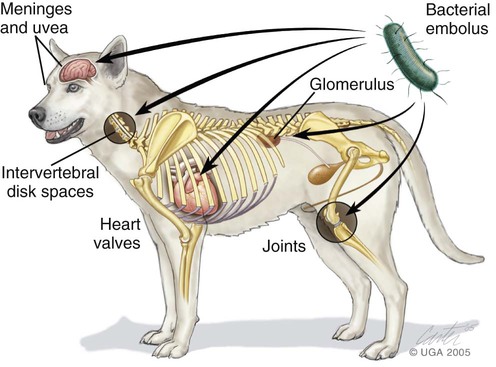
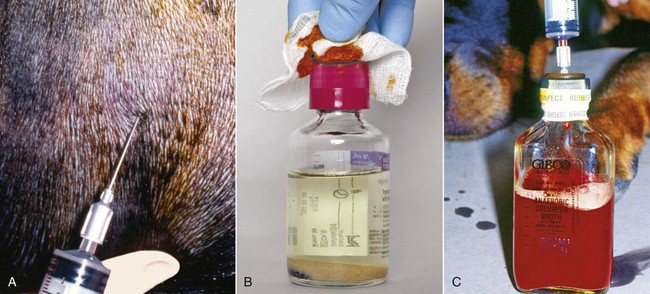
Bacteria normally are excluded from the bloodstream by host defenses. On occasion, they do circumvent these barriers, gain access to the blood, and cause a transient bacteremia, a condition that is often unnoticed in clinically healthy individuals. For example, portal and systemic bacteremia (predominantly caused by gram-negative organisms) is found in clinically healthy dogs and is magnified in dogs with acquired hepatic disease, congenital portosystemic shunts, and portal hypertension.107 The liver generally clears the bacteremia coming from the microflora-laden intestinal tract. Bacteremia, particularly in the presence of immune suppression, can lead to disastrous and overwhelming infection. Overwhelming bacteremia leads to sepsis, which often leads to decreased organ perfusion characterized by sinus tachycardia, tachyarrhythmias, hypotension, gastrointestinal (GI) damage, liver dysfunction, lactic acidosis, and oliguria. Bacteremia often leads to sepsis and may lead to septic shock. If treated quickly and aggressively, septic shock may be reversible; if not, it quickly becomes irreversible.68 Bacterial virulence and the likelihood of septic shock depend on the presence of cell capsules isolating cell wall antigens from host inflammatory cells; microbial enzyme production facilitating rapid tissue penetration; and concentration of bacteria in the bloodstream, which is related to size of the inoculum and duration of bacteremia.159,200 It is safe to assume that the prevalence of bacteremia in dogs and cats is significantly underestimated, particularly in referral and emergency hospitals. Affected dogs and cats can be any age, breed, or gender.91,92 Although bacterial prostatitis has been identified in some bacteremic male dogs, a causal relationship is difficult to prove. Nonetheless, the prostate gland should be suspected as a nidus of bacteremia in intact male dogs, especially middle-aged and older dogs.42,68 Chronic seeding of the bloodstream by sites of infection such as bone, skin, gingivae, abscesses, and the prostate gland predisposes animals to bacterial endocarditis and diskospondylitis.39,42 Bacteriuria is either a source or a consequence of bacteremia. Various factors influence the relative frequency of etiologic agents among different hospitals. Surgical and critical-care practices may experience a higher prevalence of gram-negative and anaerobic infections. The site of local infection and prior antibacterial therapy and whether the infection is nosocomial or community acquired determine the most likely offending microbes. The percentages of isolation of various organisms from dogs and cats with bacteremia and bacterial endocarditis are summarized in Table 86-1. Coagulase-positive Staphylococcus spp. are most commonly involved in dogs. Because the recognized prevalence of bacteremia in cats is less than that in dogs, most of the discussion that follows concerns dogs. A discussion of endocarditis in cats follows the information on specific bacteria identified in dogs. TABLE 86-1 Frequency of Isolation of Bacteria from Positive Blood Culturea aValues are expressed as a percentage of those cases in which organisms were isolated or detected by genetic means and represent a compilation of the cited references. The percentage of dogs for which negative isolation results were obtained is not included. bReferences 29, 39, 51, 189, 204, 212. cReferences 68, 96. Coagulase-positive staphylococci are among the most commonly isolated pathogens. Staphylococci can survive in the environment and be cultured from dried clinical material after several months. They are relatively heat resistant. Staphylococci are found in the nasopharynx and skin and can contaminate any site on the skin and mucous membranes. Multiplying staphylococci can overcome local phagocytic defenses and gain access to the lymphatics and bloodstream. Staphylococcal bacteremia can lead to metastatic infections in the heart, lungs, and bones (see Chapter 34). Staphylococci secrete numerous enzymes and toxins that are implicated in their pathogenesis. Catalase can inhibit polymononuclear oxygen-free-radical-killing activity and their toxins damage cell membranes and cause cell lysis. Manifestations of their infection vary from trivial, as in some pyodermas, to overwhelming sepsis. Coagulase-positive Staphylococcus pseudintermedius (see Etiology, Chapters 34 and 84) have been common bacteria isolated from blood cultures of dogs with bacteremia alone, diskospondylitis, and endocarditis.39,42 Common sources for bloodstream infection include abscesses, pyoderma, and wound infections. However, a source of infection is not always obvious. Physical manipulations of abscesses and cellulitis can increase tissue pressure and facilitate movement of bacteria into small veins and lymphatics. Staphylococci tend to spread from localized abscesses, wounds, and deep pyodermas into the bloodstream by invading blood vessels, producing septic thrombi, lymphatics, or incompetent lymph nodes. Metastatic foci of infection are common and often involve the spleen, kidneys, bones, joints, or heart valves. Only a small percentage of local infections caused by S. pseudintermedius gain access to the bloodstream.48 Nonetheless, enzymes such as staphylokinase, hyaluronidase, and protease can enable tissue invasion. Sepsis can result from enterotoxins that bind to T cells and macrophages, stimulating the production of cytokines. One of the targets of S. pseudintermedius is the endothelial cell.48 Organisms bind to and are internalized by endothelial cells, where cytolysins are released that can disrupt the endothelium and allow access to tissue. They can survive inside of endothelial cells and phagocytic cells. This may explain their propensity to cause recurrent and refractory bacteremia. Systemic streptococcal infections are common in dogs and cats (see Chapter 33). Streptococcal bacteremia can originate from cutaneous sites and the upper respiratory tract. Streptococcal pneumonia may be associated with a high prevalence of subsequent bacteremia. In bacteremic dogs with various underlying illnesses, blood culture results may be positive for hemolytic Streptococcus canis, non-β-hemolytic Streptococcus viridans, and enterococci. Most β-hemolytic streptococci enter the bloodstream via the skin, whereas non-β-hemolytic streptococci usually enter via breaks in the mucous membranes. Non-β-hemolytic streptococci are normal skin commensals and occasionally may contaminate improperly collected blood culture specimens. Group A streptococci (Streptococcus pyogenes) are enveloped in a hyaluronic acid capsule that retards phagocytosis by neutrophils and macrophages. Toxins produced by S. pyogenes are pyrogenic, are cytotoxic, and enhance susceptibility to the effects of endotoxin.60 Group B streptococci can cause sepsis in dogs and cats and have been incriminated as a cause of bacteremia and death in the “fading puppy” syndrome. In humans, bacteremia caused by group D streptococci (enterococci), including Streptococcus faecium, Streptococcus bovis, and Streptococcus faecalis, usually originates from the urinary tract but also can develop after manipulation of the lower bowel. S. bovis was reported to cause IE in a small-breed dog with myxomatous valvular disease after routine dental prophylaxis.216 Dogs infected with streptococci may be more likely to have mitral valve involvement and have a higher prevalence of neutrophilic polyarthritis.211 Enterococcal bacteremia, which has been reported in dogs, is especially serious because enterococci are resistant to many antibacterials. Diphtheroids, a heterogenous group of bacteria including the genus Corynebacterium, are often interpreted as contaminants when isolated from blood culture because they normally inhabit skin and mucous membrane surfaces. Corynebacterium accounts for a minority of cases of bacteremia in dogs and has rarely been associated with endocarditis. Bacillus species are frequent blood culture contaminants. However, in the immunocompromised host, Bacillus cereus and Bacillus subtilis can gain access to the bloodstream. Corynebacterium and Bacillus should be isolated from multiple blood cultures before incriminating these agents as causing a bloodstream infection. Erysipelothrix tonsillarum strains have been isolated from the heart valves of dogs with endocarditis (see Chapter 33).212 The term gram-negative bacteremia is typically applied to a hematogenous infection caused by the Enterobacteriaceae and Pseudomonadaceae. Bacteremias resulting from gram-negative agents, such as Pasteurella, Brucella, Bartonella, Serratia, and Salmonella species, which can produce similar clinical manifestations, are usually considered discrete clinical entities. Salmonella enteritidis was the most common organism isolated from bacteremic cats in one study.68 Gram-negative bacillary bacteremia usually represents a serious opportunistic infection that has developed subsequent to significant suppression of host immune defenses. Gram-negative bacteremia is often associated with high mortality, and the number of such infections has increased along with the use of invasive medical devices and immunosuppressive therapy for malignancies and inflammatory diseases. Within the family Enterobacteriaceae, Escherichia coli is the most common bloodstream isolate from animals. E. coli is abundant in the lower GI tract, which often serves as a reservoir for infection of other body sites. Intestinal epithelial damage, necrosis, or sloughing, particularly in debilitated or immunocompromised patients, can quickly lead to overwhelming and lethal E. coli–induced sepsis. The urinary tract is another source. Oropharyngeal and fecal colonization with gram-negative bacilli can increase progressively in seriously ill hospitalized patients as their clinical status deteriorates. Gram-negative bacteria not only gain access to the blood from extravascular foci but also can originate from intravenous catheters, from urinary catheters, and from septic thrombophlebitic conditions. In extravascular infections, bacteria often gain access to the blood via lymphatics or invasion of small blood vessels within a site of infection. Other sources for bloodstream invasion include drainage tubes, contaminated intravenous fluids, and contaminated aerosolization devices; disrupted mucosal barriers (e.g., after dental procedures or endoscopic examinations); and decubital ulcers. In contrast to staphylococcal bacteremia, gram-negative bacteremia in dogs and cats seldom is associated with septic thrombosis and metastatic abscess formation but is often rapidly progressive and likely to result in sepsis (see Chapter 36). Although common in the environment and occasionally present on mucosal surfaces, Pseudomonas