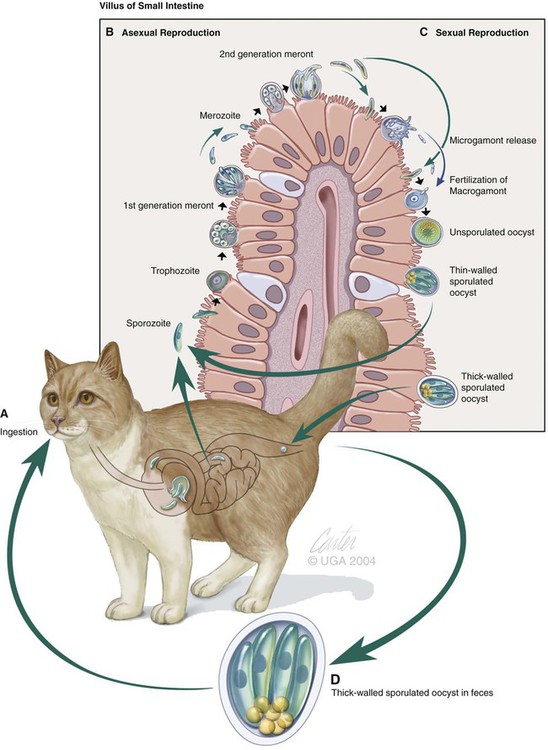
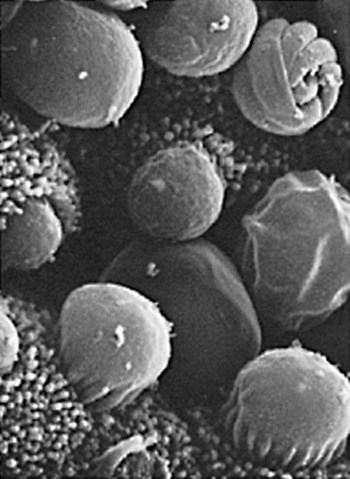
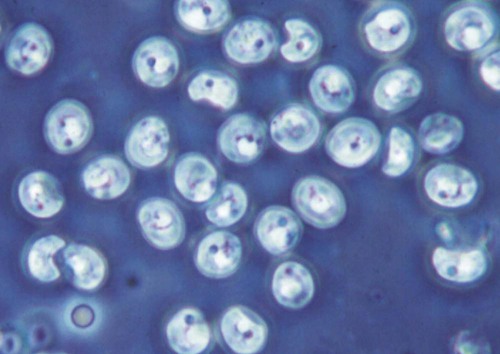
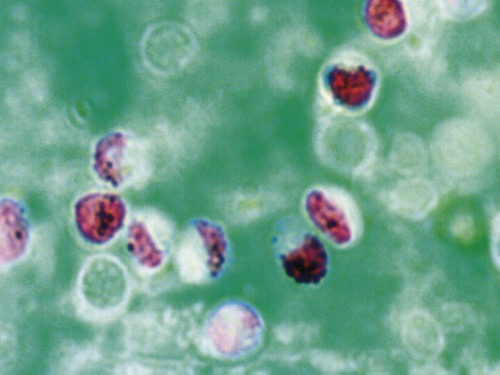
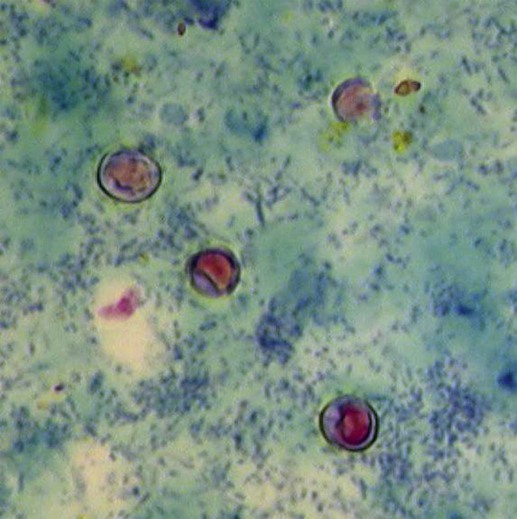
Cryptosporidium is a ubiquitous coccidian genus in the phylum Apicomplexa, class Conoidasida, suborder Eimeria, family Cryptosporidiidae, that inhabits the epithelium of the respiratory and digestive systems of reptiles, birds, and mammals. Most species may be relatively host specific. Cryptosporidium found in reptiles and birds apparently do not infect mammals. There are 16 accepted species of Cryptosporidium and almost 50 Cryptosporidium genotypes have been described in animals (Table 81-1).278,279 Most Cryptosporidium spp. are host adapted and have a narrow spectrum of natural hosts. Cryptosporidium parvum has been detected in feces from a few dogs, but in general most studies report that dogs are infected with the host-specific Cryptosporidium canis. Cryptosporidium muris has also been detected in the feces of naturally infected dogs.146 Three Cryptosporidium spp. have been detected in feces of cats: C. parvum, Cryptosporidium felis, and C. muris; however, only C. felis and C. muris have been found in naturally infected cats.65,200,216,225,226 C. canis or C. felis DNA are rarely amplified from the feces of humans (see Public Health Considerations). TABLE 81-1 Cryptosporidium Species and Host Range Modified from Ref. 278. Specific-pathogen free cats developed chronic infection after being inoculated with C. parvum.231 However, these cats showed minimal clinical signs of disease, even after the administration of glucocorticoids, suggesting that the C. parvum isolate used was minimally pathogenic in clinically healthy cats.231 In another study, C. parvum infection (of bovine origin), developed in inoculated dogs and cats.47 Dogs experimentally infected with C. muris oocysts, isolated from the stomachs of experimentally infected mice, rarely developed infections, and canids were discounted as true hosts for C. muris.11 However, when C. muris was administered to three kittens, large numbers of oocysts were shed for extended periods.112 C. canis oocysts have been inoculated to mice and to cattle but not to dogs.66 In another study, dog pups and kittens experimentally inoculated with C. parvum shed cryptosporidian oocysts after a prepatent period of 2 to 14 and 2 to 11 days, respectively.9 Patent periods lasted 3 to 33 days in pups and 2 to 25 days in kittens. None of the infected animals showed clinical illness.9 Cryptosporidium spp. infections in cats and dogs have been described in prevalence studies and in a small number of case reports. Prevalence rates for Cryptosporidium spp. infections in dogs and cats have varied between reports, which are affected by the study population and by the different diagnostic techniques that were used (Tables 81-2 and 81-3). Only a few studies have genetically characterized Cryptosporidium isolates from cats and dogs (Web Tables 81-1 and 81-2). Cryptosporidium spp. can be primary pathogens or secondary invaders of immunosuppressed individuals. Infections of the ileum are most frequent, but gastric, respiratory, and conjunctival infections have been reported in immunosuppressed hosts. As found in other species, diarrhea associated with Cryptosporidium spp. infections may be more common in young dogs and cats.167,239,239 TABLE 81-2 Prevalence of Cryptosporidium spp. in Dogs ELISA, Enzyme-linked immunoassay; FA, direct fluorescent antibody method; PCR, polymerase chain reaction. TABLE 81-3 Prevalence of Cryptosporidium spp. in Cats EIA, Enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; FA, direct fluorescent antibody method; PCR, polymerase chain reaction. WEB TABLE 81-1 Prevalence of Cryptosporidium Species/Genotypes in Cats by Genetic Markers Modified from Ref. 146. WEB TABLE 81-2 Prevalence of Cryptosporidium Species/Genotypes in Dogs by Genetic Markers Modified from Ref. 146. Cryptosporidium spp. are transmitted via the fecal-oral route. The life cycle differs from those of most other coccidians, and all stages of development occur within one host (Fig. 81-1).180 It begins with