Chapter 43 Chelating Agents
A chelating agent is a ligand (or drug) that binds to a metal ion through two or more sites to form a metal chelate that is removed from the body, usually through renal excretion. Copper (Cu) chelators are used in veterinary gastroenterology for the treatment of abnormal Cu accumulation in the canine liver.1
Pathophysiology of Copper
The liver is the central metabolic organ for the regulation of Cu body stores.2 Cu enters the body through the diet and approximately 30% is absorbed by the upper small intestine with the majority of the Cu passing out through the feces. Although the exact details of intestinal Cu absorption are not completely understood, Cu is passively taken up by intestinal epithelial cells, bound to the cytosolic protein metallothionein, and actively transported into the circulation bound to either albumin or transcuprein. Cu is then transported to the liver where it is stored, mobilized to peripheral tissues, or excreted from the body.2 Hepatic Cu is either complexed with ceruloplasmin a transport protein, incorporated in hepatic cellular pathways, or bound with metallothionein within the liver. Metallothioneins are cysteine-rich, cytosolic proteins that function to store Cu and protect the hepatocyte from Cu toxicity.2 In health, approximately 80% of the Cu that is absorbed is eventually excreted into the bile.
Normal hepatic Cu concentrations in dogs are maintained at approximately 200 to 400 µg/g dry weight liver.3 When Cu concentrations increase to levels exceeding hepatic metallothionein’s complexing capabilities, direct hepatic damage results, primarily through formation of oxygen free radicals causing either hepatocellular necrosis or apoptosis.4 The abnormal accumulation of hepatic Cu in the dog results from either a primary metabolic defect in hepatic Cu metabolism or secondary to cholestatic liver disease resulting in impaired biliary Cu excretion.1 Box 43-1 lists dog breeds documented or suspected of having a hereditary defect in Cu metabolism. Thus far, only the affected Bedlington Terrier has been identified as lacking a specific gene on chromosome 10 encoding a small cytosolic protein termed COMMD1 (MURR1) that is involved in hepatic Cu transport and biliary excretion.5 The other breeds listed in Box 43-1 do not accumulate hepatic Cu to the same extent as the affected Bedlington Terrier and most likely have a different genetic alteration in Cu metabolism. Regardless of the cause of Cu accumulation, therapy is directed at reducing hepatic Cu concentrations into the normal reference range using Cu-chelating agents. Depending on the breed, hepatic Cu concentrations, and extent of liver pathology, the choice of chelators and the dose and duration of therapy vary.
Copper Chelators
Penicillamine
Penicillamine is a thiol, a compound with a sulfhydryl group, making the molecule an active metal-chelating agent with a high affinity for Cu.6 Penicillamine was first discovered in the early 1950s when J.M. Walshe identified this unusual urinary metabolite in a human patient treated with penicillin.7 Walshe dosed himself with penicillin and found that he also excreted penicillamine in his urine. He quickly discovered that this compound had chelation properties with the thiol group because it would bind to iron. Following a dose of penicillamine, Walshe discovered he had an increase in urinary Cu excretion. Those early studies also found that the L isomer of penicillamine was toxic to growing rats but the D isomer did not have this effect.8 Once an adequate supply of the D-penicillamine was obtained, a patient with Wilson’s disease, a hereditary disease in which Cu accumulates in the tissues, was treated and showed a positive response to therapy with increased urinary Cu excretion. Over the last 50 years D-penicillamine has been the primary Cu chelator used in the treatment of Wilson’s disease.9
Mechanism of Action
By virtue of its sulfhydryl groups, penicillamine chelates a number of heavy metals, including Cu. It forms a stable water-soluble complex with Cu that is excreted through the kidneys. In vitro studies show 1 atom of Cu combines with 2 molecules of penicillamine, which in theory means 1 g of penicillamine would be followed by a maximum excretion of 200 mg of Cu.9 Clinically, Cu excretion from a 1-g dose results in the loss of only several milligrams of Cu. Prolonged penicillamine therapy in Wilson’s disease patients eventually results in a decline in urinary Cu excretion in spite of continued elevations in hepatic Cu concentrations. However, these patients for some reason continue to improve both clinically and in their liver histology.10 There is now speculation that there may actually be several pools of hepatic Cu in Wilson’s disease, some of which may not be removed by the drug. It is postulated that penicillamine may detoxify the remaining Cu in the liver, either by the formation of a hepatic chelate that is nontoxic or more likely by inducing the synthesis of hepatic metallothionein proteins that then bind and sequester Cu in the liver in a nontoxic form. Penicillamine has also been reported to have a greater affinity for Cu in tissues than in the blood.10
Penicillamine will also decrease cystinuria and has been used to prevent the formation of cystine calculi in dogs.11 It combines chemically with cystine to form a stable, soluble, readily excreted complex. Penicillamine also interferes with the formation of crosslinks between procollagen molecules, thereby exerting some weak, antifibrotic activity.10 Penicillamine may also have immunomodulatory effects and has been demonstrated to reduce immunoglobulin M rheumatoid factor and T-cell activity in human patients with rheumatoid arthritis.10 The clinical relevance of penicillamine immunosuppression in dogs is unknown.
Dosage and Toxicity
For management of Cu-associated hepatopathies in the dog, a dose of 10 to 15 mg/kg q12h of D-penicillamine (Cuprimine [capsules] or Depen [tablets]) is given orally.1 It should be administered on an empty stomach, at least 1 hour before meals or 2 hours after meals, and at least 1 hour apart from any other drug or food. This permits maximum absorption and reduces the likelihood of inactivation by chelating substances within the gastrointestinal tract.
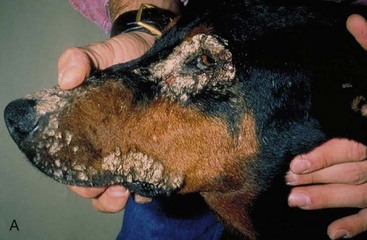
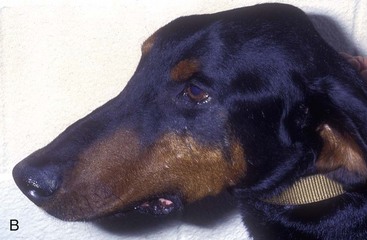
Side effects from penicillamine therapy are common in humans, with an incidence estimated as high as 30% to 50% of untoward reactions, the most common being dermatologic (Box 43-2).11 There may be some cross-sensitivity between penicillin and penicillamine in some patients. In dogs, nausea and vomiting are the most common side effects and are observed in approximately 30% of patients. These clinical signs may be minimized by reducing the dose or dividing the daily dose into three or four smaller doses. The reduced dose can usually be increased gradually over time. Alternatively, the drug can be given with a small amount of food; however, this may reduce absorption as a consequence of intestinal chelation. Very few other adverse effects have been observed or reported in dogs. The author observed skin hypersensitivity reactions (Figure 43-1 A and B) in several dogs that all have resolved with discontinuation of therapy. The author is also aware of a few anecdotal reports of renal disease following therapy. Penicillamine is reported to cause depletion of the vitamin B6 (pyridoxine) in humans. Although this has not been observed in dogs, B-vitamin supplementation during long-term therapy may be advisable.
Box 43-2
Some of the Reported Adverse Effects Associated with Penicillamine Therapy in Humans and Those Identified in Dogs
Dermatologic (pruritus, systemic lupus erythematosus–like syndrome)*
Gastrointestinal (vomiting, diarrhea)*
Hematologic (anemia, bone marrow suppression)
Renal disease (glomerulopathy, renal failure)*
Central nervous system (tinnitus, peripheral neuropathy)
Long-term Cu-chelator therapy given to a dog that is incorrectly diagnosed as having Cu hepatotoxicity can result in adverse side effects. This has been reported with trientine therapy in one dog and the author has observed similar findings in one Bedlington Terrier treated with penicillamine.12 Anorexia, vomiting, and weight loss was observed clinically with marked elevations in serum liver enzyme activity. Both dogs had marked reductions in hepatic Cu concentrations. Discontinuation of chelation therapy resulted in clinical improvement. Consequently, it is imperative to correctly diagnose a Cu storage disease based on biopsy and hepatic Cu quantitation before starting therapy and to measure hepatic Cu concentrations during therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree