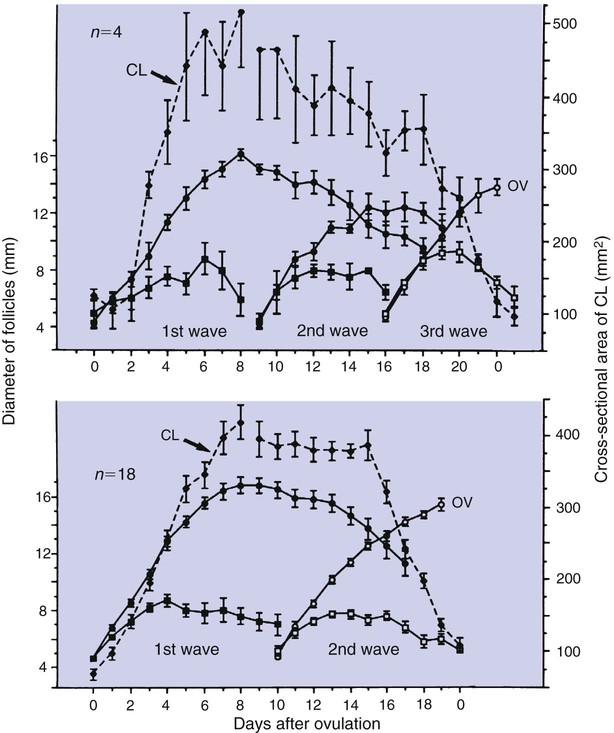
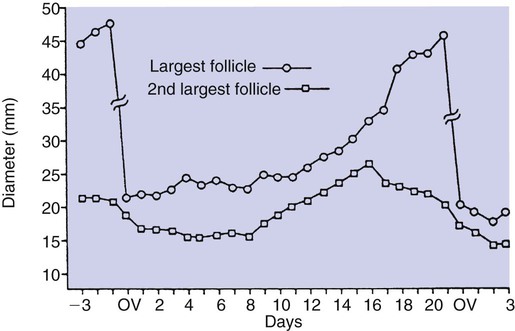
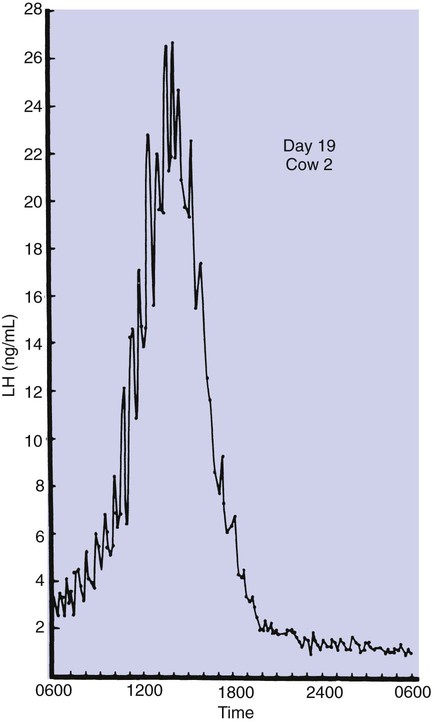
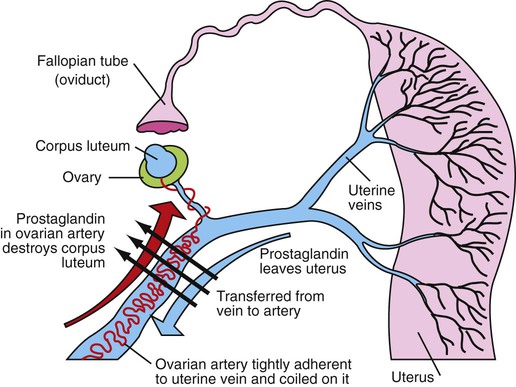
1. Ovulatory follicles are selected at the onset of luteolysis (in large domestic animals). 2. Ovulation is caused by an estrogen-induced preovulatory surge of gonadotropins. 1. The corpus luteum secretes progesterone, which is essential for pregnancy. 2. Luteinizing hormone is important for the maintenance of the corpus luteum. 3. Regression of the corpus luteum in nonpregnant large domestic animals is controlled by uterine secretion of prostaglandin F2α. 4. Changes in luteal life span in large domestic animals occur because of changes in prostaglandin F2α synthesis by the uterus. 1. In spontaneously ovulating animals, ovarian cycles have two phases: follicular and luteal; animals that require copulation for ovulation can have only a follicular phase. 2. The luteal phase is modified by copulation in some species. Until the advent of ultrasonography, it was difficult to identify growth patterns of follicles in domestic animals, especially those of follicles that develop during the luteal phase of the cycle. The concept that follicles do develop during the luteal phase was emphasized by the earlier work of Rajakowski, who described the midcycle follicle in the cow. With ultrasonography, it has been possible to define follicular growth and regression during the luteal phase of the cycle in the cow and mare. In cattle the predominant pattern is for several dominant (large) antral follicles to develop sequentially during the cycle (Figure 36-1). The follicular cycles are distinct to the extent that follicle regression usually begins (as indicated by follicle size) before the onset of the growth of the next follicle. The first dominant follicle regresses about midluteal phase, with a second dominant follicle beginning growth immediately. Whether the second dominant follicle is the ovulatory follicle, or whether a third develops, depends on the stage of the follicle at the time of regression of the corpus luteum (CL). If the second dominant follicle has begun to regress at the time of CL regression, a third follicle develops. Thus the selected ovulatory follicle is, by chance, the dominant follicle that is still in a developmental stage at the time that regression of the CL is initiated. The duration required for the development of the antral follicle to the point of ovulation has been estimated by various techniques to be about 10 days in domestic animals, perhaps slightly longer in some primates. From ultrasonographical and endocrinological studies, two different phases in final antral follicle development apparently occur in large domestic animals: a relatively slow phase that lasts 4 to 5 days, followed by a second phase of accelerated growth, again lasting 4 to 5 days, that terminates in ovulation (Figure 36-2). Because the final growth phase of follicle development can be initiated during the luteal phase, the initiation of this phase can occur under the influence of a relatively slow pulse rate of gonadotropin release that occurs during the luteal phase. The rapidly growing follicle requires exposure to a faster gonadotropin pulse rate by the third, or fourth, day in order for the follicle(s) to complete the normal growth pattern through ovulation. This situation usually occurs in conjunction with the onset of CL regression, which passively allows an increase in pulsatile rate of gonadotropin secretion (see Figure 35-4). The preovulatory surge of luteinizing hormone (LH), which begins about 24 hours before ovulation in most domestic species, including the cow, dog, goat, pig, and sheep, initiates the critical changes in the follicle that affect its endocrine organ status and result in release of the oocyte (Figure 36-3). Two important tissues, the oocyte and the granulosa, have been kept under control by the production of inhibitory substances that are probably of granulosa origin. An oocyte-inhibiting factor prevents the oocyte from resuming meiosis, and a luteinizing-inhibiting factor prevents the granulosa from prematurely being changed into luteal tissue. The impact of the LH surge blocks the production of both these factors. In most animals the resumption of meiosis results in the first division of meiosis (meiosis I), or formation of the first polar body, which is complete before ovulation. In animals with the potential for reasonably long reproductive longevity (e.g., cattle), the initiation of the meiotic process could have begun as many as 10 years or more before its completion. It is now accepted that PGF2α, a 20-carbon unsaturated fatty acid, is the uterine substance that causes regression of the CL in large domestic animals, including cattle, goats, horses, pigs, and sheep; PGF2α has no known natural role in CL regression in cats and dogs or in primates. Prostaglandin (PGF2α and PGE) therapy has been used clinically to cause luteolysis in the bitch and queen, for the treatment of pyometra, or to induce abortion. In large domestic species, regression of the CL is initiated by uterine synthesis and release of PGF2α (likely of endometrial origin) at about 14 days postovulation. The mode of transfer of PGF2α from the uterus to the ovary is thought to occur either by local countercurrent transfer or general systemic transfer. Countercurrent transfer involves the movement of molecules across the blood vascular system from higher concentrations in the venous effluent (utero-ovarian vein) to an area of lower concentration (ovarian artery) (Figure 36-4). Systemic transfer involves passage of the molecules through the general circulatory system. In some species (cow and ewe), PGF2α synthesis from a uterine horn only influences the life span of the CL in the ipsilateral ovary. In other species (sow and perhaps mare), PGF2α synthesis from one horn is sufficient to cause regression of CL in both ovaries. This effect likely occurs because of greater production of PGF2α by uterine tissue, as well as a difference in the rate of metabolism of PGF2α. PGF2α is rapidly metabolized systemically, with more than 90% changed by one passage through the lungs. Thus the system involving the use of PGF2α as the luteolytic agent in large domestic species requires that PGF2α be conserved through a special transfer system, or that it be produced in relatively large amounts.
Control of Ovulation and the Corpus Luteum
Ovulation
Ovulatory Follicles Are Selected at the Onset of Luteolysis (in Large Domestic Animals)
Ovulation Is Caused by an Estrogen-Induced Preovulatory Surge of Gonadotropins
Corpus Luteum
The Corpus Luteum Secretes Progesterone, Which Is Essential for Pregnancy
Regression of the Corpus Luteum in Nonpregnant Large Domestic Animals Is Controlled by Uterine Secretion of Prostaglandin F2α
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Control of Ovulation and the Corpus Luteum
Only gold members can continue reading. Log In or Register to continue