the ingestion of sporulated oocysts by a host.155 Oocysts excyst in the gastrointestinal (GI) tract, releasing infective sporozoites, which become enclosed as trophozoites within parasitiferous vacuoles of the microvillous surface of enterocytes (Fig. 81-2). The trophozoites proliferate asexually by merogony to produce, sequentially, two types of meronts. Within 24 hours, type I meronts (containing eight merozoites) leave the parasitiferous vacuoles to invade other epithelial cells, where they develop into more type I meronts or type II meronts (containing four merozoites). The type I meronts are thought to be capable of recycling indefinitely and, thus, the potential exists for new type I meronts to arise continuously. The type II meronts do not undergo merogony but produce sexual reproductive stages (gamonts). The zygotes formed by sexual reproduction (gametogony between male microgamonts and female macrogamonts) form either “thick-walled” or “thin-walled” oocysts, each containing four sporozoites. Approximately 20% of the oocysts produced in the gut are thin-walled oocysts that failed to form an oocyst wall. Instead, a series of membranes surround the developing sporozoites.64 These thin-walled oocysts are immediately capable of releasing infective sporozoites. Thus, C. parvum appears to have two autoinfective cycles: the first by continuous recycling of type I meronts, and the second through sporozoites released from ruptured thin-walled oocysts. The thick-walled oocysts are passed in the feces into the environment. Oocysts of cryptosporidia are highly resistant and spread via the fecal-oral route. They are sporulated when passed in feces and so are immediately infectious; in humans between 1 and 1000 oocysts are enough to cause infection.189 C. felis oocysts are shed in the feces 3 to 6 days after infection. C. felis and C. canis oocysts are similar in size; C. felis oocysts are 5 µm by 4.5 µm and C. canis oocysts are 4.95 µm by 4.71 µm.66,112 Infection occurs after oocysts are ingested from coprophagia, grooming, contaminated food, or water. It is also possible that infection occurs when dogs or cats ingest infected prey species. Cryptosporidium spp. oocysts are resistant to environmental stress and to most common disinfectants, and so infection by ingestion of contaminated food or water is common (see Prevention section). Large outbreaks can occur when a community water source becomes contaminated.62 In one report, the risk factors for cats shedding Cryptosporidium oocysts were the presence of another cat in the household shedding oocysts and the concurrent detection of Giardia spp. infection.14 There is little information regarding the pathogenesis of C. felis or C. canis in cats and dogs. The information presented here is from research done in humans, mice, and cattle after infection with C. parvum. Cryptosporidium and Giardia have very similar pathogenic mechanisms, but the molecular pathways by which Cryptosporidium causes disease are still unclear. The organism is extremely infective; as few as 100 oocysts are necessary to precipitate disease in humans.156,189 Cryptosporidium sporozoites attach to the intestinal epithelium between the cell membrane and the cell cytoplasm; this location may somewhat explain their resistance to chemotherapy.49 The pathologic changes are caused by both the parasite factors and the host-immune response against the organism. Cryptosporidiosis is thought to cause diarrhea by a combination of intestinal malabsorption of electrolytes and nutrients with hypersecretion of chloride and water.105 C. parvum is considered a minimally invasive mucosal pathogen; however, its attachment to the apical cell surface and the release of other parasite products after attachment can activate cell-signal pathways that alter cell function.40 After attachment to the host cell, C. parvum appears to “hijack” the cytoskeleton of the host cell, rearranging the host actin at the infection site, resulting in membrane protrusion to facilitate parasite internalization and recruitment of membrane transporters and channels to the parasite host-cell interface, which provide the necessary factors for parasite