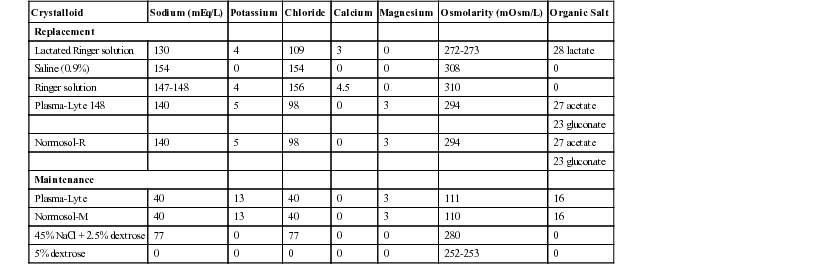
K. Gary Magdesian, Consulting Editor C. Langdon Fielding Physiologic fluid compartments consist of total body water (TBW), extracellular fluid volume (ECFV), and intracellular fluid volume (ICFV). TBW is the most clearly defined compartment because it represents the total amount of water comprising an individual. Values for TBW in adult horses have been reported to range from 0.623 to 0.677 L/kg.1–4 Values in foals are larger than adult horses and have been reported as 0.74 L/kg.5 Acute changes in TBW in clinical patients can be detected with serial body weight measurements, but this becomes less accurate over longer periods of time. ECFV represents the component of TBW that is not contained within the cells. This includes plasma volume, interstitial volume, and transcellular compartments (gastrointestinal tract, joint fluid, cerebrospinal fluid [CSF], body cavities, etc.). The ECFV has been measured using a number of techniques in horses; reported values in adult horses range from 0.214 ± 0.01 to 0.253 ± 0.01 L/kg.1,6 Estimations of ECFV in foals are significantly larger, including 0.38 to 0.40 L/kg in newborn foals and 0.290 L/kg in foals 24 weeks of age.5,7 Plasma volume has been determined to be 0.050 L/kg in healthy adult horses and 0.090 in foals at 2 days of age.5,7 ICFV is the volume of fluid contained within cells. It has been estimated as the difference between TBW and ECFV. Bioimpedance technology has also been used to estimate the volume of intracellular space in horses, whereas standard indicator dilution techniques cannot be easily applied to the ICFV.1,2 Reported values for ICFV in adult horses range between 0.356 ± 0.01 and 0.458 ± 0.06 L/kg.1,2 The ICFV of newborn foals is estimated to be 0.38 L/kg.5 Rapid changes in fluid balance and compartment volumes can occur during disease states, especially critical illness. The next section discusses the physiologic relationships between these fluid volumes. Although both the plasma volume and interstitial volume are components of the ECFV, the plasma volume is separated from the interstitial space by blood vessel walls, with constant flow of fluid and proteins into the interstitial space. The plasma and interstitial volume, therefore, are in constant flux, and a single equation (the Starling hypothesis) describes the flow between these two spaces: where Kf is the capillary filtration coefficient, being dependent on capillary surface area and hydraulic conductivity. The other five terms in the equation represent the primary determinants of fluid balance between plasma and the interstitium: 1. πp is the colloid osmotic pressure (COP) within the capillary (capillary oncotic pressure). 2. πif is the COP within the interstitium. 3. Pcap is the hydrostatic pressure within the capillary. 4. Pif is the hydrostatic pressure within the interstitium. 5. σ is the capillary reflection coefficient for proteins. There are three main determinants of net movement of fluid between the ECFV and ICFV: tonicity of the ECFV, tonicity of the ICFV, and cellular membrane permeability. The tonicity (or effective osmolality) of the ECFV is estimated by the following equation17: Under normal circumstances, sodium and chloride are the primary determinants of ECFV tonicity; the equation doubles the sodium concentration rather than including the concentration of specific anions, some of which may not be easily measured. It should be noted that BUN is not included in the tonicity formula, because BUN is an ineffective osmole. Other effective osmoles can be added to the ECFV, thereby increasing tonicity and inducing a shift of water from the ICFV to the ECFV. The tonicity of the ECFV is primarily regulated by vasopressin (ADH). Administration of fluids with a tonicity greater or less than ECFV tonicity is a means to manipulate the balance of fluid between the ECFV and ICFV. This explains why hypertonic saline causes a shift of water from the ICFV to the ECFV. Recent mathematic models have been used to predict the influence of tonicity on the absolute volumes of these fluid compartments.18 Similarly, fluid loss that has a tonicity different from the ECFV will also alter the ratio of fluid between the ECFV and the ICFV. The tonicity of the ICFV is primarily determined by the intracellular concentration of potassium and its related anions. The tonicity of the ICFV and ECFV are the same at any given