species have rarely been isolated from deeper tissues of healthy patients. They have been more common in otitis externa, dermatitis, cystitis, and respiratory infections of dogs. Because Pseudomonas organisms are opportunists, their rapid colonization with subsequent development of bacteremia is much more likely to occur after disruption of host defenses, especially cutaneous barriers (surgery, intravenous catheters, and burns), and depletion of neutrophils, as occurs in patients who are receiving chemotherapy for cancer.67 Extensive antibacterial use or contaminated intravenous fluids may also predispose patients to Pseudomonas bacteremia. Most reported cases of Pseudomonas bacteremia in dogs have been nosocomial.67 Prevention is easier than treatment of gram-negative bacteremia. The use of intravenous and urinary catheters should be limited to instances in which they are absolutely necessary. They should be inserted and maintained under scrupulously sterile conditions and removed or changed within 3 to 5 days (see Chapter 90). Strict aseptic precautions in the management of wounds, use of tube drainage systems, prevention of decubitus, and limitation of prophylactic antibacterials are all important in preventing opportunistic infections for all patients, particularly those with weakened immune systems. The risk-benefit ratio of glucocorticoid administration must be carefully evaluated. Anaerobic bacteria, particularly anaerobic gram-negative rods, are considered to be serious pathogens. Development of anaerobic bloodstream infections may be encouraged by the presence of periodontal disease, deep abscesses, granulomas, peritonitis, osteomyelitis, septic arthritis, and septic pleural effusion (see Chapter 39). Clostridium perfringens is the most common canine isolate, whereas Bacteroides and Fusobacterium are commonly isolated from cats. A mechanically correctable lesion (abscess, perforated bowel, necrotic tissue) is often the source of anaerobic bacteremia. Bacteroides may enter the bloodstream via intra-abdominal sources, such as GI and genital inflammatory diseases. Fusobacterium bacteremia often originates from infections of the respiratory tract. Actinomyces turicensis, a facultative anaerobic gram-positive bacterium, was isolated from endocarditis in a dog.138 Characteristics of anaerobic bacteremia include fever, thrombophlebitis, and icterus, particularly with Bacteroides bacteremia. Sequelae to anaerobic bacteremia include metastatic abscess formation and endocarditis. Clostridial bacteremia tends to have a relatively insidious clinical course without obvious signs of sepsis, although septic shock occurs occasionally. Bartonella spp. are gram-negative, fastidious bacteria adapted to one or more mammalian reservoir hosts and are associated with long-lasting endotheliotropic infection with relapsing intraerythocytic bacteremia (see Chapter 52). Arthropods, including biting flies, fleas, lice, and ticks, are suspected vectors of transmission. Bartonella spp. can contribute to both endothelial and intraerythrocytic infection and induce immune suppression through unknown mechanisms, and the bacteremia can be chronic, spanning months to years in duration. Arthritis, granulomatous inflammation, endocarditis, epistaxis, lymphadenomegaly, osteomyelitis, panniculitis, and vasoproliferative lesion are manifestations of Bartonella infections in humans and dogs.33 In a study of IE, dogs infected with Bartonella spp. were significantly more likely to be afebrile, have involvement of the aortic valve, and have a higher prevalence of congestive heart failure when compared to dogs infected with other pathogens.211In a retrospective study of infective endocarditis in 9 dogs, DNA of B. henselae was amplified in 7 dogs and three of these had concurrent DNA of B. vinsonii ssp. berkhoffii.78a Although not always identified, the most common sources of bacteremia include infections of the integumentary, GI (including biliary tract), genitourinary, and respiratory systems. Intravenous catheter–associated infections also occur.39,42 Bacteremia can be a complication of parenteral nutrition, and the catheter should always be considered a source of infection. Infection often begins locally in the catheter wound when the patient’s cutaneous flora invade the tract during catheter insertion and thereafter. Hub contamination is also a source of infection. Catheter-related bacteremia is confirmed when catheter and blood culture results yield the same microbe. The most common organisms are staphylococci. Catheters should always be removed and cultured immediately if bacteremia or thrombophlebitis is suspected. Catheter-related bacteremia originates from the migration of bacteria from the venipuncture into the catheter tract and along the external surface of the catheter to the intravascular tip, which becomes colonized.108 Contamination of the hub can allow migration within the catheter lumen. Teflon and polyurethane are more resistant to bacterial colonization than polyethylene or polyvinyl chloride. Some bacteria, such as staphylococci, can bind to host fibronectin that is deposited on catheters. Peripheral venous catheters have a lower risk of bloodstream infection than central venous catheters.108 Catheters impregnated with chlorhexidine or silver sulfadiazine are resistant to infection. Sterile gloves should be worn during catheter placement, and the area should be