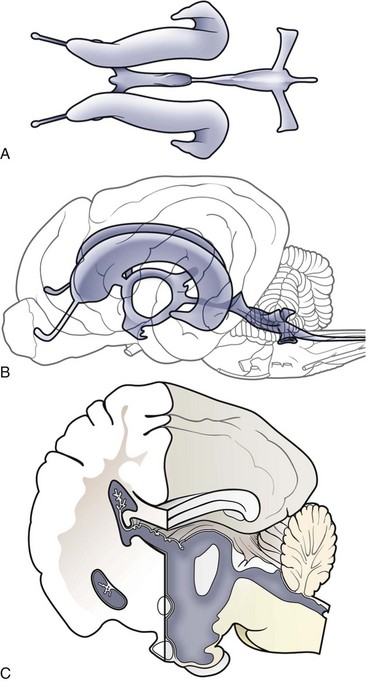
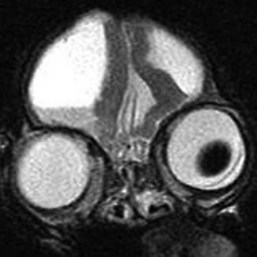
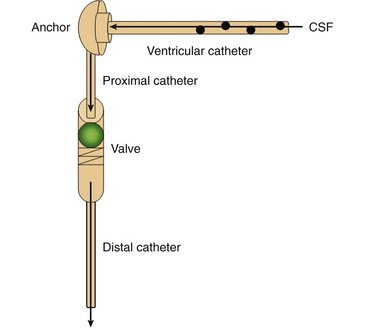
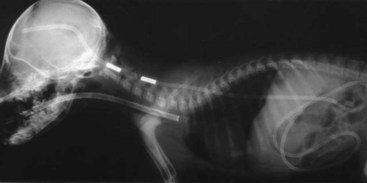
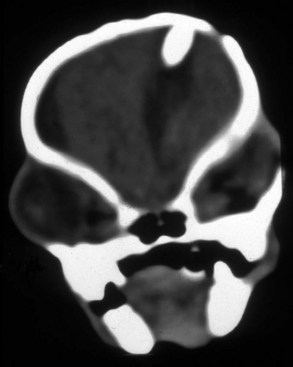
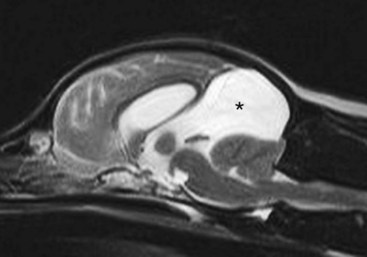
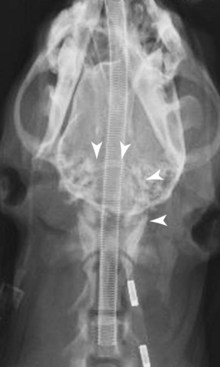
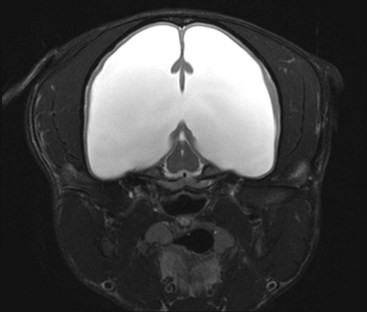
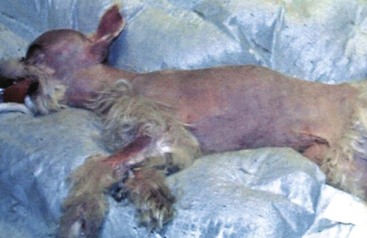
Chapter 37 Congenital hydrocephalus and intracranial arachnoid cysts are developmental disorders of dogs (primarily) and cats (occasionally) characterized by cerebrospinal fluid within the central nervous system. Hydrocephalus refers to excessive accumulation of cerebrospinal fluid within the ventricular system (particularly the lateral ventricles) of the brain that leads to clinical signs of brain dysfunction; it is most commonly a congenital disorder of young dogs and cats.8,19,49 In this text, acquired forms of hydrocephalus (e.g., neoplasia, inflammatory disease) will not be discussed in any detail; in general, surgical shunting techniques to be discussed pertain to both congenital and acquired forms of hydrocephalus. Intracranial arachnoid cyst is a congenital disorder in which a split in the arachnoid membrane leads to an accumulation of cerebrospinal fluid, typically in the caudal fossa.19,22 These disorders are discussed together in this chapter for several reasons, the most important being that medical and surgical management principles are very similar. The anatomy associated with cerebrospinal fluid flow within the brain is depicted in Figure 37-1. Cerebrospinal fluid is produced by two major processes. One process involves cerebrospinal fluid production by the choroid plexuses located in the lateral, third, and fourth ventricles. This is an energy-dependent process requiring the enzyme carbonic anhydrase. The remainder of cerebrospinal fluid production occurs via cellular elements; this fluid travels via extracellular pathways. Production of cerebrospinal fluid is constant and is unaffected by intracranial pressure. Most cerebrospinal fluid flows through the ventricular system to reach the subarachnoid space. The pathway starts in the lateral ventricles; it then goes into the third ventricle (via interventricular foramina), through the mesencephalic aqueduct, and into the fourth ventricle and reaches the subarachnoid space by flowing through the lateral apertures of the fourth ventricle. Cerebrospinal fluid is absorbed by the arachnoid villi, as well as by capillaries throughout the brain and spinal cord. The pathophysiology of central nervous system damage associated with hydrocephalus is complex and involves destruction of the ependymal lining of the ventricles, white matter damage by interstitial fluid accumulation, and eventually neuronal injury in the cerebral cortex.8,19,48 The phenomenon of excessive cerebrospinal fluid in the ventricular system of the brain occurs commonly in young dogs, especially of toy and brachycephalic breeds, and less commonly in cats. Congenital hydrocephalus is most commonly reported in dogs, especially small breeds (e.g., Chihuahua, Yorkshire Terrier, Maltese, Boston Terrier, English Bulldog, Toy/Miniature Poodle, Lhasa Apso, Pomeranian, Pekingese).8,19,48 Conventional theories regarding the pathophysiology of hydrocephalus contend that ventriculomegaly results from obstruction of cerebrospinal fluid flow within the ventricular system (e.g., mesencephalic aqueduct stenosis) and/or insufficient absorption of cerebrospinal fluid into the venous system at the arachnoid villi level. In short, these theories are based on the “bulk flow” concept of cerebrospinal fluid described earlier, in which the majority of cerebrospinal fluid is presumed to be absorbed by the arachnoid villi. A more recently proposed theory, called the hydrodynamic theory, maintains that hydrocephalus develops as the result of abnormal (reduced) intracranial compliance and the resultant effect of this compliance defect on brain capillaries. In the normal individual, brain capillaries remain open during the entire cardiac cycle (systole and diastole). This is important