time point, a homeostatic mechanism meant as a safeguard to prevent acute changes in cell volume. Any imbalance in tonicity between the two fluid spaces will result in a rapid shift of fluid in order to maintain osmolar equality. The tonicity of the ICFV can be altered over time by the cells in response to changes in ECFV tonicity; an example of such a response is the production of idiogenic osmoles during hypernatremia.19 Significant changes to ICFV tonicity during disease (resulting from damaged cell membranes) can result in accumulation of intracellular potassium, calcium, or cellular debris. Cell membranes are selectively permeable to water and ions. Changes in this permeability usually do not cause large alterations in global fluid balance during healthy states. However, cell membrane damage during disease states can result in significant fluid shifts. In fact, a shift of fluid from the ECFV to the ICFV is an indicator of cellular membrane damage. Specific disease states dictate individualized fluid plans. However, with intravenous therapy the administered fluid enters the vascular space. From there, the characteristics of the administered fluid will determine its movement from the vascular space into the interstitium and from the ECFV to the ICFV. Hyperoncotic or hypertonic fluids result in the greatest relative expansion of plasma volume, with a corresponding reduction in ICFV. Hypotonic fluids result in the smallest increase in ECFV but add volume to the ICFV, owing to a decrease in the tonicity of the ECFV. Estimates of the volumes of the different fluid spaces (whether based on clinical or laboratory values) before and during fluid therapy will allow for evaluation of the physiologic response in the clinical setting. The standard and universal administration of isotonic and hypo-oncotic fluids (such as lactated Ringer solution [LRS] or physiologic saline) to all patients regardless of disease condition ignores the available research and clinical insight to suggest otherwise. K. Gary Magdesian Current guidelines for replacement fluid therapy in human critical care emphasize the “fluid challenge” method, believed to be more effective and safer than traditional estimates of percent dehydration.1 The author’s (KGM) protocol of fluid administration in horses follows this same algorithm. Considerations for the fluid challenge technique incorporate four primary decision phases: (1) type of fluid, (2) rate and volume, (3) goals or endpoints of fluid therapy, and (4) safety limits to fluid therapy. 1. Types of fluids include crystalloids (isotonic, hypotonic, and hypertonic) and natural and synthetic colloids. There is much controversy and no consensus as to whether crystalloids or colloids are more effective or advantageous in human critical care.1 The advantages of both fluid types are capitalized on by using them together in the treatment of some cases of hypovolemia. Otherwise, because of cost differences and lack of demonstrated benefit of colloids as compared with crystalloids in terms of mortality for fluid resuscitation in critically ill human patients, the use of colloids cannot be recommended over crystalloids.2 Recent publications have suggested increased mortality and complication rates in humans receiving hetastarch as compared to crystalloids. See page 1374, Fluid Therapy for Diarrhea and Colitis in Horses. Table 44-1 lists the composition of several commercially available crystalloids that are used in equine practice. 2. The rate of fluid therapy for replacement (i.e., treatment of hypovolemia) is based on the “fluid challenge” principles, whereby a 30-minute bolus of 10 to 20 mL of isotonic crystalloid per kilogram is administered with subsequent reassessment of perfusion parameters.1 This challenge method assumes that uncontrolled hemorrhage is not present, because that would warrant hypotensive resuscitation instead. Perfusion parameters include mentation, heart rate, mucous membrane color, capillary refill time, jugular fill, extremity temperature (limbs, ears), and pulse quality. Blood or plasma lactate concentration, arterial blood pressure, urine output, and urine specific gravity are additional monitoring tools that aid in assessment for need of further fluid challenges. After the initial bolus, additional boluses—over 30 to 60 minutes depending on the degree of hypovolemia present—would be administered if perfusion parameters dictate. These