sterile. Insertion and maintenance of intravenous catheters by inexperienced staff members increase the incidence of catheter-related bacteremia. In cats, pyothorax, septic peritonitis, GI tract disease, pneumonia, endocarditis, pyelonephritis, osteomyelitis, pyometra, and bite wounds are all sources of bacteremia.26 Various factors (Box 86-1) have been cited as predisposing patients to developing bacteremia. When considering mortality from bacteremia and sepsis, the single most important factor influencing outcome after infection is the severity of the patient’s underlying disease. Death from bacteremia is much less likely to occur if the animal was healthy before the bacteremia developed. Secondary arthropathies, glomerulopathies, embolic abscesses or thrombi, and splenomegaly are more often associated with IE than with bacteremia alone (Fig. 86-1; Table 86-2). Metastatic infection can result in life-threatening complications. Virtually all dogs with IE of the left side of the heart experience multiple continuous embolizations and renal infarctions, which may lead to renal failure. Subacute and chronic bacteremia can result in sustained antigenic stimulation of the immune system and increased circulating immunoglobulin. Circulating immune complexes (CICs) may be deposited in many tissues, leading to development of polyarthritis, myositis, vasculitis, and glomerulonephritis. Young, growing animals can develop metaphyseal embolization with resultant hypertrophic osteodystrophy (see Chapter 85).203 In humans, bacteremia has been found to result in hemolysis, presumably because of modified erythrocyte antigens that develop as a result of the circulating organism. A similar association of bacteremia with immune-mediated hemolytic anemia was not found in dogs.153 Bacteria such as intraerythrocytic Bartonella species and hemotropic mycoplasmas can induce hemolytic anemia (see Chapters 52 and 31, respectively), as do many protozoan erythroparasites. TABLE 86-2 Clinical Signs in Dogs with Bacteremia or Bacterial Endocarditis Dogs with bacteremia usually display some combination of lethargy, anorexia, GI disturbances (such as vomiting and diarrhea), fever, trembling, lameness, and myalgia (see Table 86-2). The presence of lameness (which may be intermittent), joint pain, muscle pain, and stiffness can suggest either immune-mediated disease or septic embolization of various tissues, arterial thromboembolism, or hypertrophic osteopathy. Either infective or, more likely, immune-mediated arthritis can develop, and bilaterally symmetric joint involvement is more typical of immune-based arthritis. Lumbar or abdominal pain that is elicited by palpation suggests the possibility of renal or splenic inflammation secondary to septic embolization, infarction, abscess formation, or diskospondylitis. Diskospondylitis can also result in paresis or paralysis, depending on the location and degree of spinal cord compression (see Musculoskeletal Infections, Chapter 85). In younger animals, metaphyseal osteomyelitis causing malaise, fever, anorexia, swollen limbs, and reluctance to move has been observed. Occasionally, erosion of an artery occurs after septic embolization and hemorrhage results. Vasculitis and thrombophlebitis may produce hyperesthesia and lameness, with or without swelling of an extremity. In cats with bacteremia, with or without endocarditis, common clinical signs are anorexia, pyrexia, and shifting leg lameness.44 Heart murmurs can be found in those with endocarditis. In the cat, respiratory failure can occur early in the course of sepsis. In some cats, endocarditis can be more gradual, permitting the infected valves to become dystrophic.139,164 With valvular lesions, although signs of sepsis have varied, cats can develop signs of right- or left-side congestive heart failure (CHF). The leukograms of dogs with bacteremia alone and with bacterial endocarditis are similar.42 A neutrophilic leukocytosis with an appropriate left shift and monocytosis are present in most dogs with gram-positive or anaerobic bacteremia and in virtually all dogs with chronic IE at some point during the course of disease. Leukopenia with inappropriate left shift has been more common with bacteremia alone, usually in association with peracute and acute gram-negative infections. A normocytic, normochromic, nonregenerative anemia and thrombocytopenia are common with subacute or chronic bacteremia in dogs or cats.26,42 With sepsis, hemoconcentration occurs after fluid losses from the intravascular space. Serum total solids tend to decrease because of the loss of protein from the intravascular compartment. Serum chemistry abnormalities are common.42 Hypoalbuminemia (less than 2.5 mg/dL), a twofold or greater elevation of alkaline phosphatase (ALP) activity and/or bilirubin concentration, and hypoglycemia (less than 80 mg/dL) are consistent with bacteremia (also known as the “septic triad”). Hyperglycemia occurs during the early phase (hyperdynamic phase) of septic shock. Analysis of serial serum chemistries can reveal trends as described earlier. Hospitalized, stressed dogs should have a blood glucose concentration above 100 mg/dL. The liver is an important site of removal of bacteria from the blood. Increased serum ALP activity is associated with gram-positive and gram-negative infections, and bacterial toxins are associated with impaired bile metabolism and cholestasis. Hyperbilirubinemia, bilirubinuria, and