because much of cerebrospinal fluid absorption actually occurs at the capillary level, rather than in the arachnoid villi. Hydrocephalic patients are believed to have poor intracranial compliance as an underlying disorder that leads to hydrocephalus. The increased capillary pulse pressure caused by decreased compliance leads to a pulsatile transmantle pressure gradient directed from the cerebral tissue toward the lateral ventricles. These abnormal capillary pulsations occur within normal mean intracranial pressure limits. Rebound pressure from the recurring gradient, as well as hyperdynamic cerebrospinal fluid flow in the mesencephalic aqueduct, leads to ventricular enlargement over time. The end result is that hydrocephalus develops within the confines of normal intracranial pressure (i.e., normal pressure hydrocephalus).22 Hydrocephalus, especially if progressive, can cause neurologic dysfunction from compression and stretching of brain parenchyma, as well as from brain ischemia and interstitial edema. Many animals, especially of the predisposed breeds, may have hydrocephalus based on ventricular enlargement, yet have no discernible neurologic dysfunction.8,19,48 The authors do not consider these dogs to be hydrocephalic. We consider the term hydrocephalus to connote clinical signs of neurologic dysfunction. We believe that the term ventriculomegaly should be used to describe dogs and cats with subjectively large lateral ventricles and no clinical signs of dysfunction attributable to that enlargement. Common physical characteristics of hydrocephalic patients include a large, dome-shaped head, open fontanelles or larger calvarial defects, and bilateral ventrolateral strabismus (Figure 37-2). The strabismus may be due to orbital skull malformations, rather than to vestibular dysfunction, and has been referred to as the setting sun sign. It is important to realize that small-breed dogs often have persistent fontanelles with no attendant clinical signs of neurologic dysfunction. Clinical signs of neurologic dysfunction associated with hydrocephalus usually reflect a forebrain disorder and include obtunded mentation, behavior abnormalities, circling, pacing, restlessness, and seizure activity.33,38,50 In comparison with behavioral abnormalities and abnormal mentation, seizures are not commonly associated with congenital hydrocephalus. Some hydrocephalic patients may also exhibit vestibular and/or cerebellar dysfunction. Concurrent congenital abnormalities of the brain (e.g., intracranial arachnoid cyst, Dandy-Walker syndrome, Chiari-like malformation) occasionally occur in hydrocephalic dogs, accounting for cerebellovestibular dysfunction.8,19,48 The diagnosis of congenital hydrocephalus is based on a combination of characteristic clinical features, demonstration of ventriculomegaly, and the absence of other causes of encephalopathy. Ultrasonography (through a fontanelles or calvarial defects) and advanced imaging (Figure 37-3) have largely supplanted more invasive methods of documenting ventriculomegaly (e.g., contrast ventriculography). A vast majority of congenital hydrocephalus cases primarily involve dilation of the lateral ventricles (internal hydrocephalus). Occasionally, the bulk of the cerebrospinal fluid accumulation is found within the subarachnoid space overlying the brain (external hydrocephalus), with the brain being displaced axially (Figure 37-4).14 Electroencephalography has been used historically to assist in the diagnosis of congenital hydrocephalus, with affected patients typically exhibiting slow-frequency, high-voltage activity. However, these electroencephalographic findings are relatively nonspecific and seldom contribute to the diagnosis of congenital hydrocephalus.19 Figure 37-3 Axial T2-weighted brain magnetic resonance image of a dog with severe congenital hydrocephalus. The prognosis for dogs and cats with congenital hydrocephalus is variable but is generally guarded. Medical treatment of congenital hydrocephalus is aimed at reduction of cerebrospinal fluid production. Oral prednisone, at an initial dosage of 0.25 to 0.50 mg/kg q12h, may decrease cerebrospinal fluid production. Prednisone should be reduced over several weeks to the lowest possible dosage required to control clinical signs. Furosemide, a loop diuretic, decreases cerebrospinal fluid production via inhibition of the sodium/potassium/chloride co-transport system. The recommended dosage range is 0.5 to 4.0 mg/kg body weight PO q12-24h. The diuretic acetazolamide is a carbonic anhydrase inhibitor (carbonic anhydrase is a necessary enzyme for cerebrospinal fluid production) and is typically dosed at 10 mg/kg body weight PO q6-8h. Omeprazole, a proton pump inhibitor, has been shown to decrease cerebrospinal fluid production in dogs by 26%. The oral dose for dogs is 10 mg (for dogs weighing less than 20 kg) q24h and 20 mg (for dogs weighing more than 20 kg) q24h. For all of these drugs, it is recommended that the dose be tapered to the lowest dose needed to control clinical signs of disease to avoid serious side effects. Anticonvulsant drugs are administered if the patient is experiencing seizure activity.8,18,19,48 Medical therapy may be effective in some patients, whereas others require surgical shunting procedures for long-term control of clinical signs. The goal of surgical treatment for hydrocephalus is to continually divert excessive cerebrospinal fluid from the ventricles of the brain to the peritoneal cavity. The prognosis for sustained clinical improvement in neurologic status after surgical shunting procedures varies in the literature from 50% to 90% for dogs.8,19 In the authors’ experience, the success rate is approximately 75% to 80%. Potential postoperative surgical shunt complications in dogs and cats include shunt obstruction, shunt dislodgment, mechanical damage to the shunt, and shunt infection. Several shunts are available, all of which have the same basic design, including a ventricular catheter for placement into the lateral ventricle, a one-way valve, and a peritoneal catheter for placement into the peritoneal cavity (Figure 37-5). The shunts used in veterinary medicine are passive shunts that flow at a predetermined pressure level based on the valve. After the patient has been anesthetized, the head, lateral thorax, and entire lateral abdominal region are shaved and prepared aseptically for surgery with the patient in lateral recumbency (Figure 37-6). For very small patients (e.g., Chihuahuas, Yorkshire Terriers), the authors make two incisions—one curvilinear incision over the caudodorsal region of the parietal bone, and one vertical incision caudal to the last rib. For larger patients (e.g., English Bulldogs), an additional incision approximately midway between these two incisions on the lateral thorax is often required. A high-speed drill is used to drill two holes in the parietal bone: one for the ventricular catheter and one for an anchoring suture. The hole for the ventricular catheter is created to be slightly wider than the diameter of the catheter. Once the dura is exposed, it is incised with a #11 scalpel blade. The thin rim of brain parenchyma beneath the dura/arachnoid is also incised; the authors remove this small circular area of parenchyma with suction so that the ventricular catheter can be placed reliably in the lateral ventricle. The ventricular catheter is guided rostrally into the dorsal aspect of the lateral ventricle. After insertion of the ventricular catheter into the lateral ventricle, it is secured with a Chinese fingertrap suture via the anchor hole using 3-0 or 4-0 polypropylene (Figure 37-7). A small piece of Gelfoam is placed over the entry site. A grid approach to the peritoneal cavity is used, and the peritoneal catheter is tunneled from the incision over the parietal bone caudally to the incision over the lateral abdomen using a long Carmalt or Doyen forceps. After placement of the peritoneal catheter into the peritoneal cavity, it is secured to both the last rib and the abdominal musculature.8,48 Postoperative radiographs (Figure 37-8) or computed tomography (CT) scans (Figure 37-9) can be used to assess the accuracy of shunt placement postoperatively. Figure 37-6 Photograph of a patient prepared and positioned for ventriculoperitoneal shunt placement. An intracranial arachnoid cyst (also termed intra-arachnoid cyst and quadrigeminal cyst) is a developmental brain disorder in which cerebrospinal fluid is thought to accumulate within a split of the arachnoid membrane during embryogenesis. The developing neural tube is surrounded by a loose layer of mesenchymal tissue called the perimedullary mesh; this tissue eventually becomes the pia and arachnoid layers of the meninges. In normal development, pulsatile cerebrospinal fluid flow from the choroid plexuses is thought to divide the perimedullary mesh into pia and arachnoid layers, effectively creating the subarachnoid space. It is postulated that some aberration of cerebrospinal fluid flow from the choroid plexuses during this stage of development forces a separation within the forming arachnoid layer, eventually leading to the creation of an intracranial arachnoid cyst. The intra-arachnoid location of intracranial arachnoid cysts has been demonstrated via light and electron microscopy in people. The mechanisms by which an intracranial arachnoid cyst continues to expand with fluid are unknown, but several theories have been proposed. Fluid may be secreted by the arachnoid cells lining the cyst cavity. Evidence suggests that cells lining the intracranial arachnoid cyst may have secretory capacity. Fluid movement into the cyst may occur via an osmotic pressure gradient. Given that the fluid within the intracranial arachnoid cyst is nearly identical to cerebrospinal fluid, this theory is unlikely. In addition, documented cases describe people in whom small slits exist between the intracranial arachnoid cyst and the subarachnoid space; these slits act as one-way valves, diverting cerebrospinal fluid into the cyst during systole that cannot return to the subarachnoid space during diastole.6,17,19,34,44 Although intracranial arachnoid cyst has been reported to occur in several locations in human beings, all reported canine cases have been found in the caudal fossa (cerebellum and brainstem). Because intracranial arachnoid cyst is typically associated with the quadrigeminal cistern in dogs, these accumulations of fluid are often called quadrigeminal cysts in this species.17,30 Similar structures have been reported in cats.25,28 Also termed intracranial intra-arachnoid cyst, intracranial arachnoid cysts account for 1% of all intracranial masses in people and have been reported sporadically in dogs. Intracranial arachnoid cyst is often an incidental finding in humans; it has been suggested that this may also be the case for intracranial arachnoid cyst in dogs.20 In the veterinary literature, a total of 56 cases of canine intracranial arachnoid cyst have been reported.* The intracranial arachnoid cyst was thought to have been an incidental finding in approximately one third to more than one half of reported cases. A vast majority of reported intracranial arachnoid cyst cases in dogs have occurred in small breeds, with a predominance of brachycephalic dogs. The breeds reported to date include Shih Tzu (15), Maltese (4), Pug (4), Cavalier King Charles spaniel (4), Yorkshire Terrier (4), Lhasa Apso (4), Chihuahua (3), Staffordshire Bull Terrier (3), Bulldog (3), Pekingese (2), West Highland White Terrier (2), and one each of Bichon Frise, Pomeranian, Cairn Terrier, Jack Russell Terrier, Terrier Mix, Beagle, Miniature Schnauzer, and German Shorthaired Pointer.† A wide age range at clinical presentation has been noted for dogs with intracranial arachnoid cyst (2 months to 10 years), with an approximate average age of 4 years. The most common clinical signs seen with intracranial arachnoid cyst are forebrain (including seizure activity) and/or central vestibular (cerebellovestibular) dysfunction. Dogs may also present with a primary clinical sign of neck pain.17,19 The diagnosis of intracranial arachnoid cyst is typically made via CT or (preferably) magnetic resonance imaging (MRI). Intracranial arachnoid cysts can also be visualized with the use of ultrasound imaging (via foramen magnum, temporal window, and/or persistent bregmatic fontanelle), especially in younger dogs. The characteristic appearance of intracranial arachnoid cyst is a large, well-demarcated, fluid-filled structure, isointense with cerebrospinal fluid spaces and located between the caudal cerebrum and the rostral cerebellum (Figure 37-10). Because intracranial arachnoid cyst may be an incidental finding, it is important to rule out concurrent inflammatory disease (i.e., via cerebrospinal fluid examination). In the authors’ opinion, it is often difficult or impossible to discern whether intracranial arachnoid cyst in the presence of another brain disorder is purely an incidental finding. Because the presence of a large, fluid-filled structure within the cranial vault likely decreases intracranial compliance, some intracranial arachnoid cysts may be contributory, rather than an incidental finding. In one study, it was found that the degree of brain compression caused by the intracranial arachnoid cyst as measured on MRI was predictive of whether or not the patient would display clinical signs of dysfunction.26 Because this disorder is believed to represent a developmental abnormality of the intracranial ventricular cerebrospinal fluid system, it may occur concurrently with other fluid abnormalities (e.g., congenital hydrocephalus). The cyst may or may not communicate with the remainder of the ventricular system. When evidence of intracranial arachnoid cyst and another disease (e.g., granulomatous meningoencephalomyelitis [GME]) is found in the same patient, optimal response to treatment may entail treating both conditions. Medical treatment for intracranial arachnoid cyst is identical to that described for congenital hydrocephalus (e.g., corticosteroids, diuretics, anticonvulsants if indicated). Dogs with intracranial arachnoid cyst tend to respond initially to medical therapy, but the response is often temporary. Surgical management of intracranial arachnoid cyst in people is typically via cyst fenestration or cystoperitoneal shunt placement. Both procedures have been reported in dogs with intracranial arachnoid cyst.15,20,33,49,50 Five cases of fenestration have been reported in which intracranial arachnoid cyst was considered the primary disease. The extent of the fenestration performed varies among surgeons. The amount of cyst wall removed is somewhat dependent on the extent of the craniotomy performed. Three fenestration cases were reimaged post surgery; two of the three dogs had evidence of cyst persistence on MRI; however, only one of these two dogs required reoperation.33,49,50 The authors have reported successful cystoperitoneal shunting of dogs with intracranial arachnoid cyst.15 Cystoperitoneal shunting has also been successfully used in the treatment of a feline case of intracranial arachnoid cyst.25 With the exception of placement of the end of the shunt into the intracranial arachnoid cyst, the procedure is identical to that described for congenital hydrocephalus. For placement of the catheter in intracranial arachnoid cyst cases, the authors perform a wide caudolateral craniotomy, creating both rostrotentorial and suboccipital skull defects. The transverse sinus between these two defects is sacrificed and occluded with bone wax. As with congenital hydrocephalus, postoperative imaging is performed to assess the accuracy of shunt placement (Figure 37-11). The success rate for surgical management of intracranial arachnoid cyst appears to be high in human beings7 and dogs, and whether fenestration or cystoperitoneal shunting is the preferred procedure for both species remains controversial.
Congenital Brain Malformations
Congenital Hydrocephalus and Intracranial Arachnoid Cysts
Anatomy and Physiology
Congenital Hydrocephalus
Physical Examination
Diagnosis
Medical Treatment
Surgical Treatment
Intracranial Arachnoid Cyst
Physical Examination
Diagnosis
Treatment
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Congenital Brain Malformations
Only gold members can continue reading. Log In or Register to continue