are repeated until signs of shock abate, or a lack of improvement is noted with subsequent boluses, or fluid limits are reached (see later). Most animals require one to three of these boluses, and rarely are four required. Alternatively, or in addition to isotonic crystalloids, hypertonic saline (7% to 7.5%) can be administered at a rate of 4 to 5 mL/kg IV once. Plasma sodium concentration should be monitored. Colloids, because of their distribution that is limited to the central compartment, are bolused at lower volumes than isotonic crystalloids, such as 3 to 5 mL/kg for hetastarch. Up to 10 mL of hetastarch per kilogram may be administered per day, being limited by development of dose-dependent coagulopathies. If after reassessment of the perfusion parameters, additional resuscitative fluids are deemed necessary, another similar bolus is given, although each subsequent bolus is usually provided at a slightly slower rate unless the patient is markedly hypovolemic. This is repeated until signs of hypoperfusion resolve (endpoints are achieved) or safety limits are reached, at which point inotrope and/or vasopressor therapy is indicated if hypoperfusion persists. 3. The goals or endpoints of fluid therapy include both replacement and maintenance of fluid balance. Replacement refers to the replenishment of fluid deficits, primarily referring to those in the extracellular fluid compartment. Maintenance fluid therapy refers to the provision of maintenance fluid requirements to account for metabolism, insensible losses, growth, and ongoing losses. In general terms, replacement refers to replenishment of circulating volume and secondarily of interstitial fluid deficits; maintenance refers to provision of fluids after hypovolemia and dehydration have been corrected. Maintenance fluid therapy maintains both circulating volume and hydration status, including that of the intracellular compartment. In this section, replacement fluid is emphasized; the goals of replacement fluid therapy include rapid correction of hypovolemia by reversal of the signs of shock, including seven perfusion parameters: tachycardia (except in some neonatal foals), pale mucous membranes, prolonged capillary refill time, cold extremities, poor pulse quality, depressed mentation, and, in horses, reduced jugular fill. Urine production is another positive sign indicating correction of fluid deficits. Dehydration is corrected more slowly and is marked by reduced skin turgor (increased skin tent), dry mucous membranes, and dry corneal surface (reduced tear production). Additional goals of volume replacement include correction of hypotension, tachycardia, oliguria, and blood lactate.1 A decrease in urine specific gravity (in the absence of renal failure), an increase in arterial blood pressure and CVP, and improvement in venous oxygen saturation are specific endpoints. The goals of maintenance fluid therapy are different; they are to maintain a normal degree of hydration by providing for ongoing losses, including insensible losses (respiratory, cutaneous evaporative losses) and any abnormal ongoing losses such as diarrhea. Maintenance fluid rates for horses vary with ambient temperature, use, diet, and metabolic status. In general, a reasonable starting point for maintenance fluid requirements includes 2 to 3 mL/kg/h for adult horses (possibly slightly lower if off feed) and 4 to 6 mL/kg/h for neonatal foals.3,4 4. The safety limits to fluid therapy are indicators of intravascular volume overload, including supranormal CVP, a decrease in arterial oxygen saturation (Pao2), and clinical indicators of volume overload and overhydration. Once maximal CVP is achieved (10 to 12 cm H2O in neonatal foals and 15 cm H2O in adult horses), replacement bolus fluid therapy must stop; if continued, edema will form as systemic and capillary hydrostatic pressures increase in response to test boluses. A decrease in oxygen saturation in serial arterial blood samples, or in SpO2 measured with pulse oximetry, in a horse receiving fluid boluses may be consistent with development of pulmonary edema; this may precede development of tachypnea or frank edema in large airways/nostrils. Although no published reports address fluid overload in clinical equine patients, one experimental study evaluating hyperhydration before moderate-intensity exercise in horses suggested the development of arterial hypoxemia during exercise.5 These animals were administered oral fluids equivalent to 6% of body weight (approximately 26 L of isotonic fluid by nasogastric tube). Hyperhydration resulted in arterial hypoxemia, suspected to be caused by pulmonary edema associated with hyperhydration, during moderate-intensity exercise.5 Clinical indicators serving as limits to fluid therapy include visible subcutaneous edema and tachypnea; with optimal monitoring, fluid therapy will not be allowed to continue to this point. Another endpoint or limitation to fluid therapy is a lack of further improvement in perfusion parameters despite repeated fluid boluses. This would indicate that hypoperfusion is not solely due to hypovolemia and would require inotropes and/or pressor agents (e.g., dobutamine and norepinephrine, respectively). If safety limits are reached before the goals of fluid therapy are achieved, the next step is also to turn to inotrope and vasopressor therapy. See page 1374, Fluid Therapy for Diarrhea and Colitis in Horses. K. Gary Magdesian Monitoring tools provide for advanced critical care in the large animal intensive care unit. Several of these tools are pertinent to fluid therapy and provide guidance, endpoints, and safety limits. Many of these allow for direct monitoring of fluid during administration of therapy, whereas others provide indirect information through hemodynamic data. Both of these are important to overall case management of the critically ill equine patient. Central venous pressure (CVP) is the pressure within the vena cava; the term most commonly refers to that within the cranial vena cava. It is determined by blood volume as well as venous tone and cardiac contractility. With serial measurements CVP provides a limit to the administered volume of fluid. Normal CVP is approximately 2 to 12 cm H2O (1.5 to 8.8 mm Hg) in foals and 5 to 15 cm H2O (3.7 to 11 mm Hg) in adults.1–4 Subnormal to negative CVP values signify hypovolemia; however, normal values do not necessarily imply euvolemia. This is because CVP is affected by compensatory responses such as venoconstriction, and is affected by cardiac contractility and intrapleural pressures among others. CVP is easily measured in neonatal foals through the use of 20-cm central venous catheters, such as long-term single- to triple-lumen polyurethane catheters. Measurements can be obtained in adult horses with the use of 55-cm commercial CVP catheters. CVP can also be measured by passing a smaller gauge polyurethane catheter or polyethylene tubing through a 14-gauge, High CVP readings also occur with pericardial tamponade, pleural effusion, or pneumothorax, as well as false increases from catheter occlusion and air within the lines. For accurate and consistent serial results, a zero reference point should be selected and used for each measurement. The top of the sternal manubrium is a good reference point for the level of the pressure transducer or water manometer. The pressure transducer or water manometer can be taped to a fluid pole, providing a fixed zero point for repeated measurements in standing horses. It should be noted that adequacy of intravascular volume cannot be guided by any one CVP level. Precision and reliability are limited by variable zero reference points, the effects of afterload and ventricular compliance, and alterations in intrathoracic pressure.5 There is no linear relationship between intravascular volume and filling pressures, and CVP should be regarded as only an indirect estimate of volume. Serial measurements and trends over time are more useful than single numbers. CVP can be used on a clinical level for monitoring fluid therapy as follows: If the patient responds to fluid boluses without increases or with only minor increases (2 to 3 cm H2O) in CVP, it is appropriate to continue infusions until signs of hypoperfusion are reversed.5 Additional guidelines used in human patients are as follows: If the CVP increases to 3 to 7 cm H2O (2 to 5 mm Hg), the infusion should be paused and perfusion reevaluated after a 10-minute wait. If the change in CVP was an increase of 7 cm H2O (5 mm Hg) or greater after the fluid bolus, the infusion is stopped.5 In this regard CVP is a better “upper limit” to fluid therapy rather than an actual goal to strive for, other than normalization in horses with low CVP values. Arterial blood pressure measurements provide some insight into perfusion status, particularly when used in conjunction with clinical signs and lab work, such as blood lactate concentration. It can be measured either directly through the use of arterial catheters or indirectly with a blood pressure cuff on the tailhead. Arterial lines can be placed in the great metatarsal artery of recumbent foals and the transverse facial artery in standing adult animals for direct measurements. Indirect measurements are best performed with oscillometric blood pressure monitors that provide systolic, diastolic, and mean arterial pressure (MAP). Cuffs are provided by the manufacturer and vary in width and length with size of the patient. Arterial blood pressure in healthy neonatal foals is highly variable and depends on breed, gestational age, and size of foal. In general, normal mean arterial blood pressure (direct) is 84.4 ± 3.7 mm Hg at 1 day of age and up to 101.3 ± 4.4 mm Hg at 14 days of age.2 Indirect blood pressure readings in Thoroughbred foals has been reported as 144 ± 15, 74 ± 9, and 95 ± 13 mm Hg for systolic, diastolic, and mean pressures, respectively.6 Direct blood pressure has been reported for adult horses: 126 to 168 (systolic)/85 to 116 (diastolic) with a range for the mean arterial pressure of 110 to 133 mm Hg.7–10 Indirect blood pressure in adult horses is 111.8 ± 13.3 mm Hg for systolic pressure and 67.7 ± 13.8 mm Hg for diastolic pressure.11 Blood pressure values should not be regarded as the sole criteria for intervention. Rather, they should be used in conjunction with perfusion parameters, blood lactate concentration, and urine output in deciding whether there is need for further fluid administration or inotrope and vasopressor support. The exact blood pressure value that should trigger intervention in horses and foals is unknown and likely varies with the individual patient; however, end organ perfusion requires a mean arterial pressure of 60 mm Hg, thereby making that a reasonable general target.
Critical Care and Fluid Therapy

Equine Fluid Physiology
Plasma and Interstitial Balance
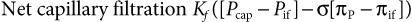
COP is determined by the concentration of plasma proteins, primarily albumin, and their ability to attract ions. Normal COP in adult horses is approximately 20 mm Hg (19 to 26 mm Hg) and in neonatal foals is 18.8 ± 1.9 (15 to 22.6) mm Hg.8,9 Hypoproteinemia with resultant decreased oncotic pressure can occur during a variety of diseases in horses but is most often a result of loss or decreased production. Losses most commonly occur through the diseased gastrointestinal tract in large animal species (protein-losing enteropathies); losses may also occur as a result of glomerular diseases or large accumulations of protein-rich effusions within body cavities or the interstitial space. Decreased production of protein (specifically albumin) occurs in response to systemic inflammation because albumin is a negative acute-phase protein.10 Hypoproteinemia also occurs uncommonly because of liver disease.11 Decreases in COP are also commonly observed during intravenous fluid therapy in horses undergoing general anesthesia.12
Interstitial COP is more difficult to measure than plasma COP, but estimates of this number include 12 to 15 mm Hg in other species under experimental conditions.13 Changes in total plasma protein concentration are likely to also affect the interstitial protein concentration and therefore alter both oncotic pressure components in Starling’s equation. This may explain why some horses with significant hypoproteinemia do not show clinical signs of interstitial fluid accumulation (i.e., edema). That is to say, the gradient between capillary and interstitial oncotic pressure is only minimally affected when both have decreased proportionally, especially with time. It has been speculated that acute changes in plasma total protein concentration, however, may lead to edema formation more often than chronic hypoproteinemia; this is because of a relatively larger decrease in plasma protein concentration as compared with that in the interstitium with acute disease. Conversely, administration of hyperoncotic intravenous fluids (fluids with a COP greater than 20 cm H2O) can potentially shift the balance of oncotic pressure back to the vascular space by raising plasma COP. However, the colloid molecules from these fluids must remain within the vascular space to have this effect, which may be negated by significant alterations in capillary permeability.