icterus are the hallmarks of reactive hepatopathy of sepsis (see Chapters 36 and 89). Hypoalbuminemia is a common manifestation of most types of bacteremia.42 Subacute and chronic bacteremia can result in transcapillary leakage as a result of immune-mediated or embolic vasculitis or bacterial toxins. Sepsis also has been associated with reduced hepatic synthesis of albumin, and as many as 50% of bacteremic dogs can have increased Bromsulphalein retention, suggesting either reduced hepatic function or reduced hepatic arterial blood flow. Bile acid levels can also be increased, suggesting hepatic insufficiency. Hypocalcemia is often observed and usually attributed to hypoalbuminemia. The mechanism of hypoglycemia involves the effects of bacteria or bacterial toxins on the intermediary metabolism of glucose. In contrast, hyperglycemia has been correlated with a higher postoperative mortality in dogs than normoglycemia or hypoglycemic septicemic dogs.41 This finding can be related to the fact that hyperglycemia has been seen in patients with early, severe septicemia, and hypoglycemia develops in more chronically affected cases. Hypercoagulability leading to DIC is a common sequela of bacteremia. Evidence of DIC (low fibrinogen, prolonged prothrombin time and activated partial thromboplastin time, increased fibrinogen degradation products and D-dimer levels) is consistent with advanced sepsis wherein major organ failure, including cardiovascular collapse, can be imminent.113,130,130 Blood lactate levels are often increased in septic patients. Hemoconcentration and shock cause decreased oxygen delivery to the tissues, resulting in anaerobic metabolism. Cellular oxidative respiration is inhibited by an endotoxin or a mediator.199 Septic patients tend to be in a hypermetabolic state, requiring increased oxygen delivery to tissues. Bacteremia is only occasionally diagnosed by direct microscopic examination of leukocytes in blood smears. Direct Gram stains of peripheral blood are usually unrewarding because the number of microorganisms present is often much lower than the 105/mL necessary for detection. Wright stains of buffy-coat smears can increase the rate of detection. Acridine orange is more sensitive than Gram stain, because organisms can be detected at 104/mL concentrations. Slides must be handled carefully to prevent inadvertent contamination, suspected when extracellular bacteria are seen. In the most severe cases, cats have had prehepatic jaundice, associated with erythrocyte destruction, and neutrophilia with a left shift.26 Cats with sepsis often exhibit hypoxemia, hypercapnia, and metabolic acidosis. In addition, they often have hypoalbuminemia, low serum ALP activity, and hyperbilirubinemia.26 The definitive diagnosis of bacteremia requires compatible clinical signs and laboratory data and the isolation of the offending microbe from blood cultures. Primary or secondary sites of infection, urine, and joint fluid may also contain the organism. Culture of joint fluid is usually unrewarding because the arthropathy is usually immune-mediated rather than septic. Preferably, the bacteria should be isolated from more than one blood sample. In addition, a source of infection should be sought and attempts made to isolate an organism from that site. Negative blood culture results in bacteremic patients can occur as a result of prior antimicrobial therapy, chronic low-grade infections such as those associated with diskospondylitis and endocarditis, Bartonella infections (requiring specialized handling), intermittent shedding of the organism, causative agents other than bacteria, uremia, and right-sided endocarditis. Clinicians should test all patients with negative blood culture results for Bartonella spp. (see Chapter 52). Polymerase chain reaction (PCR) performed on blood samples can prove useful in the identification of bacteremia. There has been some success in dogs with IE using panbacterial PCR primers targeted to amplify the 16s ribosomal bacterial DNA.101 When an etiologic diagnosis is established with positive blood culture results, more appropriate and effective antibacterial therapy can be used. Clinicians often have the opinion that blood culture attempts are unrewarding. However, a common cause of negative blood culture results is the absence of bacteremia. When blood culture samples are taken from patients with a high likelihood of bacteremia, and when the samples are handled properly, positive results are common. Timing, volume, number of specimens and proper laboratory processing are important for positive results. Prior antibacterial therapy, fastidious microbes, intracellular microbes, and failure to culture for anaerobes are factors leading to false-negative results. Adhering to recommended guidelines for obtaining and processing blood culture specimens can make the diagnostic yield from blood cultures rewarding. Although empiric multiple-antibacterial therapy often has been instituted in critically ill patients before obtaining culture results, the effort and expense required for blood culture have been justifiable because improper treatment may increase mortality from bacteremia. Furthermore, prior administration of antimicrobials appears to slow but not prevent bacterial isolation.68 Bacteremia is usually continuous, although low level. Intermittent bacteremia usually reflects established infections extrinsic to the bloodstream. Samples should be taken from either a freshly placed, meticulously maintained jugular catheter or from several veins.40,68 Duration of antimicrobial therapy is an important factor in the detection of bacteria.104 Therapy for only 2 to 3 days may not interfere; however, longer courses of therapy require the use of antibacterial removal devices or discontinuation of therapy before sampling. Suppression of bacteremia often persists longer than antibacterial blood levels. One of the most critical factors is taking an adequate sample volume, because the concentration of organisms in bacteremic specimens is small. In addition, multiple sample collections have been recommended in an attempt to detect an intermittent bacteremia. For dogs and cats, it has been suggested that at least three blood cultures be obtained over 24 hours.39,41 In the case of critically ill, acutely septic patients, the three blood cultures should be obtained over 30 to 60 minutes before instituting antimicrobial treatment. However, for small animals, this may be too much blood. Taking large volumes (20 mL) per sample, as recommended for human patients, is often difficult in dogs and is impossible in small dogs and cats. The idea that bacteremias are intermittent or are correlated with fever spikes has also not been well substantiated. Therefore, taking at least two specimens of sufficient volume from different vascular sites within a 10-minute interval may be sufficient to determine whether bacteria are present in the blood and whether positive blood culture results are owing to true bacteremia or contamination during collection. At least 5 to 10 mL of blood should be collected for each culture, because the chances of obtaining a positive result are directly related to the volume of blood cultured. The concentration of organisms is relatively low (fewer than 5/mL) in the blood of most patients. It is recommended that as large a volume as practical be taken to maximize the chance of culturing an offending organism. A minimum of 20 mL is taken from large dogs, 10 mL from intermediate-size dogs, and 5 mL from cats and smaller dogs. If possible, a second specimen should be taken from a second venipuncture site within a short interval of the first. A blood-culture broth ratio of 1:10 must be maintained to counteract the bactericidal activity of serum. Anticoagulant and antiphagocytic effects of broth additives are diminished if dilution of blood in media is less than 1:8. Therefore, the proper size of blood culture bottle should be used for the intended blood sample volume. Before venipuncture, thorough skin antisepsis, as for surgery, is the most effective means of avoiding culture contamination (Fig. 86-2, A).109 Small numbers of bacteria may persist inside hair follicles and sweat and sebaceous glands, which may be penetrated by the needle. If the vein must be palpated after skin disinfection, a sterile glove should be worn. To minimize the risk of contamination, blood samples should not be drawn through an indwelling intravenous catheter unless it is a recently and appropriately placed jugular catheter. Arterial blood specimens offer no advantage over venous blood specimens. Finding the same organism at two different surgically prepared sites reduces the likelihood that the organism is a contaminant, especially when sampling intervals are close. The culture bottle diaphragm is disinfected with alcohol or iodine before sample inoculation (Fig. 86-2, B). Blood is inoculated immediately and directly into culture media using a syringe and new needle or a blood transfer set (Fig. 86-2, C). Only commercial culture bottles packed under vacuum and fitted with rubber diaphragms should be used for routine blood cultures to minimize the risk of contamination (Table 86-3). Air must not be allowed to enter into vacuum bottles during blood injection. The blood should be dispersed in the culture medium by gently inverting the bottle two or three times. Blood culture bottles should be inoculated and can be maintained at room temperature to avoid killing temperature-sensitive bacteria; however, incubation at 37° C is often used in the laboratory. TABLE 86-3 Commercially Available Blood Culture Systems Bacterial growth can be suppressed in blood culture vials containing blood from patients receiving antibacterials. Anionic and cationic resins have been incorporated into blood collection vials for antibacterial removal before broth culture. The effectiveness of removal varies.58,194 In the laboratory, culture bottles are examined daily for evidence of microbial growth, which includes turbidity, hemolysis, gas production, or colony formation. In aerobic culturing, the broth should appear turbid within 24 hours of inoculation. In 95% of all instances wherein bacteria are isolated from blood culture media, isolation occurs within 7 days. Longer incubation may be necessary for specimens from patients who previously received antibacterial therapy or those with endocarditis caused by fastidious organisms such as Bartonella (see Chapter 52). In addition to visual inspection, routine (blind) subcultures onto solid culture media usually are performed for antimicrobial susceptibility testing between 7 and 14 hours after blood collection and again after 48 hours of incubation. It can be difficult to determine whether a positive culture result signifies actual bacteremia or simply indicates contamination. Contamination is best distinguished from bacteremia if multiple blood specimens are cultured. Knowledge of the normal canine and feline bacterial skin flora is helpful in interpreting blood culture results. Coagulase-negative staphylococci, β-hemolytic streptococci, Micrococcus species, and Acinetobacter species are normal skin commensals on the dog. S. pseudintermedius is normally present on canine hair, whereas Micrococcus organisms, β-hemolytic streptococci, and Acinetobacter species are normally present on feline skin. Recovery of diphtheroids, Bacillus species, and coagulase-negative staphylococci usually signifies contamination unless they are isolated from multiple specimens. Nonhemolytic streptococci and β-hemolytic streptococci from single cultures are also of uncertain significance. In all cases the significance of positive blood culture results should be interpreted in light of the patient’s clinical status and the potential sources for bacteremia. The insect cell culture medium Bartonella Alpha Proteobacteria Growth Medium, or BAPGM (Galaxy Diagnostics, Durham, NC; www.galaxydx.com), combined with PCR testing now allows better growth and detection of Bartonella in animals as compared to other diagnostic tests currently available (see Chapter 52).34 Because of the high prevalence of Bartonella organisms in the blood of clinically healthy cats, the only way to confirm that the endocarditis is cause by Bartonella is by using immunologic stains on, or performing a postmortem culture of, valvular tissues.22 On the basis of predisposing infections or other factors, time course of infection, and known patterns of associated bacteria and their antimicrobial susceptibilities, the antibacterials most likely to be effective can be predicted (Table 86-4). This knowledge is important when blood culture results are negative and in critically ill patients before the return of blood culture results. Subsequently, appropriate adjustments in therapy may be necessary. In dogs and cats, staphylococci and streptococci are the most commonly encountered gram-positive pathogens, whereas the Enterobacteriaceae, such as E. coli and Klebsiella, and Proteus and Pseudomonas organisms are the most common gram-negative offenders. TABLE 86-4 Choice of Antimicrobial Therapy for Bacteremia or Endocarditis aamg, Aminoglycoside; amp, ampicillin, amoxicillin; amp-clav, ampicillin-clavulanate, amoxicillin-clavulanate; azith, azithromycin; brp, β-lactamase–resistant penicillin; carb, carbenicillin; 1cep, first-generation cephalosporins; 2cep, second-generation cephalosporins; 3cep, third-generation cephalosporins; chlor, chloramphenicol; clin, clindamycin; doxy, doxycycline; met, metronidazole; pen, penicillin; quin, quinolone; rif, rifampin; ticar, ticarcillin; ticar-clav, ticarcillin-clavulanate; tms, trimethoprim-sulfonamide; vanc, vancomycin. bMay combine aminoglycoside with ticarcillin or carbenicillin for maximum efficacy. cOnly certain cephalosporins in this group, such as ceftazidime, are effective against Pseudomonas species. Patients with bacteremia must be closely monitored (see also Sepsis, Chapter 36). Although clinical and hematologic evidence of improvement often occurs initially, relapse is common. Acquired antimicrobial resistance can develop rapidly. Clinical signs of resurging bacteremia include fever, which can be transient, deterioration of mucous membrane color, increasing capillary refill time, increasing rectal to toe-web temperature differential, decreasing blood pressure, and tachycardia. Detection of these signs of early deterioration indicates a need for intensification of therapy, including adjustments of antibacterial administration. It also should prompt a search for a persistent focus of infection (e.g., abscess, catheter) that may be treatable. For additional information on specific drugs discussed next, see Chapter 30 and the Drug Formulary in the Appendix. The penicillin family of antibacterials includes the narrow-spectrum penicillin G; intermediate-spectrum ampicillin; and the extended-spectrum carbenicillin, ticarcillin, ticarcillin-clavulanate, and piperacillin (Table 86-5). A common misconception is that ampicillin is a broad-spectrum antibacterial. Most coagulase-positive staphylococcal isolates are resistant to penicillin and ampicillin, and these are poor empiric choices for serious and life-threatening infections. Penicillin G should be restricted to streptococcal and some gram-positive anaerobic infections. TABLE 86-5 Antimicrobial Dosages for Bacteremia with or without Endocarditis in Dogs and Cats
Cardiovascular Infections
Bacteremia
Etiology
Epidemiology
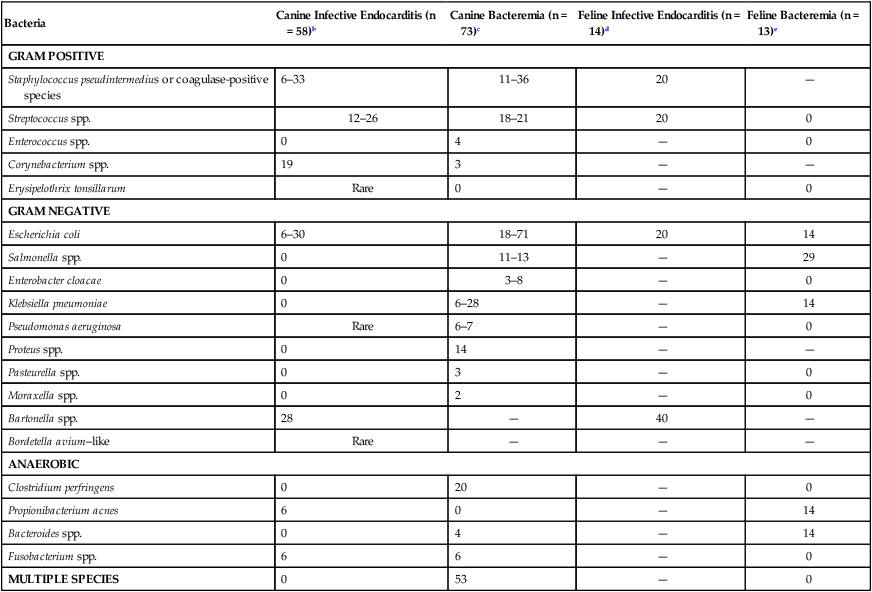
Bacteria
Canine Infective Endocarditis (n = 58)b
Canine Bacteremia (n = 73)c
Feline Infective Endocarditis (n = 14)d
Feline Bacteremia (n = 13)e
GRAM POSITIVE
Staphylococcus pseudintermedius or coagulase-positive species
6–33
11–36
20
—
Streptococcus spp.
12–26
18–21
20
0
Enterococcus spp.
0
4
—
0
Corynebacterium spp.
19
3
—
—
Erysipelothrix tonsillarum
Rare
0
—
0
GRAM NEGATIVE
Escherichia coli
6–30
18–71
20
14
Salmonella spp.
0
11–13
—
29
Enterobacter cloacae
0
3–8
—
0
Klebsiella pneumoniae
0
6–28
—
14
Pseudomonas aeruginosa
Rare
6–7
—
0
Proteus spp.
0
14
—
—
Pasteurella spp.
0
3
—
0
Moraxella spp.
0
2
—
0
Bartonella spp.
28
—
40
—
Bordetella avium–like
Rare
—
—
—
ANAEROBIC
Clostridium perfringens
0
20
—
0
Propionibacterium acnes
6
0
—
14
Bacteroides spp.
