol to facilitate absorption. Trade names: Fulvicin P/G, Grisactin Ultra, Gris-PEG. Effective dosages are higher than manufacturer suggests and may be toxic. Note: manufacture of this drug may be discontinued. Experimental infection studies have emphasized that the ideal treatment regimen is composed of three elements (topical, systemic, and environmental treatment), each of which has a somewhat different role. Topical therapy reduces contamination on the haircoat (and thus environmental contamination) and results in a faster mycologic cure than systemic therapy alone.263 However, with the exception of lime-sulfur or enilconazole, topical therapy appears to do little to accelerate complete clinical resolution in the animal. Systemic therapy, in contrast, benefits the individual animal by reducing the number of weeks to complete cure. Environmental treatment also reduces the chances that the infection will spread to other animals or humans in the same household. Clipping of the haircoat will mechanically remove fragile hairs that will otherwise fracture and release spores into the environment and onto the haircoat. Furthermore, it allows for thorough penetration of topical medications, reducing the amount and duration of treatment. Clipping of the entire haircoat is the optimal treatment in all cases of dermatophytosis; however, this is not always possible or practical. Clipping is time consuming and often requires sedation and can be irritating to cats. If not done carefully, thermal burns from electric clipper blades can also occur. Clipping of localized lesions can be easily accomplished using children’s scissors, which are disposable. For more generalized lesions, clipping of the haircoat with a No. 10 clipper blade is usually adequate. One of the authors (KAM) has successfully treated shelter cats for mild to severe generalized dermatophytosis without clipping of the haircoat using a combination of twice weekly topical therapy and itraconazole (ITZ).198,215 However, success was only achieved with thorough application of the topical solution, concurrent use of a systemic drug, and confinement of the pet. In some cattery situations, it may be mandatory for successful eradication. Clipping of the haircoat may temporarily irritate the skin and exacerbate lesions; however, these complications should not be used as a reason to not clip the haircoat. To date, no evidence has been found to support the use of localized or spot treatment for dermatophytosis in dogs or cats. In fact, evidence has been found suggesting that use of spot treatment products alone may predispose individuals to chronic subclinical infections.31 Rather, whole-body shampooing, dipping, or rinsing with topical antifungal agents is much preferred. General topical treatment recommendations are summarized in Box 56-1. Generalized topical therapy, in addition to clipping of the haircoat, will help decrease contamination of the environment by hairs and spores, decrease the chance of spread of the disease to other animals and humans, and help speed mycologic cure of the infection. The choice of topical antifungal treatment is important because studies have shown that many common topical agents are ineffective.42,70,191,197,282 The relative efficacies of various topical antifungal drugs is summarized in Table 56-4. In vitro and in vivo studies have shown that the most consistently effective topical antifungal whole-body treatments are lime-sulfur, enilconazole, and miconazole (the last with or without chlorhexidine).* Captan, povidone-iodine, and chlorhexidine are consistently ineffective against M. canis and should not be used in lieu of the following products listed. TABLE 56-4 Efficacy of Various Topical Agents Recommended for Treatment of Dermatophytosis in Dogs and Catsa aIn vitro tests performed with infected hair. bCombined 2% miconazole/chlorhexidine shampoo has been more effective than either agent alone. Lime-sulfur has been extensively tested in in vitro infected spore models and, when used at concentration of 8 ounces/gallon of water (1:16 dilution), has shown superior antifungal activity.94,197 Lime-sulfur treatment alone, twice weekly at this concentration, has been used by one of the authors (KAM) to successfully treat dermatophytosis in single- and multiple-cat households when combined with or without whole-body clipping and appropriate environmental treatment. Lime-sulfur is very safe and can be used on newborn kittens and puppies. There are many formulations of lime-sulfur available, and no difference in the sporicidal efficacy against M. canis was found among them when tested in vitro.84In one study involving cats (see under discussion of miconazole-chlorhexidine rinse below), lime-sulfur original formulation was more effective than a similar preparation with odor modification.214a Enilconazole topical solution is also an effective treatment; unfortunately, it is not available in the United States and is licensed only for use in dogs and horses. Because of its superior antifungal activity, the safety and efficacy of enilconazole have been evaluated in cats.115,122 In two studies, enilconazole was evaluated as a sole topical therapy (after whole body clipping) for the treatment of naturally occurring M. canis infection in Persian cats.79,122 In one study, cats were dipped with either water (control) or 0.2% (2 mg/mL) enilconazole twice weekly for 8 weeks.79 In the enilconazole-treated cats, results of fungal cultures were negative as early as 5 weeks after initiation of therapy and remained negative to the end of the 10-week monitoring period. In contrast, 75% of control cats still had culture-positive results at the end of 10 weeks of monitoring. In the second study, 22 Persian cats in a cattery were treated with 0.2% enilconazole every 3 days for a total of eight applications.122 All cats improved clinically and had culture-negative results by day 28 of therapy. In both studies, cats were observed for adverse effects, and serum biochemistry panels were monitored. Enilconazole was well tolerated but may have been associated with hypersalivation, anorexia, weight loss, emesis, idiopathic muscle weakness, and slightly elevated serum alanine aminotransferase activities. Anecdotal reports have surfaced of uncommonly severe toxicity, and even death, occurring in cats after topical application of enilconazole. These cases are thought to be associated with ingestion of the solution by the cat via grooming after application. Enilconazole appears safe for use on cats if the animals are fitted with an Elizabethan collar for a few hours after each treatment, to prevent grooming, until the cat is dry. See the Drug Formulary in the Appendix for further information. Miconazole is also an effective topical antifungal in both