Vascular hydrostatic pressure represents the pressure of plasma within the vessels pushing out toward the interstitium. A number of factors influence this pressure, including blood volume, vascular tone, and central venous pressure (CVP). This outward pressure on the vessel walls serves as the driving pressure for the continuous flux of fluid and protein out of the vessels into the interstitium. Pathologic increases in hydrostatic pressure can be caused by right-sided heart failure or other obstructive processes, such as venous thromboses, which can lead to edema formation. Pulmonary capillary hydrostatic pressure increases with left-sided heart failure and significant pulmonary vasoconstriction. Administration of intravenous fluids can also increase capillary hydrostatic pressure. Fluid losses resulting from diuretic administration and acute blood loss are examples of changes that result in decreased capillary hydrostatic pressure.
The hydrostatic pressure of the interstitium varies in different tissues but often is overlooked as a significant contributor to fluid balance between the vascular and interstitial spaces. The importance of the interstitial hydrostatic pressure as it pertains to fluid balance has been reviewed by Reed and Rubin.13,14 Degeneration of the interstitial collagen network may cause a decrease in interstitial hydrostatic pressure, resulting in a shift of fluid into the interstitium. Inflammatory cytokines have been implicated as a cause for these changes in interstitial hydrostatic pressure. Severe burn injury can also result in marked negative pressures within the interstitium, causing a draw of fluid into the interstitial space and subsequent edema formation. In the future there may be practical therapeutic options to manipulate interstitial hydrostatic pressure and alter fluid balance.
Changes in capillary permeability can dramatically increase the flux of fluid and protein from the intravascular space into the interstitium.15 If this increase in flow cannot be balanced by a corresponding increase in lymphatic return, edema results. Increased vascular permeability has been regarded as a major mechanism of edema formation; however, it may be only one component of the significant fluid shift that results from a systemic inflammatory response syndrome (SIRS). Adult horses and neonatal foals may have significant differences in interstitial composition and vascular permeability. It has also been proposed that foals have an increased capillary filtration coefficient as compared with adults.16 These hypotheses may explain the propensity of neonatal foals to form edema relatively easily in response to overzealous fluid administration. Because of this increased risk, careful monitoring and serial assessment of the balance between plasma volume and the interstitium are warranted when treating neonatal foals with fluid therapy.
Extracellular to Intracellular Fluid Volume Relationship
Tonicity of the ECFV.

Tonicity of the ICFV.
Cellular Membrane Permeability.
Effects of Fluid Physiology on Clinical Fluid Therapy
General Principles for Fluid Therapy in Critical Care
Critical Care and Fluid Therapy Monitoring Techniques
Central Venous Pressure
 -inch standard intravenous catheter. CVP can be measured with a disposable water manometer (for spot readings) or using a pressure transducer for continuous waveforms. Interpretation of CVP waveforms requires knowledge of component waves and descents, including the a, c, and v waves and x and y descents. The a wave represents atrial contraction. The mean of the a wave is the appropriate point at which to measure CVP (at expiration). The c wave is associated with tricuspid valve closure and a bulging of the valve into the right atrium, and the v wave is caused by atrial filling from venous return. The x descent occurs after the a wave and represents the fall in pressure associated with atrial relaxation. The y descent occurs after the v wave, representing a drop in CVP caused by ventricular relaxation and reopening of the atrioventricular valves.
-inch standard intravenous catheter. CVP can be measured with a disposable water manometer (for spot readings) or using a pressure transducer for continuous waveforms. Interpretation of CVP waveforms requires knowledge of component waves and descents, including the a, c, and v waves and x and y descents. The a wave represents atrial contraction. The mean of the a wave is the appropriate point at which to measure CVP (at expiration). The c wave is associated with tricuspid valve closure and a bulging of the valve into the right atrium, and the v wave is caused by atrial filling from venous return. The x descent occurs after the a wave and represents the fall in pressure associated with atrial relaxation. The y descent occurs after the v wave, representing a drop in CVP caused by ventricular relaxation and reopening of the atrioventricular valves.
Arterial Blood Pressure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree