(1)
Department of Psychology and Program in Behavioral Neuroscience, Western Washington University, Bellingham, WA, USA
Abstract
The thesis of this chapter is that the unconscious (basic) craving process, consisting of activation within limbic incentive motivation and memory systems, is ultimately responsible for relapse behaviors. The chapter first includes a brief discussion of current clinical conceptions of craving. This is followed by an argument for what aspect of human craving animals may experience. This leads to a description of various animal models (primarily using rats) that indirectly measure activity of a basic craving mechanism. A final section is provided with examples of the utility of animal models of craving illustrated with translational evidence. It is argued that basic craving is amenable to study by animal models of craving that measure motivated drug seeking behavior. Furthermore, reflection on a distinction between conscious (subjective) craving and basic craving leads to the following possible conclusions: one could treat the conscious craving and this would provide some benefit to the addict. But what would remain is the basic craving response to drug-paired stimuli, situations, and even drug-focused thought processes. Reducing basic craving using pharmaco- or behavioral therapies based upon animal model findings may ultimately be more effective at reducing relapse behaviors.
Key words
CravingIncubationReinstatementMotivationHabitAutomaticityDrug seekingRelapse1 Introduction
The purpose of this chapter is to review animal models of craving. Since the definition and usefulness of craving as a construct in humans has been challenged (1, 2), the chapter first includes a brief discussion of current clinical conceptions of craving. This is followed by an argument for what aspect of human craving animals may experience. This leads to a description of various animal models (primarily using rats) that indirectly measure activity of a basic craving mechanism. A final section is provided with examples of the utility of animal models of craving illustrated with translational evidence.
2 What Is Meant by “Craving”?
Craving is an often used, but inconsistently defined, construct. For example, the World Health Organization (WHO) ICD 10 lists “strong desire to take the drug” as a possible symptom of dependence syndrome, yet does not specifically list craving (3). A subsequent WHO expert meeting on the “craving mechanism” provided a now often-cited statement from 1992:
Drug craving is the desire for the previously experienced effects of a psychoactive substance. This desire can become compelling and can increase in the presence of both internal and external cues, particularly with perceived substance availability. It is characterized by an increased likelihood of drug seeking behaviour and, in humans, of drug-related thoughts (4, p. 5).
A less clear statement arose from a meeting of experts brought together by the National Institute on Drug Abuse. In this statement (2), the group agreed that drug-paired stimuli were one cause of craving and there was consensus that:
…craving is a subjective state in humans that is associated with drug dependence…the majority of measurements involve self-report questionnaires which range in complexity from endorsements of simple yes-no statements (‘I crave drug x’) to questionnaires that have attempted to analyze multiple aspects of craving…(e.g. urges, desires, wanting) (p. 128).
Such flexible definitions of craving have been criticized (rather tongue-in-cheek) by Stephan Tiffany and colleagues (5) in a summary of consensus statements appearing in the literature in the 1990s:
Although we do not know what craving is and we can establish no consensus about the best way to measure it or manipulate it, we certainly believe that more research should be conducted on this possibly, but not necessarily, important construct (p. S178).
While self-report is a typical procedure to measure craving, it is important to distinguish self-reported craving from other measures that, along with self-reported craving, are subsumed under the broad categorization of “cue-reactivity.” Some of the confusion expressed by consensus groups likely stems from use of this term interchangeably with subjective craving when, in fact, there are critical distinctions. Cue reactivity might be assessed as a change in a physiological measure, a change in self-reported mood or craving, or relapse itself.
Typical physiological measures in response to drug-paired cues include changes in galvanic resistance and heart rate (6). Less typical are measures of facial EMG responses (7). Physiological changes do not always covary with craving self-report (8), and the effect sizes for these measures are relatively modest compared to subjective reports (6). These apparent weaknesses have been used as to criticize the utility of physiological measures of cue reactivity as indicators of craving (6).
Subjective assessments of craving are complicated by a host of issues. In survey studies, craving is sometimes used to define itself (e.g., “are you craving cocaine right now”) or is assumed to be expressed as a desire, or an urge (9). Even more, urge is sometimes measured as an “intention” to take drug vs. as an “expectancy of a positive outcome” (10–12). These differences in assessment and theoretical orientation are also, by nature of subjective report, complicated by reactivity problems (e.g., demand characteristics) (2). For both physiological and self-report measures in the laboratory, there also exists the dilemma that the addict is aware that any craving induced in the laboratory will most likely not be satisfied with actual drug availability (11, 13, 14). And even if this were so, the abstinence-focused patient would likely experience and report distress when confronted with a relapse-provoking situation. These anxiety-provoking effects likely distort the emotional valence of self-report outcomes. Finally, there is the problem of distorted memory when an addict reports craving that has preceded a lapse or relapse. For example, smokers overreported stress antecedent to a lapse when comparing recall of stress over the past several weeks compared to a computerized diary they had kept (15).
Despite these complications in defining craving, it is clear that craving of some sort is experienced by many addicts and this state causes suffering (16). What is less clear, however, is how craving relates to actual relapse to drug taking. Review of some well-cited clinical literature on relapse can easily leave one with the impression that craving is the key to understanding relapse, especially protracted relapse. For example, from detailed observation and survey evaluation of 30 outpatient cocaine abusers attempting prolonged abstinence, Gawin and Kleber (16) created a three-phase model of cocaine abstinence that relies heavily on craving as an antecedent to relapse. In this model (Fig. 1), craving increases over the course of the first 10 weeks of withdrawal and functions as a trigger to initiate relapse behaviors that precede a return to binge cocaine self-administration. Protracted withdrawal (abstinence) is punctuated by craving that may be activated by conditioned cues. Thus, the addict is always at “risk” for relapse due to the risk of craving.
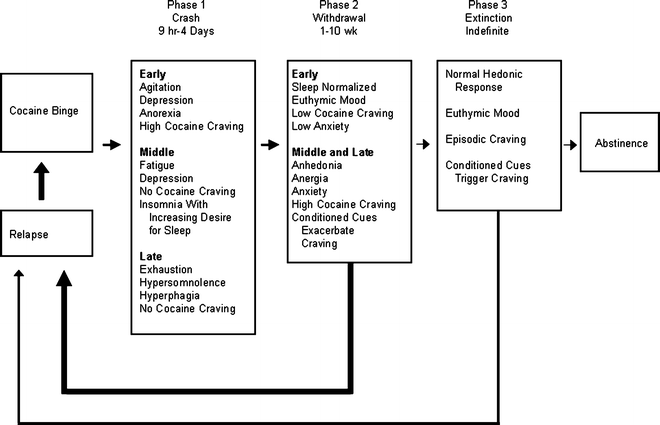

Fig. 1.
A model for cocaine relapse that includes craving. In this model, craving is lowest during physical withdrawal but may be elicited in protracted withdrawal when the addict comes into contact with drug-paired cues (Reprinted with permission from (16). © (1986) American Medical Association. All rights reserved).
In contrast to this popular model of relapse, Tiffany and Carter (17) provide several examples of published studies indicating a potentially spurious relationship between self-reported craving and relapse. Even more, in one study, urge to drink in alcoholics actually predicted less relapse (18). How might the presumed importance of craving in relapse be reconciled with the fact that self-reported craving does not reliably predict relapse? Tiffany proposed a model that, in a sense, moves subjective craving to a cognitive epiphenomenon (1). This cognitive processing model supposes that actual drug relapse is due to unconscious, “automatic” behavioral responses that were developed during the establishment of the addiction. This automatic relapse is proposed to be “mindless” and resembles the theoretical construct of habit described by William James 100 years before as:
In a habitual action, mere sensation is a sufficient guide, and the upper regions of brain and mind are set comparatively free (19, pp. 115–116).
In the cognitive processing model, it is the recognition, in cognitive awareness, of this automatic response that is the subjective craving reported by addicts (Fig. 2). Tiffany and others have provided some evidence to support the concept that this subjective craving, and the thinking required to either combat it or go with it, requires a degree of cognitive processing that disrupts other processing. It is suggested that this interference may produce the psychic distress experienced by many addicts experiencing craving (20).
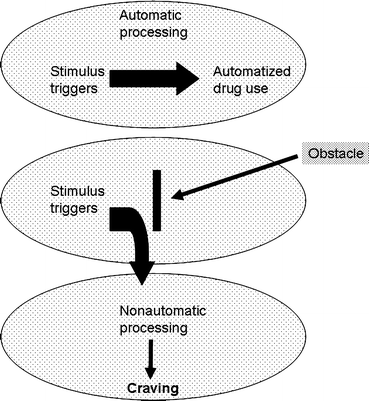

Fig. 2.
The cognitive processing model of craving and relapse. This model supposes that craving is a cognitive reaction to disruption of an automated drug seeking process generated by the addict coming into contact with drug-paired stimuli. This “automatized” behavior may be subconscious or “mindless” (Reprinted from (120)).
From a systems perspective to understanding addiction, it would then seem critical to identify the system(s) underlying automaticity. The term automaticity is limited, however, in that it does not encompass other factors relevant to the sensitivity to reward. For example, drug seeking behavior is not simply a habit. Craving has clear motivational components. Motivational state can, in turn, be regulated by drive state and motivation can become attached to incentive stimuli. As drug seeking behaviors are guided and driven by stimuli imparted with incentive by previous association with drug taking, the drug seeking would be largely a manifestation of activity of the limbic circuitry underlying drive, motivation, and incentive salience (21–24). An analogous phenomenon could be situational-invoked fear in post-traumatic stress disorder as a manifestation of activation of central amygdala and central stress systems (25). Perhaps the system that produces drug seeking should be referred to as the “craving mechanism” (4). Within this framework in the present chapter, basic craving will be used to refer to this mechanism in part to separate it from the subjective craving typically assessed in clinical studies. As will be outlined below, activation of this basic system by drug-paired cues (exteroceptive or interoceptive) arguably serves as the beginnings of behavioral, and ultimately cognitive, manifestations of craving. It will be argued that subjective craving could be considered an epiphenomenon of engaging the basic craving system.
The basic craving system is essentially the brain reward system (21) interacting with memory systems that signal, for example, current reward valuation and contextual or discrete cue salience due to previous association with a reward (26). As such, reward is a construct entangled with incentive motivation and memory and has been defined by Wise as:
“an object or event that elicits approach and is worked for; its analogue is ‘a reinforcer’…In addition to their reinforcing effects, rewarding and reward-associated stimuli have proactive, drive-like effects. Such stimuli cause motivational arousal and increase the probability of response initiation when the primary reward has not yet been earned or directly sensed” and “the common term ‘reward’ is often used to denote the undifferentiated effects of reinforcement and motivational arousal” (27, pp. 2–3).
From a systems perspective, the study of reward and incentive motivation provides a strong framework for studying appetitively motivated behaviors such as drug seeking driven by craving. Craving is essentially incentive motivation for drug (28). This definition invokes the reward system, but also memory systems. These memory systems include those embedded in reward including reinforcement (see (29)) and systems that support interoceptive cue–drug associations (see (30)) as well as exteroceptive contextual and discrete (exteroceptive or interoceptive) cue–drug associations. It is clear that some of the memory components are involved more at a level of indicating when an incentive stimulus has been recognized (e.g., basolateral amygdala) and in which context (e.g., hippocampus) (31, 32). Other systems appear to be involved in evaluating the remembered reward valence of the stimulus (e.g., orbitofrontal cortex) (33).
Overall, the result of activation of the basic craving system is to mediate drug seeking in the presence of drug-paired stimuli as well as the reinforcing efficacy of a drug when the drug is present. In the latter case, interoceptive effects of the drug could stimulate craving (34). Therefore, for the addict, one can describe the likelihood of relapse as the perceived (perhaps unconsciously) reinforcing efficacy of the drug when the drug is available. For Gawin:
“Initiation of a cocaine binge thus depends on an interaction between drug availability, environmental stimuli (conditioned cues), and the withdrawal status of the dependent abuser.” and “such (conditioned cocaine) cravings appear after the appearance of varied, idiosyncratic objects or events that were temporally paired with prior cocaine intoxications, and these appearances are experienced as partial memories of cocaine euphoria.” (italics added) (35, p. 1582).
3 Animal Models Suggest a Craving Mechanism
Animal models are an obvious choice for exploring behavioral and neurobiological substrates of basic craving due to the level of experimental control and the fact that most laboratory species (e.g., rats) are without the level of conscious reflection of humans. In fact, animal models might best serve to model the various aspects of craving, rather than provide a comprehensive model of the human addict (35, 36). Recently Miczek and de Wit echoed this limitation in a paper on translational issues and requirements for an animal model. Eight principles for effective animal models are listed – the first suggests that the scope of a model be restricted to a “core symptom” of the clinical disorder (37). Basic craving could be argued to be a core symptom of addiction.
So what aspects of basic craving can be examined with animal models? Several theoretical models for relapse have been presented over the years and basic craving could be argued to be a component of each. The models can be organized into two groups according to whether they frame relapse as driven by factors associated with drug withdrawal (dependence) or by factors that are drug-like (reward). It has been demonstrated, at least for morphine, that the neuronal substrates of dependence and reward are dissociable (38). For this “duality of craving” (39), dependence mechanisms are sometimes referred to as opponent processes while reward mechanisms are proponent processes (40).
Opponent processes driving a craving mechanism include conditioned withdrawal and conditioned compensatory responses. Conditioned withdrawal-induced craving was first presented as a situation where an individual would experience withdrawal symptoms if they were to return to the context or to confront cues initially paired with drug intake. In Wikler’s formulation, this conditioned withdrawal state would then induce drug seeking and relapse (41). While a compelling hypothesis, it has not been borne out by research studies (e.g., (42)). A more likely drug conditioning scenario would be that stimuli paired with drug taking could come to produce conditioned compensatory responses to the unconditioned effects of the drug. This is a withdrawal model that follows from Siegel’s findings that rats experience drug-opposite responses when placed in an environment where drug has previously been delivered. These conditioned compensatory responses have a large effect of producing drug tolerance (30), but their role in craving and relapse has not been substantiated. There is evidence that spontaneous or precipitated withdrawal can raise brain stimulation reward threshold (43, 44) and slightly increase drug self-administration (44), but such a change in threshold has not been linked to craving in the absence of drug. Even more, spontaneous or precipitated withdrawal from heroin does not lead to reinstatement of extinguished heroin seeking in rats (45, 46) and while naltrexone-induced withdrawal was found to increase cue-induced heroin seeking when given immediately following heroin self-administration, a similar pretreatment was not effective at altering cue reactivity 2 weeks following heroin self-administration (47). Likewise, early withdrawal from several drugs of abuse actually appears to better predict relatively low levels of craving in both humans (16) and rats (48). Overall, opponent processes do not clearly account for craving behaviors.
A proponent process, however, has been presented as a more likely mediator of craving. The motivation to experience drug-like effects has been identified as a key factor in relapse (10, 35) and has been most clearly identified as a predictor of drug seeking in animal models of addiction (48, 49). The basic craving mechanism described in the earlier section of this chapter is proponent and is derived from the wealth of theoretical considerations and empirical findings on incentive motivation published over the past several decades. Incentive motivation, simply stated, is the reward valence attributed to an object or place due to its pairing with a reward in the past (27). Exposure to that object or place will produce approach behaviors, indicative of activation of a reward mechanism (21). It is important to recognize that these conditioned behaviors are not simply conditioned reflexes, but conditioned behavioral responses heightened by drive and motivational factors. For example, hunger can increase drug seeking (50) and responding for the presentation of a drug-paired stimulus is taken as a measure of the conditioned reinforcing properties of the stimulus (51).
Figure 3 incorporates a proponent-driven basic craving mechanism into a relapse model that acknowledges both conscious and unconscious dimensions of craving. From this basic craving model, it is suggested that for humans, drug-craving actions could occur independent of conscious awareness akin to the mindless relapse of Tiffany. Conscious craving would then be a conscious reflection of the underlying mood/motivational state and behavioral activation; a “cognitive interpretation” of the activation of the basic craving mechanism (52).
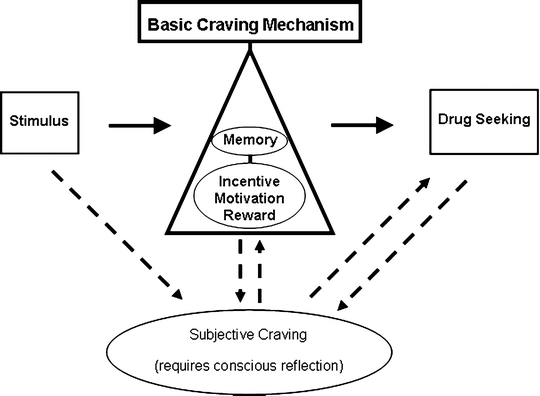

Fig. 3.
An incentive motivational model of a basic craving mechanism. This model, its creation influenced by (28) and (121), supposes that drug-paired stimuli are detected and valued by memory systems (e.g., basolateral amygdala and orbitofrontal cortex) and drug seeking responses are initiated with their vigor determined by their incentive salience (e.g., via activity of meso-accumbal dopamine projections). The drug seeking responses are therefore an indirect measure of the activity of the basic craving mechanism. The model also allows the possibility of interaction between a basic craving mechanism and subjective craving and for subjective craving to produce drug seeking independent of the basic craving mechanism.
Understanding these basic craving processes could then arguably be the critical focus for craving research. Behavioral and pharmacological interventions would be aimed at these underlying processes rather than targeting the cognitive symptoms. For example, one approach to managing addiction is to reduce the relative reinforcing effect of a drug by contingency management. Contingency management involves decreasing the relative value of the drug by increasing the value of remaining abstinent (53). This isn’t to say that conscious craving would not take on a life of its own in humans. Obsessive thoughts are a debilitating aspect of addiction and they could be disconnected from actual motivation to take the drug – akin to cravings vs. urges in Marlatt’s cognitive behavioral model of relapse (10). It is also possible that these drug memories could prime the subconscious basic craving system (Fig. 3). Activation within this system would then result in the increased probability of drug seeking and increased reinforcing efficacy of drug when it is available.
4 Assessing Craving Using Nonhumans
Animal models are well-suited for identifying core behavioral components, and underlying neurobiological substrates, of the basic craving mechanism. An animal model “peels away” several layers of complexity that appear to confuse the measurement of craving in humans. A recent example of this is the observation that a “lapse” in rats increases the probability of a relapse (54). A current cognitive-based explanation for this effect in humans is that the abstinence violation of the lapse allows greater rationale for relapse by the addict (10). Since the same effect is seen in rats, the parsimonious explanation would be that there is a change in reinforcing efficacy of drug or drug-paired stimuli due to the lapse – a higher-level cognitive explanation is not necessary.
In this section, several animal models for assessing the proponent aspects of craving will be identified and described in brief. These models could be argued to indirectly measure the basic craving construct outlined in the previous section (Fig. 3). Several of these models are discussed in detail in other chapters in this volume (see Chaps. 6, 13, and 17).
For the following section, the operational definition of basic craving is intentional, motivated approach to, and/or response for, a drug or a drug-paired stimulus.
Using this definition, a change in basic craving is inferred by an increase in drug seeking behavior in the absence of drug (55) or an increase in reinforcing efficacy of the drug when it is available (56). A key aspect of this definition is that it does not simply define automatic behavior or habit. The intent of the definition is to capture how craving is an acquired motivation (28). This operational definition implies a necessary role for a central motivational mechanism that interacts with feedback on the incentive value of stimuli in the environment. The strength of the models described below is in how they tap into key aspects of the basic craving mechanism. The models described are categorized according to whether the drug is present, and to the main conditioning features of the model (e.g., classical or operant or both). Not all models that measure craving are presented here due to space considerations. In addition, notably absent are models that assess how impulse control and higher-order memory systems modulate craving, including those that tap into the role of the prefrontal cortex and orbitofrontal cortex in regulating drug seeking behavior. Such models of impulsivity (57) and reward revaluation (33) are providing important insight into how cortical regions can ultimately “decide” (or fail to decide) on how basic craving will manifest as overt behavior.
One consistent finding from the models described below is that key elements and projections of the mesolimbic dopamine pathway mediate the craving-related behavior being modeled (drug seeking). Relevant examples of this corroboration are provided for each model. Excellent reviews of the validity levels of most of the following models may be found in (28, 58).
