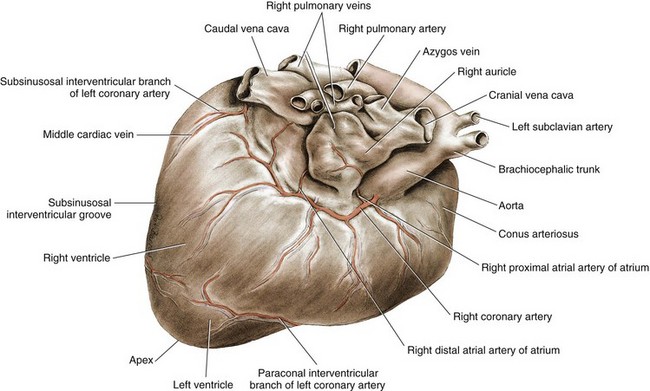
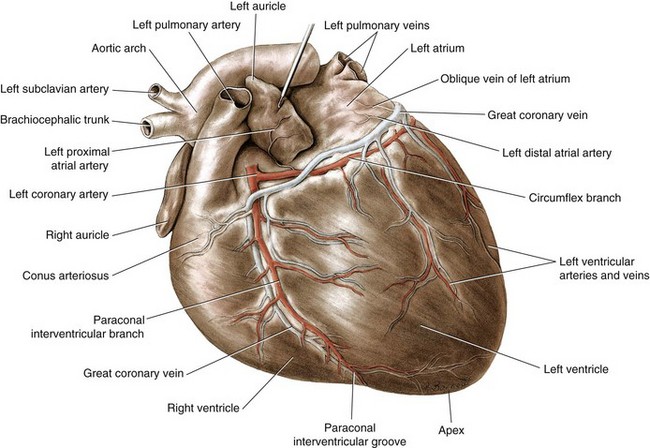
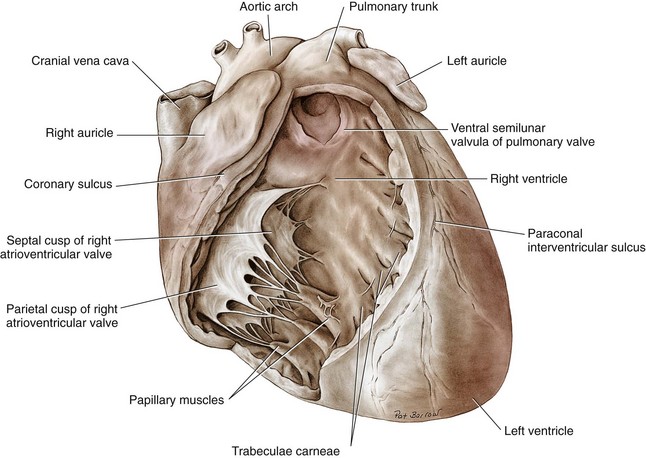
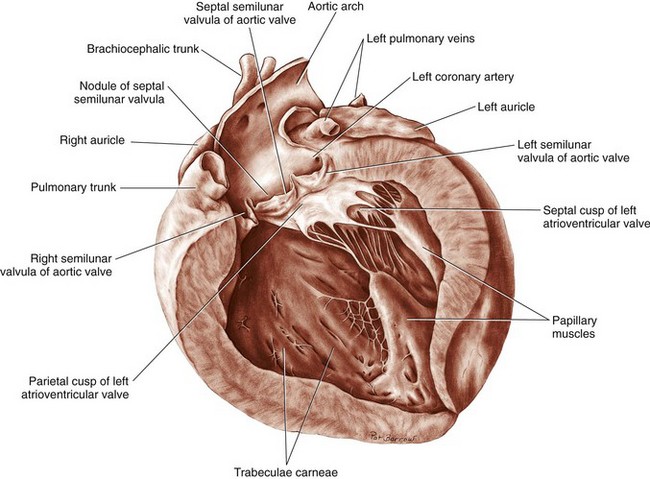
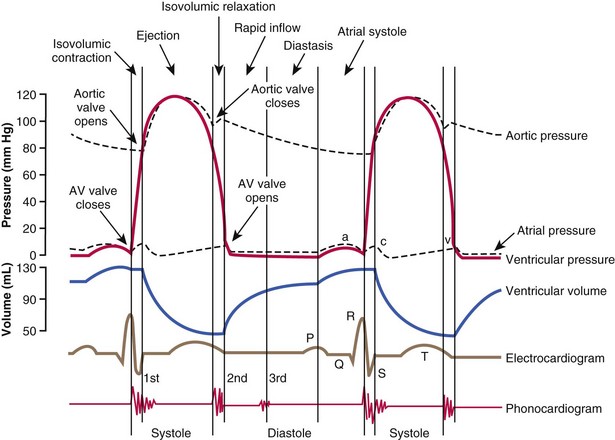
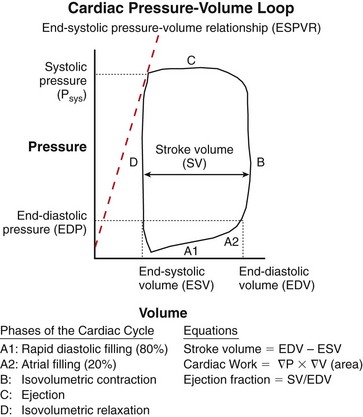
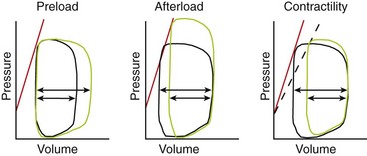
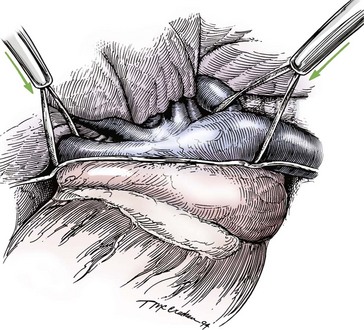
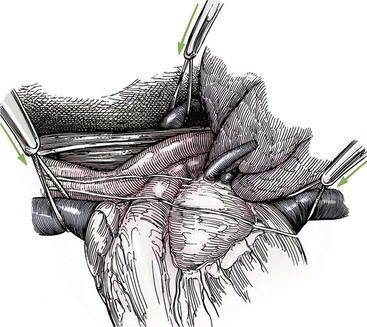
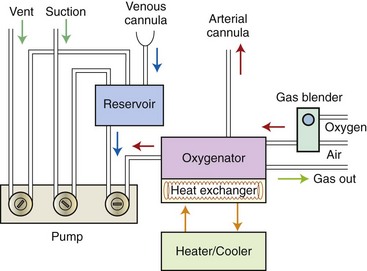
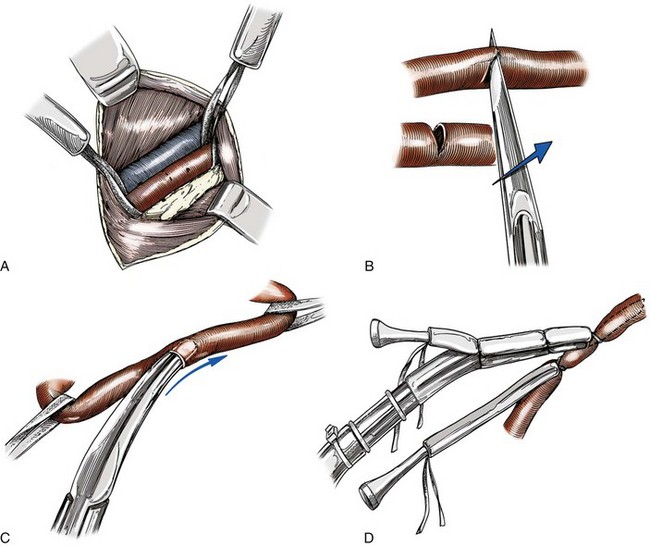
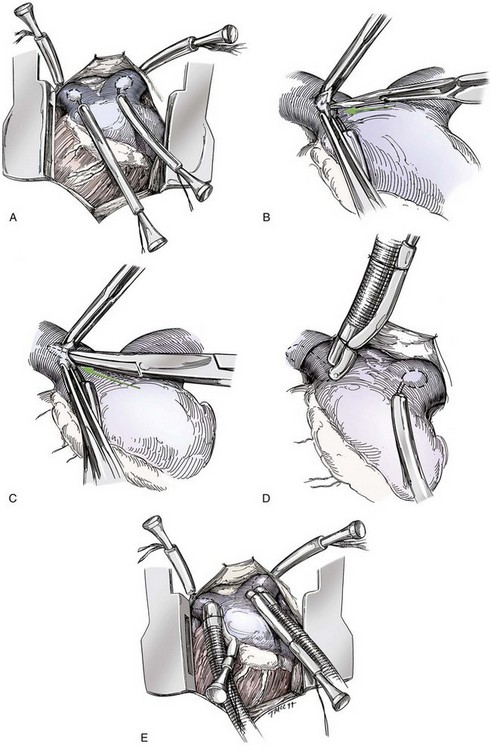
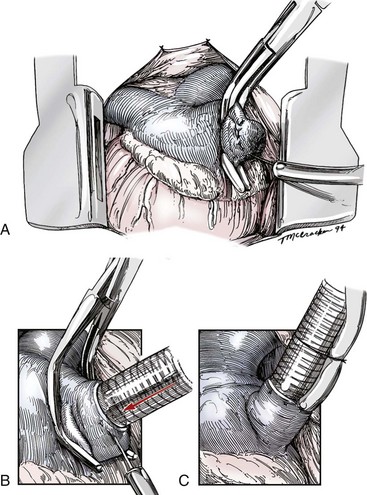
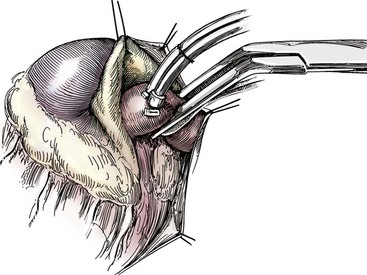
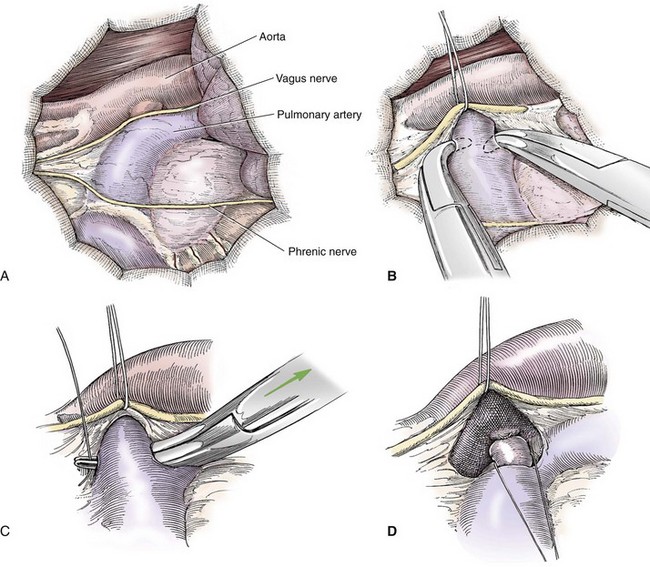
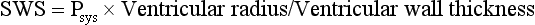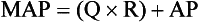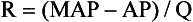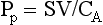Chapter 106 The mammalian heart is a muscular organ consisting of two atria, two ventricles, and several great vessels. The right atrium and left atrium are divided by an atrial septum and receive blood from the systemic and pulmonary venous circulations, respectively. Each atrium has a cranial projecting blind sac known as an auricle (Figures 106-1 and 106-2). Blood is carried from the systemic circulation to the right atrium by the cranial and caudal vena cavae. In dogs and cats, the azygous vein usually drains into the cranial vena cava close to its junction with the right atrium. These major veins and the right atrium are readily accessible from the right lateral aspect of the heart (see Figure 106-1). Blood is carried to the left atrium by multiple pulmonary veins located on the dorsal aspect of the heart. Blood flows from the right and left atrium into their respective ventricles through the right atrioventricular (tricuspid) and left atrioventricular (mitral) valves (Figures 106-3 and 106-4). The right ventricle pumps blood to the pulmonary arterial circulation via the main pulmonary artery or trunk. The pulmonic (pulmonary) valve is situated between the right ventricle and main pulmonary artery. The left ventricle pumps blood to the systemic arterial circulation via the aorta. The aortic valve is located between the left ventricle and aorta. The ventricles are separated by the interventricular septum, which consists of an inconspicuous membranous portion and a dominant muscular portion. The left ventricle comprises the left caudodorsal aspect of the cardiac apex. The left ventricular chamber has the geometric shape of a prolate ellipsoid. Inflow and outflow portions of the left ventricle are separated by the septal leaflet of the mitral valve and run approximately parallel to each other. The right ventricle comprises the right cranioventral aspect of the cardiac apex. It has distinct inflow and outflow portions that are oriented approximately 45 degrees to each other. The outflow portion of the right ventricle wraps around the cranial aspect of the heart, which positions the pulmonic valve and main pulmonary artery to the left of the aortic valve and ascending aorta. The pulmonic valve and main pulmonary artery are accessible from the left lateral aspect of the heart (see Figure 106-2). The tricuspid and mitral valves in dogs and cats are composed of two dominant leaflets: a septal leaflet and a mural (or parietal) leaflet. These leaflets are supported by chordae tendineae attached to the papillary muscles. The left ventricle has two prominent papillary muscles, and each receives chordae tendineae from each leaflet of the mitral valve (see Figure 106-4). The anatomy of papillary muscles supporting the tricuspid valve is variable (see Figure 106-3). The pulmonic and aortic valves (semilunar valves) have a trileaflet structure consisting of three unsuspended valve cusps and a valve sinus behind each cusp. The aortic valve has a right coronary, left coronary, and noncoronary valve cusp and sinus based on the origin of the right and left coronary arteries. The heart is supplied by the right and left coronary arteries that arise from the right and left coronary aortic ostia, respectively. The left coronary artery is dominant and divides into a circumflex branch and a paraconal interventricular branch (see Figure 106-2). The circumflex branch courses in the atrioventricular groove that separates the atria and ventricles. The paraconal branch descends along the paraconal interventricular groove that serves as an external landmark of the cranial division between the left and right ventricle. The great coronary vein originates in the paraconal interventricular groove. There it runs parallel to the paraconal branch of the left coronary artery before turning and coursing with the circumflex branch in the atrioventricular groove. The great coronary vein drains into the coronary sinus that, in turn, empties into the right atrium just ventral to the caudal vena cava. Cardiac Cycle and Pressure-Volume Relationship The cardiac cycle encompasses the electrical, pressure, volume, flow, and valve motion events that occur during one complete cardiac systole and diastole. The temporal relationships of these events are demonstrated by plotting each independently versus time (Figure 106-5). During each cardiac cycle, the heart accomplishes two fundamental kinds of external work. It generates pressure (i.e., potential energy), and it ejects volume (i.e., kinetic energy). The relationship of these two events is illustrated by plotting instantaneous ventricular pressure and volume against each other to generate a ventricular pressure-volume plot (Figure 106-6). Pressure-volume plots form the basis of current understanding of cardiac physiology. Each loop of a pressure-volume plot represents one complete cardiac cycle and consists of two filling phases, an isovolumetric contraction phase, an ejection phase, and an isovolumetric relaxation phase. Under normal physiologic conditions, cardiac filling is divided into rapid diastolic filling (passive) and atrial filling (atrial systole). The principle pressure endpoints are end-diastolic pressure (EDP) and peak systolic pressure (Psys). The principle volume endpoints are end-diastolic volume (EDV) and end-systolic volume (ESV). The difference between EDV and ESV is the stroke volume (SV). The area inside a pressure-volume loop represents the external work done by the heart in one cardiac cycle. The ejection fraction is the SV divided by EDV. SV is a major determinant of cardiac output (i.e., cardiac flow). SV, in turn, is determined by three important independent variables: preload, afterload, and contractility. Preload encompasses the Frank-Starling principle of the heart. On a cellular basis, preload is determined by the amount of diastolic strain on each cardiomyocyte. Within limits, the greater the diastolic strain, the more forceful the cardiac contraction. On a whole heart basis, preload is reflected by the EDV and EDP (Figure 106-7). On a beat-to-beat basis, the greater the EDV and EDP, the greater the preload and SV. Factors that determine the beat-to-beat EDV and EDP, and therefore preload, are the mean filling pressure of the circulation and vascular resistance. Mean filling pressure of the circulation is determined by the blood volume and venous vascular tone and has a direct relationship with preload. Vascular resistance has an inverse relationship with preload. Thus, the determinants of preload reside within the circulation and not in the heart. Cardiac remodeling can also change the EDV. Chronic changes in EDV attributable to remodeling do not represent changes in cardiac preload. Thus, on a beat-to-beat basis, afterload is determined by Psys. On a chronic basis, cardiac remodeling (e.g., ventricular dilatation) also affects afterload. Thus, afterload is affected by events outside (i.e., Psys) and inside (i.e., cardiac remodeling) the heart. Afterload has an inverse relationship with SV. The greater the cardiac afterload (e.g., Psys), the less the SV (see Figure 106-7). Contractility, also known as inotropic state, represents the intrinsic contractile state of the heart independent of preload and afterload. On a beat-to-beat basis, contractility is largely a function of the amount of sympathetic (β) influence on the heart. Contractility is also influenced by the diseases of the myocardium and cardiac drugs. Changes in cardiac mass (e.g., cardiac hypertrophy) also have a direct effect on the global contractility of the heart. Contractility has a direct relationship with SV. The greater the contractility or inotropic state, the greater the SV. On a pressure-volume loop, contractility is reflected by changes in the slope of the end-systolic pressure-volume relationship (ESPVR) (see Figure 106-7). Vascular resistance cannot be measured directly but can be calculated by knowing Q, MAP, and AP: Anesthesia is an important component of cardiac surgery. The following are intended to be general guidelines59 rather than in-depth protocols, which are beyond the scope of this chapter. Consultation with a veterinary anesthesiologist is warranted for advanced cardiac surgeries. Before induction, an intravenous catheter is placed for administration of fluids and drugs. Jugular and arterial catheters are placed as needed after anesthetic induction. In general, most patients are premedicated with an opioid (e.g., hydromorphone, oxymorphone, methadone) and a benzodiazepine (e.g., midazolam, diazepam). Acepromazine should generally be avoided because it has the potential to result in prolonged hypotension. Similarly, alpha-2 agonists cause significant cardiovascular depression and should be avoided. An anticholinergic drug (e.g., glycopyrrolate, atropine) is administered to animals with bradycardia. Patients are preoxygenated, and anesthesia is induced with drugs such as fentanyl or etomidate, which demonstrate a wide cardiovascular safety margin. Anesthesia is maintained with balanced protocols consisting of combinations of an inhalant, such as isoflurane or sevoflurane in oxygen; an opioid, such as fentanyl; a benzodiazepine (e.g., midazolam); and a neuromuscular blocking agent, such as atracurium besylate. Monitoring includes electrocardiography, direct arterial blood pressure, end-tidal CO2, pulse oximetry, body temperature, central venous pressure, arterial blood gases, electrolytes, packed cell volume, total protein, and lactate, depending on the degree of cardiac dysfunction present. Positive-pressure ventilation is maintained during surgery with or without positive end-expiratory pressure (PEEP). Inflow occlusion is a strategy for performing brief open cardiac repairs. It involves interruption of venous blood returning to the heart and a brief period of complete circulatory arrest. The principal advantages are its simplicity; lack of need for specialized equipment; and associated minimal cardiopulmonary, metabolic, and hematologic derangement after surgery.49 The main disadvantages of inflow occlusion are limited time provided to perform the cardiac procedure, motion of the surgical field, and unavailability of a fallback or rescue strategy if something delays completion of surgery. As a result, cardiac surgery performed during inflow occlusion must be meticulously planned and executed. Inflow occlusion can be accomplished from a left or right thoracotomy or median sternotomy, depending on the procedure to be performed. Tourniquets are placed on the venae cavae and azygous vein to accomplish inflow occlusion. Care should be taken to exclude the right phrenic nerve during placement of tourniquets on the venae cavae to avoid injury during occlusion. Direct access to the venae cavae and azygous vein for inflow occlusion is obtained readily from a right thoracotomy (Figure 106-8) or median sternotomy. Access for inflow occlusion is more difficult from a left thoracotomy, particularly when the heart is enlarged. With the left approach, the venae cavae and azygous vein are accessed by dissecting through the mediastinum (Figure 106-9). Cardiopulmonary bypass is accomplished with a heart–lung machine. Major components of a heart–lung machine include three to five pumps, a temperature-controlled circulating heater/cooler water bath, an oxygen blender, a gas flowmeter, and an anesthetic vaporizer. The primary bypass circuit consists of a reservoir, pump, membrane oxygenator, heat exchanger, and circulating heater/cooler water bath (Figure 106-10). A membrane-type oxygenator should be used to minimize injury to the blood. A pediatric-size oxygenator is preferred in dogs that weigh less than 40 kg to minimize volume necessary to prime the circuit. During cardiopulmonary bypass, blood is drawn away from the patient to the reservoir by gravity flow. Blood is then pumped through the oxygenator under pressure and returned to the patient by means of a roller or centrifugal pump. The heater/cooler water bath is used to control body temperature by means of a heat exchanger built into the primary circuit. Shed blood in the operative field is salvaged and returned to the reservoir by one or two suction lines driven by additional roller pumps. Cannulation for cardiopulmonary bypass can be accomplished from a right or left thoracotomy or median sternotomy, depending on the cardiac procedure being performed. Oxygenated blood is returned to the patient through the arterial cannula and line. Arterial cannulation is performed before cardiac manipulations and other cannulation. If bleeding occurs before initiation of cardiopulmonary bypass, blood can be salvaged from the operative field and returned to the patient by the arterial cannula and line. Arterial cannulation of a femoral or carotid artery is preferred over direct aortic cannulation in dogs (Figure 106-11) and should be performed before the thoracic cavity is opened. If a thoracotomy approach is used, the femoral artery on the opposite side (down) limb is chosen for cannulation. A straight arterial cannula, ranging in size from 8 to 14 Fr, is used. Blood is diverted from the right heart to the cardiopulmonary bypass circuit by means of one or two venous cannula(s) and a venous line. Venous cannulation can be accomplished by several configurations, depending on the cardiac surgery undertaken. Bicaval venous cannulation is required for surgeries performed through a right atrial cardiac approach (e.g., septal defect repairs and tricuspid valve surgery) (Figure 106-12). Alternatively, venous cannulation can be accomplished by introducing a single, two-stage, cavoatrial cannula through the right auricle (Figure 106-13). Cavoatrial cannulation is the only option for venous cannulation for cardiopulmonary bypass from a left thoracotomy. Hemodilution is desirable during cardiopulmonary bypass to counter effects of increased blood viscosity during hypothermia.43 The goal is to decrease the hematocrit to approximately 25% to 28%. Hemodilution is accomplished by priming the bypass circuit with a calculated volume of crystalloid solution. The hematocrit decreases when the patient’s blood mixes with the circuit prime. A balanced, pH-adjusted (7.4) crystalloid solution should be used to prime the circuit (Box 106-1). Whole blood can be added to the prime solution if the volume of crystalloid prime is insufficient to adequately prime the circuit without causing excessive hemodilution. Other additives to crystalloid prime include NaHCO3 and heparin. Before cannulation and initiation of cardiopulmonary bypass, the animal must undergo complete anticoagulation by administration of heparin (see Box 106-1). The activated clotting time is monitored every 30 minutes during bypass and kept above 480 seconds. Perfusion flow rates during bypass depend on several factors, including patient size, temperature, and hematocrit (see Box 106-1). Standard cardiopulmonary bypass perfusion strategies with mild to moderate hypothermia can be used for dogs weighing more than 10 kg.53 For small dogs (<10 kg), deep hypothermic (15° to 18° C) low-flow (20 mL/kg/min) cardiopulmonary bypass may be a more appropriate strategy to decrease the adverse effects associated with bypass.57 Ultimately, perfusion flow should be kept at the lowest level that meets the needs of the patient, with a goal of maintaining a venous oxygen saturation of 70% or greater and normal lactate concentrations. MAP, which should be 50 to 70 mm Hg during bypass, usually decreases dramatically for several minutes after bypass initiation because of sudden hemodilution. Phenylephrine is administered as necessary to increase vascular resistance and maintain adequate MAP. Esophageal and rectal temperatures are monitored continuously during cardiopulmonary bypass. Dogs are cooled to between 25° and 28° C during the cardiac surgery using the heater/cooler water bath and heat exchanger in the pump circuit. Requirement for inhalation and other anesthetic drugs decreases during hypothermia. During cardiopulmonary bypass, the oxygenator receives continuous flow of a mixture of oxygen and nitrogen from an oxygen blender. The fraction of inspired oxygen (FIO2) should be adjusted to keep PaO2 above 120 mm Hg during cardiopulmonary bypass. PaCO2 is adjusted during cardiopulmonary bypass by varying total gas flow (L/min) to the oxygenator by means of the flowmeter. Blood gases should be measured every 30 minutes during cardiopulmonary bypass. An α-stat strategy (temperature uncorrected blood gas measurements) for acid–base management, which permits relative alkalosis as the patient cools, is most appropriate for patients on cardiopulmonary bypass.43 Metabolic acidosis during cardiopulmonary bypass should be corrected by administration of NaHCO3. Complete cardiopulmonary bypass is accomplished by cross-clamping the ascending aorta (Figure 106-14). Protection of myocardium from ischemic injury during aortic cross-clamping is accomplished by immediate cessation of electromechanical activity and rapid myocardial cooling. This is achieved by administration of cold cardioplegia solution into the coronary circulation just after the aortic cross-clamp has been placed. The solution contains a high concentration of K+ that arrests the electrical and mechanical activities of the myocardium, thereby greatly reducing its metabolic requirement. Cooling myocardium to approximately 4° to 8° C further decreases its metabolic requirement. Sanguineous crystalloid cardioplegia solutions are superior to pure crystalloid cardioplegia solutions.5 A sanguineous crystalloid cardioplegia solution is made by blending a crystalloid cardioplegia solution (see Box 106-1) with heparinized blood from the bypass circuit. Cardioplegia solution is delivered by a cannula placed in the ascending aorta before aortic cross-clamping. All electrical activity on the electrocardiogram should cease after cardioplegia administration. Cardioplegia is repeated every 20 minutes while the aorta is cross-clamped. Ideally, aortic cross-clamp time should not exceed 90 minutes. After closure of cardiac incisions, the aortic cross-clamp is removed, and coronary circulation is reestablished. If ventricular fibrillation occurs, the heart should be electrically defibrillated with direct current and internal paddles. If initial attempts at defibrillation are unsuccessful, then defibrillation should be repeated every few minutes as the patient is rewarmed until a normal rhythm is restored. Rewarming should begin as the cardiac surgery is being completed, usually while cardiac incisions are being closed. After the patient is rewarmed to 37° C and an effective cardiac rhythm established, a gradual process of weaning from cardiopulmonary bypass is begun. During this period, calcium chloride and inotropic drugs are usually required to support cardiovascular function (see Box 106-1). After the animal has been fully weaned from cardiopulmonary bypass and is hemodynamically stable, cannulae are removed in reverse of the order in which they were introduced. Protamine sulfate (see Box 106-1) is administered to reverse the heparin anticoagulation. Protamine has a very potent hypotensive effect in dogs and must be administered slowly. The activated clotting time should return to less than 150 seconds. Administration of fresh whole blood is indicated after cardiopulmonary bypass. Cardiopulmonary bypass initiates a systemic inflammatory response that has a profound effect on management after surgery.43 The first 12 hours after cardiopulmonary bypass are most critical. Major problems that can occur during this period include hemorrhage, hypoxemia, circulatory collapse, cardiac arrhythmias, low urine output, and electrolyte and acid–base abnormalities. Hemorrhage is a major postoperative concern after cardiopulmonary bypass and can be surgical, biologic, or both. Surgical hemorrhage results from bleeding from cardiotomy sites and is best prevented by careful inspection of those sites before thoracotomy closure. Even with perfect closure of cardiotomy sites, significant bleeding can occur after cardiopulmonary bypass because of biologic effects of cardiopulmonary bypass. Dilutional and consumptive thrombocytopenia, acquired platelet dysfunction, consumptive and dilutional coagulopathy, and fibrinolysis all contribute to biologic bleeding after surgery. In most cases, hemorrhage after cardiopulmonary bypass is biologic and is best managed conservatively with supportive care. Administration of fresh whole blood is indicated to restore red blood cells, platelets, and clotting factors and should begin in the operating room. Additional stored blood or plasma may be necessary in the postoperative period. Shed blood can be collected, washed with a cell washer, and returned to the patient as necessary. Some degree of hypoxemia is invariably present after cardiopulmonary bypass because of pulmonary injury and increased pulmonary vascular permeability.43 This well-recognized condition is referred to as pump lung. Ventilatory support with PEEP (5 to 8 cm of water) for 4 to 12 hours after surgery is generally necessary. Circulatory support after cardiopulmonary bypass should include volume expansion to keep central venous pressure between 4 and 10 mm Hg, which should maintain adequate cardiac output without causing congestion. Because of the generalized increase in vascular permeability after cardiopulmonary bypass, volume deficits should be restored primarily with plasma or blood; hematocrit should be kept above 30%. Administration of high volumes of crystalloid fluids should be avoided after cardiopulmonary bypass. Inotropic support may be necessary after surgery to maintain adequate cardiac output and mean systemic blood pressure. Ventricular tachycardia often occurs after cardiopulmonary bypass and should be controlled with lidocaine. Urine output should be monitored for 12 hours after surgery to ensure adequate renal function. Hypokalemia and hypocalcemia are frequently encountered after surgery and should be corrected. Patent ductus arteriosus is the most common congenital heart defect seen in dogs, accounting for 25% to 30% of congenital malformations.18,30 The defect also occurs in cats but with a much lower prevalence.50 Patent ductus arteriosus is seen more commonly in purebred dogs, with a predilection for females. Poodles, Keeshonds, Maltese, Bichon Frises, Yorkshire terriers, Cocker spaniels, Pekingese, collies, Shelties, Pomeranians, Welsh corgis, and other breeds have an established predisposition for patent ductus arteriosus.18 A heritable basis for patent ductus arteriosus has been established in poodles and Welsh corgis.68,71 The heritable defect is hypoplasia and segmental asymmetry of the ductus muscle mass that results in failure of ductus contraction.17,19 Patent ductus arteriosus allows left-to-right shunting of blood from the aorta to the pulmonary artery. The result is severe volume overload of the left heart, leading to left ventricular and atrial dilatation, progressive myocardial deterioration, and left-sided congestive heart failure. Progressive functional mitral regurgitation further overloads the left ventricle. Atrial fibrillation may occur as a late sequela. A majority of dogs and cats with untreated patent ductus arteriosus die from progressive heart failure before 1 year of age.36 Based on poor long-term prognosis without correction, patent ductus arteriosus closure is indicated in all dogs and cats with left-to-right patent ductus arteriosus, with few exceptions. Options for patent ductus arteriosus closure include percutaneous placement of embolization coils or Amplatzer ductal occlude devices or surgical ligation.1,40 Surgical ligation can be undertaken in dogs and cats any time after 8 weeks of age and, preferably, before 16 weeks of age. Older animals should undergo surgery as soon as possible after the diagnosis. Even animals with severe secondary myocardial failure and functional mitral regurgitation will benefit from surgery. Animals that present in congestive heart failure should be treated with diuretics and then undergo surgical ligation as soon as they are stable, usually within 24 to 48 hours. Animals with pulmonary hypertension can undergo patent ductus arteriosus ligation as long as pulmonary artery pressures have not reached systemic levels. Surgical ligation of a right-to-left or bidirectional patent ductus arteriosus is contraindicated. Standard surgical correction of left-to-right patent ductus arteriosus is accomplished by ligation of the ductus arteriosus (Figure 106-15). Patent ductus arteriosus ligation is accomplished through a left fourth thoracotomy in dogs and a left fourth or fifth thoracotomy in cats. The left vagus nerve courses over the ductus arteriosus and serves as an anatomic landmark for identification of the ductus arteriosus. The vagus nerve is isolated at the level of the ductus and gently retracted with one or two sutures. Occasionally, a persistent left cranial vena cava may overlie the ductus arteriosus. In this case, the vein should be isolated and retracted with the vagus nerve. A persistent left cranial vena cava should not be ligated or divided. The ductus arteriosus is isolated by blunt dissection without opening the pericardium. Heavy (1) silk ligatures are passed around the ductus using right-angled forceps. The ductus arteriosus is closed by slowly tightening and tying each silk ligature. Closure of patent ductus arteriosus with hemostatic clips has been reported,26 but this method is not preferred because of the risk for significant residual ductal flow and recanalization of the patent ductus arteriosus.
Cardiac Surgery
Anatomy
Physiology
Stroke Volume (Preload, Afterload, Contractility)
Cardiac Output, Blood Pressure, and Vascular Resistance
Anesthesia
Strategies for Cardiac Surgery
Inflow Occlusion
Cardiopulmonary Bypass
Surgical Conditions of the Heart
Pathophysiology
Indications for Surgery
Patent Ductus Arteriosus Ligation
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cardiac Surgery
Only gold members can continue reading. Log In or Register to continue