development.40,49 On the epithelial cells, Toll-like receptors identify the foreign Cryptosporidium and activate downstream signaling pathways activating nuclear factor κB to trigger a series of immune responses.40 The expression of enteric beta defensin, a peptide component of the innate defenses of the intestinal tract, was upregulated by 5- to 10-fold in calves after C. parvum infection.49 The release of nuclear factor κB–associated cytokines and chemokines plays an important role in the inflammation caused by cryptosporidiosis.40 Interleukin-8 and other proinflammatory cytokines are upregulated during C. parvum infection.40 However, transforming growth factor-β, which is an anti-inflammatory cytokine, is also upregulated during infection and seems to play a protective role in limiting the epithelial damage produced by infection with C. parvum.49 Malabsorption and maldigestion are caused by loss of the epithelial brush border and diffuse microvillus shortening that is mediated by activated T lymphocytes.29 As with giardiasis, brush border injury in cryptosporidiosis is likely to be the result of a host-mediated event, rather than from an effect from direct exposure to parasite products. An increased number and activation of the CD8+ Γδ T-cell populations have been reported in the intraepithelial compartment during intestinal infestation with Cryptosporidium.29 Intestinal permeability is also increased in cryptosporidiosis.105 Cryptosporidia disrupt epithelial tight junctions in a mechanism similar to that of Giardia or other enteropathogens.29,30 Loss of epithelial barrier function activates host immune pathologic pathways, which increases the levels of apoptosis in the intestinal epithelial cells and facilitates the activation of intra- or subepithelial immune cells.29 Several proteases specific to Cryptosporidium are involved in host cell invasion; however, the specific role of these proteases in infection is uncertain.29 Host enterocytes produce antimicrobial products that modulate the pathophysiologic pathway, including nitric oxide, and a newly discovered antiapoptotic mechanism that involves a glucose-mediated absorbtion.29 Alterations of expression of microRNAs (miRNAs), a new class of small regulatory RNA molecules, have been found in epithelial cells after C. parvum infection.40 These miRNAs are apparently involved in the posttranscriptional regulation of epithelial antiparasitic responses.40 Loss of microvilli, degeneration of host epithelial cells, and atrophy of the villi have been described in heavy infections of cats. These alterations may predispose to malabsorption or other GI dysfunction.78,131,131 Moderate small intestinal lymphocytic infiltrates were detected in one cat infected with Cryptosporidium spp., in the absence of concurrent infections.131 Histologic findings of intestine from infected dogs revealed severe crypt damage with hyperplasia, dilation, marked villous atrophy, and loss of glands from the mucosa. The lamina propria had been infiltrated with lymphocytes, plasma cells, and neutrophils.10,50,50 In one report involving an infected puppy, organisms, identified as C. canis by polymerase chain reaction (PCR), were more prominent within the gastric region.167 Host immunity is likely important in the development of infection and clinical illness after exposure to cryptosporidia, although the association between risk factors has been confusing. Many cats shedding Cryptosporidium oocysts, or having Cryptosporidium spp. antigens or DNA in their feces, do not show clinical signs of disease.6,8,8 In New York State, 10 of 263 (3.8%) cat fecal samples had positive test results for Cryptosporidium spp. by enzyme-linked immunosorbent assay (ELISA); however, diarrhea was not significantly associated with their detection.246 In northern Colorado, the presence of oocysts or antigen in feces was detected in 11 of the 206 (5.4%) fecal samples.100 Only 4 of the 11 cats with Cryptosporidium had diarrhea.246 In an uncontrolled study population of cats with diarrhea, 6 of 179 (3.3%) and 52 of 179 (29%) were shown to be infected by Cryptosporidium spp. by direct immunofluorescent antibody (FA) assay or the PCR method, respectively.233 However, further studies, evaluating corresponding groups of cats without diarrhea, will be required to determine the pathogenic potential of C. felis in wider groups within the cat population. Because of the isolation of cryptosporidia from clinically healthy animals, finding the organism in an animal with clinical illness must always be viewed with caution as an indication of a direct role of the parasite. In the small number of reported cases of suspected clinical cryptosporidiosis in cats, small-bowel diarrhea, associated with large volumes, anorexia, and weight loss, was the most common clinical sign of disease. Vomiting has been uncommon in cats with cryptosporidiosis unless concurrent abnormalities exist. Most cats with diarrhea and Cryptosporidium spp. infection have been immune suppressed, had preexisting disease of the intestines, or had co-infections.26,78,79,131,203 Cats may have had immune suppression associated with feline leukemia virus or feline immunodeficiency virus infection or were under other conditions of stress.19,78,79,172,203 Some cats with suspected clinical cryptosporidiosis have had co-infections, which makes it difficult to determine whether Cryptosporidium spp. are the primary cause of the observed clinical signs. Cystoisospora spp., Toxocara cati, coronavirus, Giardia, Tritrichomonas foetus, and Campylobacter had been documented in cats with Cryptosporidium spp. in feces and diarrhea.* However, co-infection with other parasites has also been reported in healthy cats with Cryptosporidium spp. infections.19 Noninfectious diseases associated with cryptosporidiosis in cats are lymphoma and inflammatory bowel disease.26,131,131 In a case report of a cat with concurrent inflammatory bowel disease, the inflammatory changes resolved after the parasite was successfully treated with tylosin at a dose of 11 mg/kg orally (PO) twice daily for 28 days, suggesting that the inflammation was due to the parasitic infection.131 Some cats with chronic Cryptosporidium spp. infections had stopped shedding oocysts in feces only to have shedding recur after the administration of glucocorticoids, which suggests that low-level infection can persist for months in this species.8,165 Cryptosporidial infection was identified in a cat with chronic diarrhea.66a Over 1 year later the cat developed recurrent vomiting. Gastroduodenoscopic findings of mucosal edema and cryptosporidia were found by histopathologic examination in the gastric and duodenal mucosae and were identified by PCR as Cryptosporidium muris and C. felis, respectively. C. muris normally inhabits the gastric glands of mice; however, gastric infection has been reported in humans.198 Cryptosporidium spp. have also been detected in dogs with and without GI signs of disease. The diarrhea is usually small bowel, characterized by high-volume, low-frequency stools and significant weight loss. Fresh blood, tenesmus, and discomfort can be reported in some chronic cases. In a study in northern Colorado, 5 of 130 (3.8%) dogs had positive results for Cryptosporidium spp. by direct FA; 4 of the dogs had diarrhea.91 In an uncontrolled study, 2 of 113 (1.7%) and 19 of 113 (16.8%) of dogs with diarrhea were shown to be infected by a Cryptosporidium spp. by direct FA or PCR methods, respectively.233 These results and those of others suggest that dogs are commonly exposed to the organism. However, there is very little information in the veterinary literature concerning cryptosporidiosis in dogs. It may be that C. canis is very host adapted in dogs and rarely associated with disease in healthy dogs. There are several published case reports of puppies with diarrhea thought to be from infections with Cryptosporidium that had co-infections of parvovirus, distemper, parasitism, or lymphoma.10,50,69,257,271 Intestinal malabsorption was reported in an adult dog with cryptosporidiosis.84 One of the authors (MRL) had been consulted about a 3-month old papillon dog with chronic diarrhea. The dog had positive test results for Giardia spp. for several months after successive treatments with metronidazole, fenbendazole, and Giardia vaccine. Cryptosporidium spp. co-infection was confirmed, and tylosin was administered at 11 mg/kg, PO, every 12 hours for 28 days. By day 9 of the treatment, stools were normal and the dog had negative test results for both parasites by direct FA. The medication was discontinued, and a week later the dog appeared to be healthy. In a study of Cryptosporidium spp., isolates originally obtained from a patient and a pet dog were found to have cattle and dog genotypes, respectively.2 C. muris infection was identified in the stomach of a dog that had a chronic history of vomiting, and profuse diarrhea that was observed during physical examination.59a Histopathologic findings of stomach biopsy specimens were gastritis associated with cryptosporidia and numerous spiral bacteria compatible with Helicobacter spp. The dog was treated empirically for gastric helicobacteriosis with metronidazole and omeprazole and had clinical improvement during a 6-week follow-up period. Cryptosporidium oocyst shedding can be sporadic, and so a negative assay result may not completely exclude the infection. Although microscopic morphology can be used to document Cryptosporidium spp. infection, it is not a reliable tool for designating infecting species.60 Despite the high rate of infection in clinically healthy animals, cryptosporidiosis in cats and dogs has probably been underdetected. This results from the relative insensitivity of some of the available tests, and because the species infecting dogs and cats generally shed relatively few oocysts. For example, in a study of naturally infected cats, the mean numbers of oocysts passed by cats with and without diarrhea were 1817 oocysts per gram of feces and 191 oocysts per gram of feces, respectively.260 In comparison, infected calves, less than 21 days old, shed an a mean of 90,867 oocysts per gram of feces.187 The threshold of positive detection of Cryptosporidium oocysts was a minimum of 106 oocysts per g of feces using unconcentrated fecal smears.267 Thus, oocyst concentration procedures such as Sheather’s sucrose flotation, zinc sulfate flotation, and saturated sodium chlorine methods are needed to increase the test sensitivity.120a Variations in the consistency of the feces influence the level of detection. In watery fecal samples containing 5000 oocysts per gram of feces, oocysts were identified in 90% by direct FA examination and 60% by acid fast staining. For 100% identification in formed fecal samples, 50,000 oocysts per gram of feces were needed for positive results with FA examination, whereas 500,000 oocysts per gram of feces were needed for acid-fast stain detection.265 Because cats and dogs generally only shed 103 oocysts per gram of feces, even concentration procedures are considered insensitive for detecting infection. Because oocysts are slightly smaller compared with erythrocytes, and because of transparency, unstained preparations using conventional light microscopy do not permit accurate identification (Fig. 81-3).207 A number of stains can make Cryptosporidium spp. oocysts more apparent during microscopic examination; those used most frequently are safranin–methylene blue stain, Kinyoun, Ziehl-Neelsen and dimethyl sulfoxide–carbol fuchsin (Figs. 81-4 and 81-5). In the modified Ziehl-Neelsen acid-fast staining (MZN) technique, the oocysts are stained with carbol-fuchsin and the dye is retained in the decolorizing step with the acid alcohol.205 The acid fastness of the oocysts allows differentiation from fecal material that is visualized by counterstaining with malachite green or methylene blue. In this technique, Cryptosporidium oocysts appear as pink to bright red spheres approximately 5 µm in diameter.205 In one study of human feces, the average detection limit using MZN after concentration of the feces was 5 × 105 oocysts/gram.265 However, because cats and dogs shed only low numbers of oocysts, this technique may not be very sensitive in those species. Direct FA detection procedures, using monoclonal antibody reagents, can be more sensitive and specific than acid-fast or other staining methods. In one study using human feces, the average detection limit using the MZN was 5 × 105 oocysts per gram of feces and the threshold of detection using direct FA method was 5 × 104 oocysts per gram of feces.265 Additionally, direct FA results are easier to interpret for inexperienced laboratory personnel, and screening by direct FA requires less time than some of the other staining techniques.265 The main disadvantage, compared to other staining procedures, is that direct FA staining has a higher cost and requires a fluorescent microscope. A commercially available direct FA test kit (Merifluor Cryptosporidium/Giardia, Meridian Bioscience, Cincinnati, OH) that simultaneously detects Giardia cysts and Cryptosporidium oocysts has been frequently evaluated for use in dogs and cats. In one study, this direct FA method had a lower sensitivity than the MZN staining technique when a single fecal specimen from a cat was tested.154 However, the sensitivity of direct FA equaled that of MZN when two to four consecutively collected samples were tested. Oocyst fluorescence intensity varied from an intense apple-green to a weak-green color fluorescence. This direct FA test has been titrated using C. parvum oocysts in human feces, and the variation in oocyst fluorescence may be due to antigenic diversity within Cryptosporidium spp. or to infection with more than one Cryptosporidium spp.154 Unfortunately, the Cryptosporidium species infecting the cats of that study were not determined. However, when the same feline samples were tested with a polyclonal antibody, a higher sensitivity was achieved, indicating that antigenic differences exist between C. parvum of humans and species infecting cats.154 In a study in humans, the sensitivity of different direct FA methods ranged between 94% and 100% when compared to MZN staining and ELISA, and specificity rates of the direct FA method were 100% compared to MZN and ELISA.205 None of these human assays have been completely validated for C. felis or C. canis; therefore, when used for the detection of Cryptosporidium spp. in feline and canine samples, false-negative results may be expected. A PCR assay was shown to be more sensitive than direct FA testing for the detection of C. parvum in experimentally infected cats.231 The direct FA method had a sensitivity threshold of around 104 to 105 oocysts per gram of feces when used with C. parvum–spiked feline feces.231 A number of different ELISA tests are available for detection of C. parvum antigens in feces. The available assays were titrated for use with human feces; therefore, sensitivity and specificity of each assay for use with dog or cat feces is unknown. The sensitivity of three fecal ELISA and direct FA tests were compared to MZN staining as the gold standard in assaying samples from cats.154 Using samples collected on day 1 of the study, the sensitivity of the three ELISA methods were 89%, 80%, and 15%. The sensitivity of these assays increased when samples from 2 consecutive days were examined.154 In another study, a comparison was made between the sensitivity of a fecal ELISA kit and carbol fuchsin stain in the detection of Cryptosporidium in canine and feline samples.42 Of 270 dog samples, 26 (9.5%) had positive test results for Cryptosporidium by microscopy, and 8 of 270 (2.95%) samples had positive test results with the fecal ELISA.42 In the same study, none of 100 cats had positive results for Cryptosporidium using microscopy, but 22 of 100 (22.4%) cats had positive results using fecal ELISA. Because observation of organisms using microscopy is considered the reference standard, it is unknown whether the ELISA results of this study were false positive.42,100,100 In general, ELISA methods have been shown to have lower specificity (90.3% to 100%) in assays of human specimens compared to direct FA methods.205 In tests using human-based ELISA test kits on feline specimens, specificities were high (greater than 99%), but sensitivities ranged from 72.7% to 91.2% compared to direct FA testing.166 Two lateral-flow devices designed for point-of-care use for detection of C. parvum in human feces were found to be insensitive for the detection of Cryptosporidium spp. in feline feces.12 This may relate to antigenic differences between C. felis and C. parvum.
Cryptosporidiosis and Cyclosporiasis
Cryptosporidiosis
Etiology and Epidemiology
Species
Major Host
Minor Host
Cryptosporidium muris
Rodents, Bactrian camels
Humans, rock hyrax, mountain goats
Cryptosporidium andersoni
Cattle, Bactrian camels
Sheep
Cryptosporidium parvum
Cattle, sheep, goats, humans
Deer mice, pigs
Cryptosporidium hominis
Humans, monkeys
Dugongs, sheep
Cryptosporidium wrairi
Guinea pigs
Cryptosporidium felis
Cats
Humans, cattle
Cryptosporidium canis
Dogs
Humans
Cryptosporidium meleagridis
Turkeys, humans
Parrots
Cryptosporidium baileyi
Chicken, turkeys
Cockatiels, quail, ostriches, ducks
Cryptosporidium galli
Finches, chickens, capercaillies, grosbeaks
Cryptosporidium serpentis
Snakes, lizards
Cryptosporidium saurophilum
Lizards
Snakes
Cryptosporidium molnari
Fish
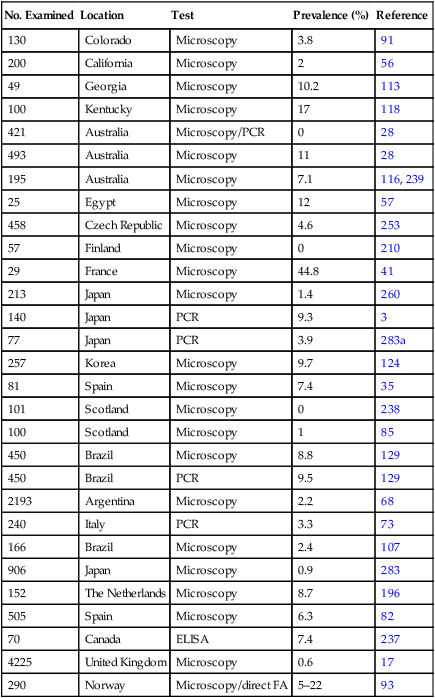
No. Examined
Location
Test
Prevalence (%)
Reference
130
Colorado
Microscopy
3.8
91
200
California
Microscopy
2
56
49
Georgia
Microscopy
10.2
113
100
Kentucky
Microscopy
17
118
421
Australia
Microscopy/PCR
0
28
493
Australia
Microscopy
11
28
195
Australia
Microscopy
7.1
116, 239
25
Egypt
Microscopy
12
57
458
Czech Republic
Microscopy
4.6
253
57
Finland
Microscopy
0
210
29
France
Microscopy
44.8
41
213
Japan
Microscopy
1.4
260
140
Japan
PCR
9.3
3
77
Japan
PCR
3.9
283a
257
Korea
Microscopy
9.7
124
81
Spain
Microscopy
7.4
35
101
Scotland
Microscopy
0
238
100
Scotland
Microscopy
1
85
450
Brazil
Microscopy
8.8
129
450
Brazil
PCR
9.5
129
2193
Argentina
Microscopy
2.2
68
240
Italy
PCR
3.3
73
166
Brazil
Microscopy
2.4
107
906
Japan
Microscopy
0.9
283
152
The Netherlands
Microscopy
8.7
196
505
Spain
Microscopy
6.3
82
70
Canada
ELISA
7.4
237
4225
United Kingdom
Microscopy
0.6
17
290
Norway
Microscopy/direct FA
5–22
93
No. Examined
Location
Test
Prevalence (%)
Reference
418
Australia
Microscopy
0
159
40
Australia
PCR
10
159
162
Australia
Microscopy
1.2
227
41
Canada
ELISA
7.3
237
135
Czech Republic
Serology
57
253
16
Egypt
Microscopy
6.2
57
10
France
Microscopy
0
41, 248
13
Japan
Microscopy
38.5
112
507
Japan
Microscopy
20