4.1 Self-administration and Priming
Self-administration of a drug is a hallmark of the rewarding effects of the drug (see Chaps. 2–5). Motivational aspects, aspects central to basic craving, can be measured by rate of self-administration but also rate of responding in the absence of the drug (i.e., extinction) (59) including responding in the initial drug-free components of fixed interval and second-order schedules of reinforcement (51, 60). Procedures that assess responding in the absence of drug are discussed in paragraphs below. Interpretation of responding for drug has been criticized for the dependence of the measure on rate of responding. Fixed ratio responding (fixed number of responses required for each drug delivery) is typically identified as the most problematic. That is, there may be confusion when interpreting a change in response rate (e.g., following pharmacological manipulation) as being due to a change in incentive motivation or due to a motor side effect of the drug (61). This criticism is less tenable in certain situations when considering that relatively low doses of a dopamine antagonist actually increase operant behavior reinforced by a psychostimulant (27). Despite this fact, a popularly employed alternative to the fixed ratio procedure that is argued to be less sensitive to the rate-dependency problem (62, 63) is the progressive ratio (PR) procedure (64). The typical procedure under the PR schedule is for the number of responses required for drug delivery to increase after each drug delivery; often the increases occur along a logarithmic scale (65). Use of the PR procedure with rats and nonhuman primates has revealed insights into mesolimbic brain regions involved in incentive motivation (61, 66, 67) and has provided a useful procedure for comparing the relative reinforcing efficacy of different drugs of abuse (64). In Fig. 4, individual response records are provided where a rat responded for different doses of cocaine on a PR schedule of reinforcement. Higher doses supported increased effort, suggesting increased motivation to obtain the drug.
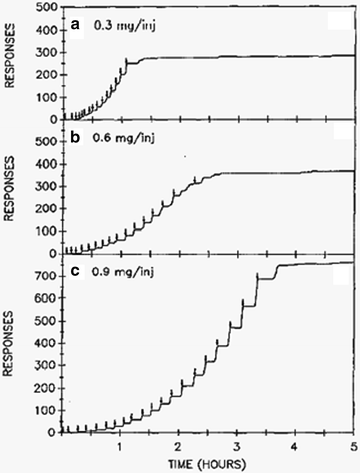

Fig. 4.
Event records of progressive ratio responding for cocaine by a rat. Downward arrows indicate self-infusions of cocaine. Higher doses of cocaine support higher ratios (number of responses required for cocaine delivery) of responding. This is taken as an indication that the higher doses have greater reinforcing efficacy. Achieving higher break points (final ratio that supports sustained responding) is also taken as a measure of the motivation to self-administer the drug (Reprinted from (65) (Fig. 1) with kind permission of Dr. Roberts and Springer Science + Business Media).
Reinstatement is a general term to describe the reactivation of a previously extinguished behavior (55) (Relapse is explored in depth in Chap. 17). Priming-induced reinstatement describes a reestablishment of either drug taking or drug seeking following noncontingent delivery of drug. Similar to Davis and Smith (68) with morphine, de Wit and Stewart found that cocaine effectively reinstated extinguished lever responding initially supported by cocaine (49). In Fig. 5, rats that decreased lever responding due to non-reinforcement (extinction) reinstated lever responding following reexposure to cocaine. This “taste” of drug appears to function as a prime of the basic craving mechanism.
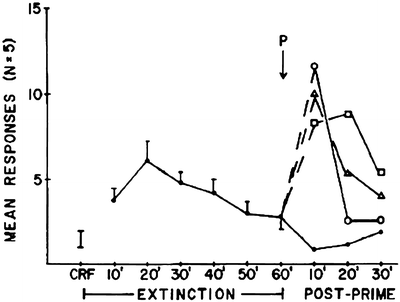

Fig. 5.
Cocaine-primed reinstatement of extinguished cocaine seeking by rats. Noncontingent injections of cocaine (●, saline; ❍, 0.5 mg/kg; Δ, 1.0 mg/kg; ❑, 2.0 mg/kg) reactivate extinguished responding suggestive of the prime serving as a cue that drug is now available. Means ± SEMs are indicated on the figure (Reprinted from (49) (Fig. 1) with kind permission of Dr. Stewart and Springer Science + Business Media).
Furthermore, the reactivated responding reflects activation of dopaminergic mesolimbic circuitry (e.g., nucleus accumbens) (69), already identified as components of the reward and incentive motivation circuitry (23, 70). While self-administration and priming studies are central to addiction and craving research, procedural issues complicate interpretation of findings from these studies. In particular, the drug is present during the behavioral evaluation (except for prior to the first injection – see start of this section). Stimulative or depressive effects of the self-administered drug could confound interpretation of effects of other pharmacological manipulations (62, 63). Second, related only to self-administration, is that these models do not model relapse after extended periods of abstinence.
4.2 Place Preference
A way around the problem of confounding effects of the self-administered drug on measures of basic craving is to use procedures where craving is assessed in the absence of the drug. Conditioned place preference (CPP) is one procedure (CPP is explored in depth in Chap. 6). CPP could be described as motivated approach to a drug-paired environment due to previous association of that environment with drug. In its simplest form, a CPP suggests that a location has been associated with the rewarding effects of a drug. As stated above, the selection of this location would require both incentive motivation and memory. Response requirements from the subject for CPP are minimal, in part due to the fact that the learning is a stimulus–stimulus (Pavlovian) association and requires no behavioral output initially. It is interesting that CPP has been demonstrated not only in mammals, but also in the invertebrates Drosophila (71) and planaria (72). Figure 6 depicts a methamphetamine-produced CPP in a planaria. The figure indicates locomotor path during training and testing. An initial bias to a quadrant is lost and preference to another quadrant is created when that quadrant was paired with methamphetamine application.
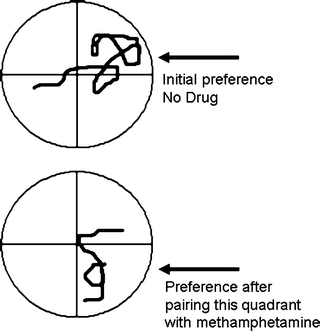

Fig. 6.
Establishment of a conditioned place preference for methamphetamine in the flatworm planaria. Association of a previously avoided quadrant with methamphetamine led to a subsequent place preference for the quadrant. The figure indicates tracings of the worm’s locomotion during the tests (Redrawn from (72) with kind permission of Dr. Watanabe).
Both Drosophila and planaria have rudimentary dopamine systems and have qualitatively similar locomotor responses to several drugs of abuse as mammals (73). While the CPP and the basic craving it suggests in these species might be debated to be more homologous than analogous compared to mammals, it is a thought-provoking example of how simplistic animal models might be used in addiction research. In addition, from an evolutionary perspective, the putative conservation of the basic learning and underlying neurochemistry of basic craving suggests a remarkable degree of adaptive fitness.
As noted above, and in contrast to self-administration procedures, testing for craving in CPP is done in a drug-free state. In addition, variations of the CPP procedure have been created making it amenable to study as a “relapse” model – the CPP reinstatement procedure (74). Most CPP in rats is dependent upon mesolimbic dopamine (75, 76).
4.3 Discriminative and Pavlovian Stimuli
Pavlovian associations also can gate drug-craving behavior by functioning as “occasion setters” and by enhancing or reinstating drug seeking behaviors (77). These stimuli, sometimes discrete and sometimes contextual, come to discriminate for the subject when drug is available. In various procedures, they have been found to increase drug seeking – an indication of basic craving. Discrete stimuli may become discriminative stimuli if they are consistently present during drug availability and consistently absent when drug is not available. Weiss and colleagues have used such a model to demonstrate that rats will respond on a lever in the absence of drug when a stimulus that previously indicated drug availability is presented. Figure 7 depicts the results of one of their studies (78) wherein rats responded in the presence of a cocaine-predictive tone (S+) but not in the presence of a saline-predictive light (S−). S+ responding was attenuated by pretreatment with the dopamine D1 receptor antagonist SCH23390.
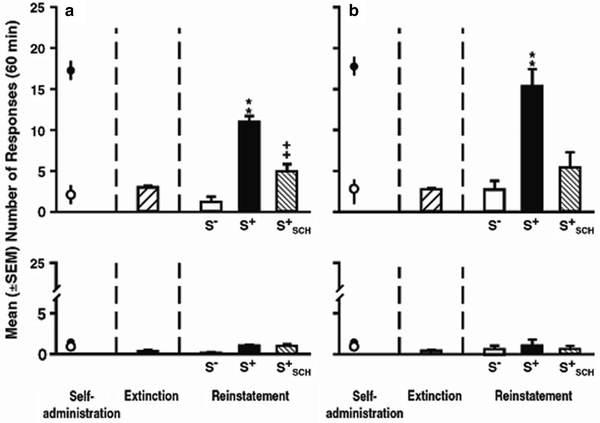 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


