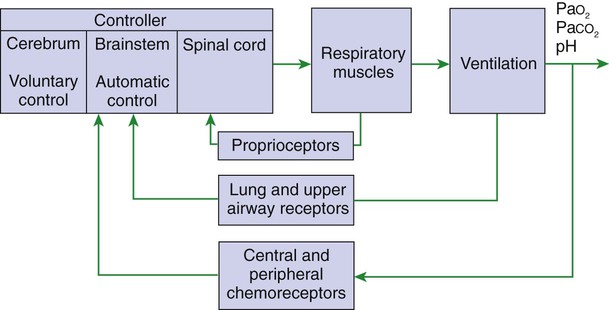
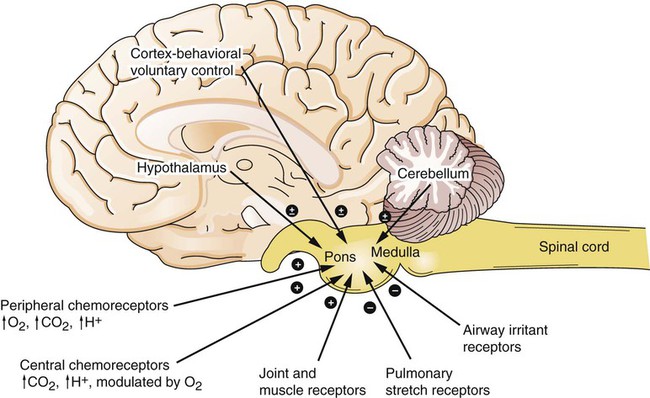
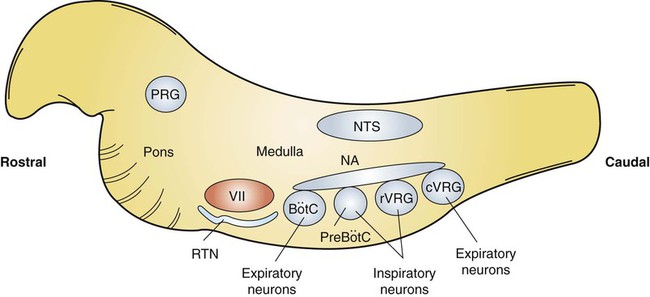
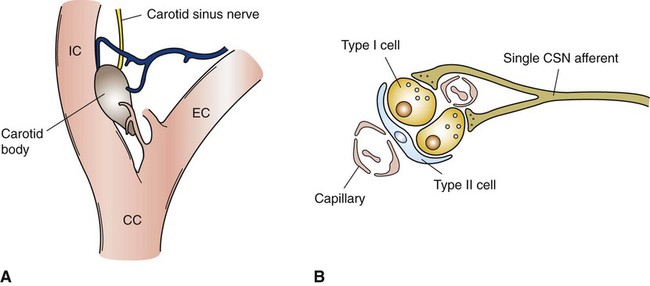
1. Respiration is regulated to meet the metabolic demands for delivery of oxygen and removal of carbon dioxide. Central control of respiration 1. Respiratory rhythmicity originates in the brainstem with inputs from higher centers. Pulmonary and airway receptors 1. Pulmonary stretch receptors, irritant receptors, and juxtacapillary receptors can influence the rhythm of breathing. 2. Muscle spindle stretch receptors monitor the effort exerted by respiratory muscles. 1. Hypoxia, acidosis, and hypercapnia are all potent stimuli for ventilation. 2. Peripheral chemoreceptors are the only receptors monitoring blood oxygen levels but also respond to changes in carbon dioxide and hydrogen ion concentrations. 3. The ventilatory response to carbon dioxide is mediated by central chemoreceptors. 4. Integrated breathing involves the central pattern generator, and inputs from chemoreceptors and vagal pulmonary afferents. 5. Ascent to high altitude is accompanied by a decrease in inspired oxygen tension and consequently by hypoxemia, which leads to an increase in ventilation. 6. During exercise, ventilation must increase because the tissues demand more oxygen and produce more carbon dioxide. Figure 49-1 provides a diagram of feedback control for the respiratory system. The central controller generates the signals that regulate the activity of the respiratory muscles, which by contracting give rise to alveolar ventilation. Changes in alveolar ventilation affect blood gas tensions and pH, which are monitored by the chemoreceptors. These receptors send signals back to the central controller so that necessary adjustments can be made to ventilation. Mechanoreceptors in various parts of the respiratory system monitor the degree of stretch of the lungs and changes in the airways and vasculature. Stretch receptors (proprioceptors) in respiratory muscles monitor the effort of breathing. Early attempts to understand the brain’s role in the regulation of breathing indicated that rhythmic respiration originates in the medulla and pons of the brainstem but is also affected by other afferent information from the lungs, chemoreceptors, and elsewhere. After many years of investigation it is becoming clear that the apparently simple, in and out nature of breathing is the result of a complex neural network known as a central pattern generator (CPG) located in the brainstem. This CPG may actually involve rhythmic and nonrhythmic subnetworks that are modulated by inputs from peripheral and central chemoreceptors, pulmonary stretch receptors, and others (Figure 49-2). The status of these subnetworks is affected by sleep, wakefulness, and changes during early development. The CPG includes neurons in the pontine respiratory group (lateral parabrachial and Kolliker-Fuse areas) and several medullary areas especially the Bötzinger and pre-Bötzinger complexes, the retrotrapezoid nucleus (RTN), and the rostral ventral and caudal ventral respiratory groups (rVRG and cVRG, respectively) (Figure 49-3). A dorsal respiratory group of neurons located in the nucleus tractus solitarius relays information to the CPG from peripheral chemoreceptors, pulmonary stretch receptors, bronchial irritant receptors, and other visceral information. The carotid bodies (Figure 49-4, A) are located close to the bifurcation of the internal and external carotid arteries, and the aortic bodies are located around the aortic arch. The latter appear to be most active in the fetus and of little importance in the adult. The aortic bodies are supplied by the vagus nerve, and the carotid bodies are supplied by a branch of the glossopharyngeal nerve. Fibers within the nerves supplying the peripheral chemoreceptors are primarily brainstem afferents, with a few parasympathetic and sympathetic efferent fibers to blood vessels.
Control of Ventilation
Respiration Is Regulated to Meet the Metabolic Demands for Delivery of Oxygen and Removal of Carbon Dioxide
Central Control of Respiration
Respiratory Rhythmicity Originates in the Brainstem with Inputs from Higher Centers
Chemoreceptors
Hypoxia, Acidosis, and Hypercapnia Are All Potent Stimuli for Ventilation
Peripheral Chemoreceptors Are the Only Receptors Monitoring Blood Oxygen Levels but Also Respond to Changes in Carbon Dioxide and Hydrogen Ion Concentrations
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Control of Ventilation
Only gold members can continue reading. Log In or Register to continue