260
608
Japan
Microscopy
3.8
6
1079
Japan
Microscopy
2.8
283
55
Japan
PCR
12.7
283a
60
The Netherlands
Microscopy
4.6
196
57
Scotland
Microscopy
12.3
183
235
Scotland
Microscopy
8.1
178
258
Scotland
Serology (IgG)
74
180
50
Spain
Microscopy
4
82
145
United Kingdom
Microscopy, direct FA
15.2
90
1355
United Kingdom
Microscopy
1
258
344
United States (CA)
Microscopy, direct FA
4.7
166
180
United States
Microscopy, direct FA
3.3
233
600
United States
Serology
8.3
164
206
United States (CO)
Microscopy/ELISA
5.4
100
263
United States (NY)
Microscopy
3.8
247
173
United States (NC)
Microscopy, direct FA
6.5
186
250
United States (MS)
Microscopy, direct FA
12
14
250
United States (MS)
PCR
4.8
14
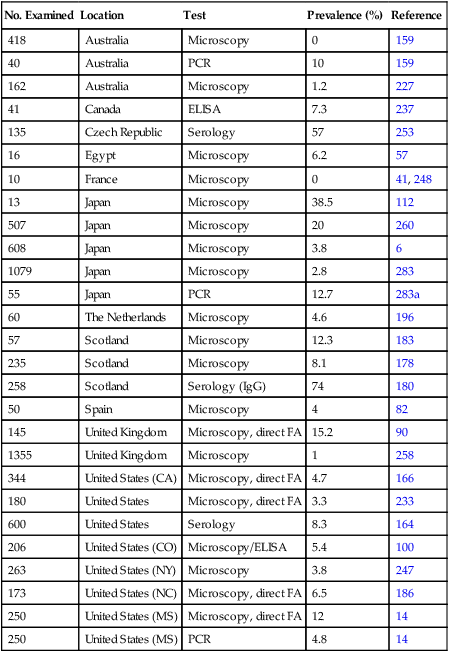
Cryptosporidium Species/Genotypes (No. of Isolates Examined)
Country
Reference
Cryptosporidium felis (2)
Australia
227
C. felis (4)
Australia
174
C. felis (7)
Brazil
254
C. felis (5)
Colombia
225
Cryptosporidium muris (1)
Colombia
225
C. felis (2)
Czech Republic
223
C. felis (1)
Czech Republic
92
C. muris (1)
Czech Republic
200
C. felis (1)
Portugal
4
C. felis (18)
United States
65
C. felis (14)
United States
232
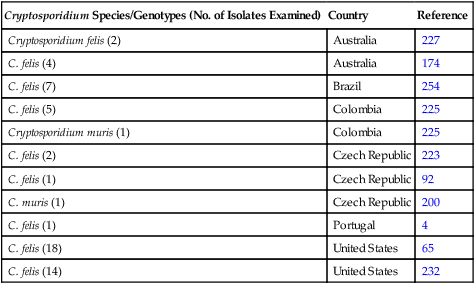
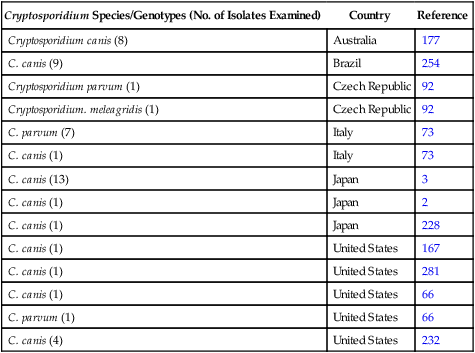
Cryptosporidium Species/Genotypes (No. of Isolates Examined)
Country
Reference
Cryptosporidium canis (8)
Australia
177
C. canis (9)
Brazil
254
Cryptosporidium parvum (1)
Czech Republic
92
Cryptosporidium. meleagridis (1)
Czech Republic
92
C. parvum (7)
Italy
73
C. canis (1)
Italy
73
C. canis (13)
Japan
3
C. canis (1)
Japan
2
C. canis (1)
Japan
228
C. canis (1)
United States
167
C. canis (1)
United States
281
C. canis (1)
United States
66
C. parvum (1)
United States
66
C. canis (4)
United States
232
Pathogenesis
Clinical Findings
Cats
Dogs
Diagnosis
Fecal Microscopic Examination
Concentration Techniques
Cytologic and Histologic Staining Techniques
Immunostaining Methods
Fecal Antigen Detection by Enzyme-Linked Immunosorbent Assays
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cryptosporidiosis and Cyclosporiasis