in vitro and in vivo studies.158,229 Synergism between miconazole and chlorhexidine has been demonstrated in vitro,229 and shampoos with this combination of ingredients are documented to hasten mycologic cure.263 Using an isolated infected spore model, a miconazole (5.2%)–chlorhexidine gluconate (5.9%) commercial rinse was found to be as sporicidal as lime-sulfur.199 In a nonrandomized study, cats were treated with oral ITZ with topical therapy of either lime sulfur, lime sulfur with odor modification, or miconazole-chlorhexidine commercial rinse with mycologic cure rates at 42 days after treatment of 87.1%, 59.2% or 52.6% respectively.214a It is important to note that miconazole-based products are most effective when used as adjunct therapy and not as sole therapy.
Cutaneous Fungal Infections
Dermatophytosis
Etiology
Pathogenic Dermatophytes
Fungal Microflora on the Hair and Skin
Epidemiology
Pathogenesis
Transmission and Predisposing Factors
Virulence Factors
Immunologic Factors
Dermatophyte Antigens
Acquired Immunity During Infection
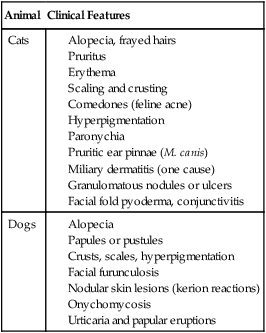
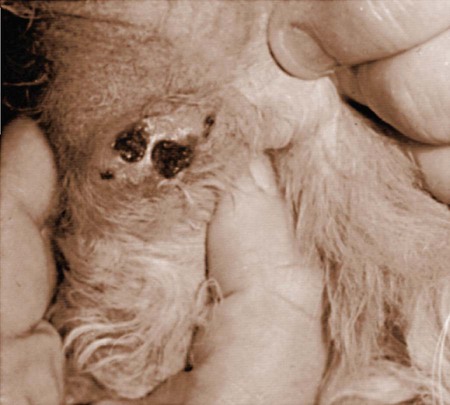
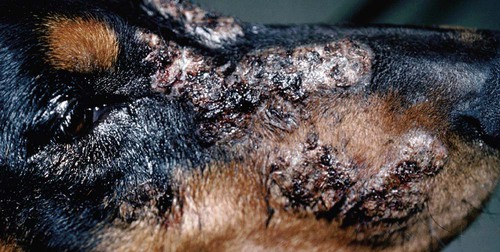
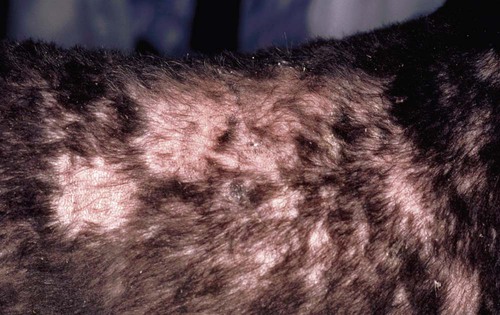
Clinical Findings
Cat
Dog
Diagnosis
Wood’s Light Examination
Direct Microscopic Examination
Fungal Culture
Media, Incubation, and Interpretation
Organism
Colony Characteristics
Microscopic Appearance
Microsporum canis
White top surface, yellow-orange reverse pigment undersurface; flat with depressed center; cotton or wool consistency
Macroconidia: spindle- or canoe-shaped; each contains ≥6 cells with thick walls with outer spines on surface and terminal knob
Young colonies may have <6 cells
Microconidia: single cell and may be common or rare
Microsporum gypseum
Cinnamon brown top surface, yellow to tan reverse pigment undersurface; flat colonies; face powder consistency
Macroconidia: rowboat-shaped; each contains <6 cells with thin walls
Microconidia: single cell
Trichophyton spp.
White to cream top surface, tan to brown to red reverse pigment under surface
Macroconidia: rare; cigar-shaped
Microconidia: common; often spinal hyphae
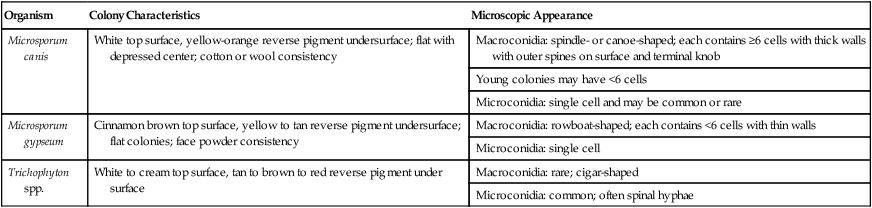
Microscopic Confirmation
Therapy
Druga
Species
Doseb (mg/kg)
Route
Interval (hours)
Durationc (weeks)
Ketoconazoled
D
10
PO
24
4–8
Itraconazolee
D
5–10
PO
24
4–8
C: daily therapy
10
PO
24
4–8, or until cured
C: continuous/pulse
10
PO
24
4, then every other week until cured (~8–10 weeks)
C: low-dose cycle
1.5–3
PO
24
Variablef
Terbinafineg
B
30–40
PO
24
3–18
Griseofulvin
Microsizeh
B
25–50
PO
24i
6–10
Ultramicrosizej
B
10–30
PO
24i
6–10
Clipping of the Haircoat
Topical Therapy
Topical Agent
Available Formulations
Comments on Efficacy
Chlorhexidine
Shampoo, rinse
No advantage over control treatment in experimental clinical infection; inferior in in vitro testsb
Clotrimazole
Cream, lotion
Creams and lotions not formulated to penetrate infected hair
Enilconazole
Rinse, fogger
Superior in in vitro tests
Ketoconazole
Shampoo, cream
Tested in animal models, fair efficacy; creams not formulated to penetrate infected hair
Lime-sulfur
Rinse
Superior at 1:16 dilution in in vitro tests
Miconazole
Shampoo, cream
Superior in in vitro testsb
Povidone-iodine
Shampoo, rinse, ointment
Poor performance in vitro, not recommended
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cutaneous Fungal Infections