0
4
—
14
Fusobacterium spp.
6
6
—
0
MULTIPLE SPECIES
0
53
—
0
Staphylococci
Streptococci
Gram-Positive Aerobic Bacilli
Gram-Negative Bacilli
Anaerobic Bacteria
Bartonella
Pathogenesis
Sources of Infection and Risk Factors
Time Course
Clinical Signs
Bacteremia Alone (n = 77)
Infective Endocarditis (n = 45)
Fever (>39.4° C [102.9° F])
75
70
Lameness: total
19
34
Lameness: shifting
6
18
Vomiting
17
35
Heart murmur
6
74
Ventricular arrhythmia
5
27
Clinical Findings
Dogs
Cats
Diagnosis
Clinical Laboratory Findings
Dogs
Cats
Blood Culture
Indications
Technique
Indicated Use
Collection Media
Manufacturer
Aerobic and anaerobic
BBL SEPTI-CHEK with trypticase soy broth
Becton Dickinson Microbiology Systems, Franklin Lakes, NJ
Pediatric patients
BBL SEPTI-CHEK with brain heart infusion
Becton Dickinson Microbiology Systems, Franklin Lakes, NJ
Antimicrobial therapy
BBL SEPTI-CHEK with resins culture bottle
Becton Dickinson Microbiology Systems, Franklin Lakes, NJ
Centrifugation lysis
ISOLATOR SYSTEM
Wampole Laboratories, Princeton, NJ
Liquid Media
Interpretation of Results
Therapy
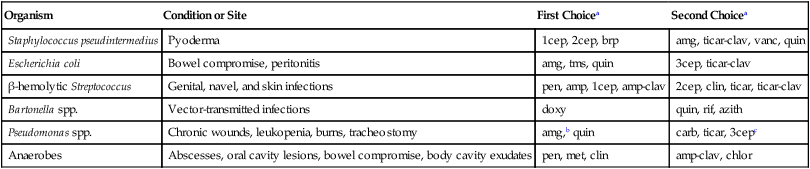
Organism
Condition or Site
First Choicea
Second Choicea
Staphylococcus pseudintermedius
Pyoderma
1cep, 2cep, brp
amg, ticar-clav, vanc, quin
Escherichia coli
Bowel compromise, peritonitis
amg, tms, quin
3cep, ticar-clav
β-hemolytic Streptococcus
Genital, navel, and skin infections
pen, amp, 1cep, amp-clav
2cep, clin, ticar, ticar-clav
Bartonella spp.
Vector-transmitted infections
doxy
quin, rif, azith
Pseudomonas spp.
Chronic wounds, leukopenia, burns, tracheostomy
amg,b quin
carb, ticar, 3cepc
Anaerobes
Abscesses, oral cavity lesions, bowel compromise, body cavity exudates
pen, met, clin
amp-clav, chlor
Antibacterials
Druga
Species
Doseb
Route
Interval (hours)
Duration (days)c
Penicillin
B
20–40 × 103 U/kg
IV
4–6
7–14
Imipenem
B
10 mg/kg
IV
8
7–14
Carbenicillin
B
40–50 mg/kg
IV
6–8
7–14
Piperacillin
B
30 mg/kg
IV
6
7–14
Ampicillin
B
20–40 mg/kg
IV, SC
6–8
7–14
Amoxicillin-clavulanate
B
20 mg/kg
PO
12
Follow-up
Ticarcillin
D
50 mg/kg
IV, SC
8
7–14
Ticarcillin-clavulanate
D
50 mg/kg
IV
8
7–14
C
40 mg/kg
IV
6
7–14
Cefazolin (first generation)
B
20–30 mg/kg
IV, SC
8
7–14
Cefapirin (first generation)
B
15–30 mg/kg
IV, SC
8
7–14
Cefoxitin (second generation)
B
30 mg/kg
IV
6–8
7–14
Cefuroxime (second generation)
D
15–30 mg/kg
PO, IV
8
7–14
Cefotaxime (third generation)
B
20–80 mg/kg
IV
8
7–14
Ceftiofur sodium (third generation)
B
2.2–4.4 mg/kg
SC
12
Follow-up
Gentamicind
B
4–6 mg/kg
IV
24
7–14
Amikacind
B
7–10 mg/kg
IV
24
7–14
Trimethoprim–sulfonamidee
D
15 mg/kg
IV
8–12
7–14
B
30 mg/kg
SC, PO
12–24
Follow-up
Metronidazole
B
8–15 mg/kg
IV, PO
8
5–7
Clindamycin
B
10 mg/kg
IV
8–12
7–14
B
10–11 mg/kg
PO
12
Follow-up
Chloramphenicol
D
15–25 mg/kg
IV
6–8
7–14
C
10–15 mg/kg
IV
6–8
4–7
Ciprofloxacinf
B
10–15 mg/kg
PO
12
14
Enrofloxacinf
D
5–7 mg/kg
IV
24
4–7
D
5–15 mg/kg
PO
24
Follow-up
C
5 mg/kg
PO
24
Follow-up
Azithromycin
B
5–10 mg/kg
PO
12
Follow-up ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree