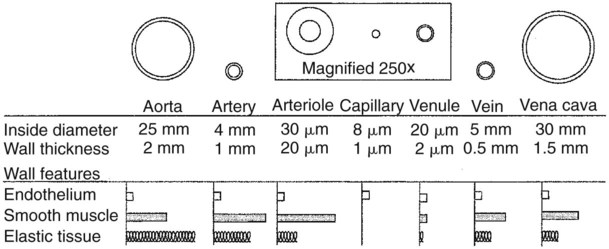
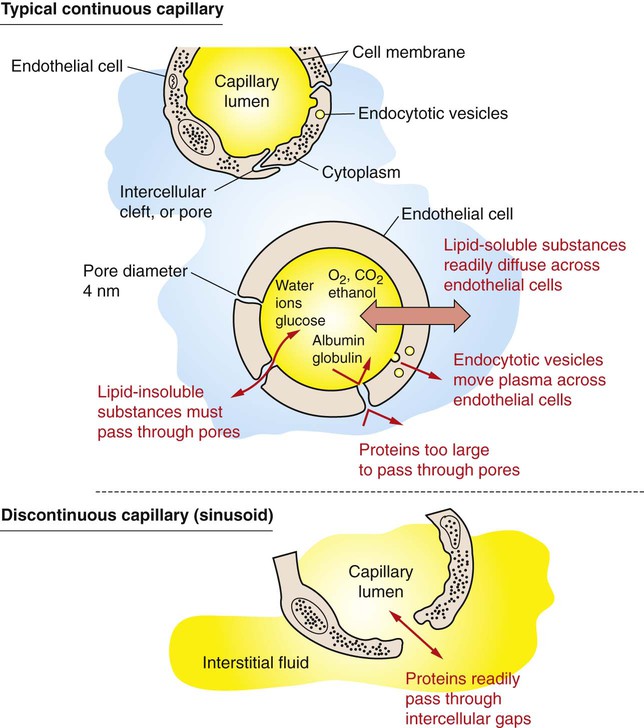
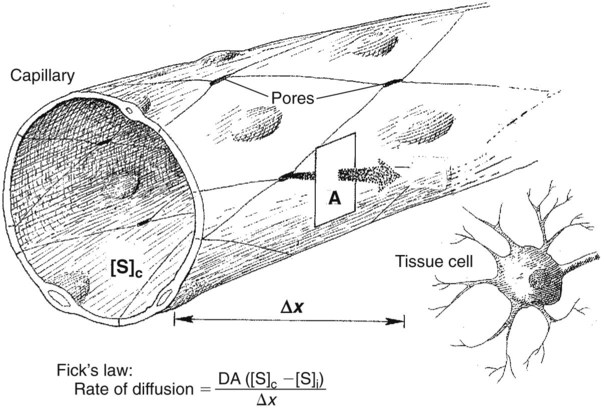
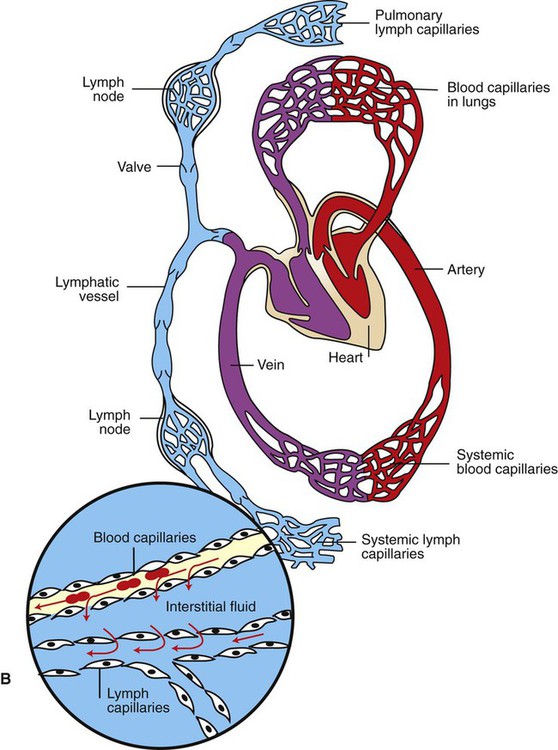
1. Capillaries, the smallest blood vessels, are the sites for the exchange of water and solutes between the bloodstream and the interstitial fluid. 2. Lipid-soluble substances diffuse readily through capillary walls, whereas lipid-insoluble substances must pass through capillary pores. 3. Fick’s law of diffusion is a simple mathematical accounting of the physical factors that affect the rate of diffusion. 4. Water moves across capillary walls both by diffusion (osmosis) and by bulk flow. 5. The Starling equation quantifies the interaction of oncotic and hydrostatic forces acting on water. 6. Several common physiological changes alter the normal balance of Starling forces and increase the filtration of water out of capillaries. 7. Edema is a clinically noticeable excess of interstitial fluid. Figure 23-1 shows the contrasting features of the walls of the various types of blood vessels in the systemic circulation. The distinguishing feature of the walls of the aorta and large arteries is the presence of a large amount of elastic material along with smooth muscle. These vessels are called the elastic vessels; elasticity is necessary because the aorta and large arteries must distend with each pulsatile ejection of blood from the heart. The arterial walls are also strong and quite stiff (low compliance). There is no contradiction in saying that the arteries are elastic and have low compliance. Elasticity denotes distensibility and an ability to return to the original shape after the distending force or pressure is removed. Compliance is a measure of how much force or pressure is required to achieve distention. The arteries are elastic, but a high pressure (systolic pressure) is required to distend them. Capillaries form a network (see Figure 18-4). In most tissues the capillary network is so dense that each cell of the tissue is within 100 µm of a capillary. However, not all the capillaries of a tissue carry blood at all times. In most tissues the arterioles alternate between constriction and dilation, so blood flow is periodically reduced or even stopped in most capillaries. Also, in some tissues (e.g., intestinal circulation), tiny cuffs of smooth muscle encircle capillaries at the points where they branch off from arterioles. Contraction of these precapillary sphincters can reduce or stop the flow of blood in individual capillaries. When the metabolic rate of a tissue increases (and therefore its need for blood flow increases), the arterioles and precapillary sphincters still constrict periodically, but they spend more time in the dilated (relaxed) state. This increases the fraction of capillaries in which blood is flowing at any one time. At maximal metabolic rate (e.g., maximal exercise in a skeletal muscle), blood flows through all the capillaries all the time. Sending blood flow to all the capillaries not only increases the total blood flow through a tissue but also minimizes the distance between each cell of the tissue and the nearest capillary carrying blood by bulk flow. Both these effects speed up diffusional exchange between the capillary blood and the tissue cells. The rate of diffusional exchange between capillary blood and the surrounding interstitial fluid depends both on the properties of the substances being exchanged and on the features of the capillary wall. Small, lipid-soluble substances (e.g., dissolved oxygen and carbon dioxide, fatty acids, ethanol, and some hormones) readily dissolve in the cell membranes of the endothelial cells that form the capillary walls. Such lipid-soluble substances can diffuse very rapidly through the endothelial cells from blood to interstitial fluid, or vice-versa. In contrast, lipid-insoluble substances (e.g., ions, glucose, and amino acids) do not dissolve in cell membranes and so cannot diffuse through the endothelial cells. Instead, such substances must pass through the pores, or clefts, that exist between the endothelial cells (Figure 23-2). These pores create tiny, water-filled channels between the capillary blood and the interstitial fluid. The diffusional movement of lipid-insoluble substances across capillary walls is much slower than the movement of lipid-soluble substances, because the lipid-insoluble substances are restricted to passage through the capillary pores, which constitute only about 1% of the total wall surface area of a typical capillary. The capillaries in most tissues are called continuous capillaries because the endothelial cells form a continuous tube, except for the tiny, water-filled pores between the endothelial cells. In typical continuous capillaries, the diameter of the pores is about 4 nm, which is large enough to permit the passage of water and of all the small solutes in plasma and interstitial fluid. The plasma protein molecules, however, are a little too large to pass through pores of this size. Blood cells, of course, are far too large to pass through such small openings (see Figure 18-7). The main route for the delivery of plasma proteins into the interstitial fluid is through the three-step process of transcytosis. The first step is pinocytosis (a form of endocytosis), which involves the invagination of the capillary endothelial cell membrane to form an intracellular vesicle that contains plasma, including plasma proteins (see Figure 23-2). Second, some of these vesicles cross the capillary endothelial cell from the side facing the bloodstream to the side facing the interstitial fluid. In the third step, these vesicles fuse with the membrane of the endothelial cell on the interstitial fluid side; the vesicles discharge their contents into the interstitial space. This third step is called exocytosis. The delivery of plasma constituents into the interstitial fluid by transcytosis is extremely slow, compared with the diffusion of lipid-soluble substances through endothelial cells, or the passage of small, lipid-insoluble substances through capillary pores. The size of the capillary pores, or clefts, varies from tissue to tissue. Two extremes are found in the brain and the liver. In brain capillaries, the junctions between adjacent endothelial cells are so tight that only water and small ions (e.g., Na+ and Cl−) can pass through them; not even glucose or amino acid molecules can pass through these tiny pores. Yet brain neurons require glucose to carry out their normal metabolism. Glucose is moved across the brain capillary endothelial cells by means of specialized protein carrier molecules that are embedded in the cell membranes of the endothelial cells. The energy to drive this facilitated diffusion comes from the glucose concentration difference between the blood and the brain interstitial fluid. The tight junctions between endothelial cells in brain capillaries create a barrier between the bloodstream and the brain tissue that is called the blood-brain barrier (also discussed in Chapter 15). One function of the blood-brain barrier is to protect brain neurons from exposure to toxic substances that may be in the blood. In the liver, the clefts between capillary endothelial cells are so large that these vessels are called discontinuous capillaries (or sinusoids). Even plasma proteins such as albumin and globulin can readily pass through these large clefts, which typically exceed 100 nm in width (see Figure 23-2, bottom). Large gaps between endothelial cells are an appropriate feature for capillaries in the liver because the plasma proteins are produced by liver cells (hepatocytes). The large gaps between endothelial cells permit the newly synthesized protein molecules to enter the bloodstream. The large gaps are also appropriate for the role of the liver in detoxification. Some toxins become bound to plasma proteins in the bloodstream, and then are removed from the blood by the liver and chemically changed into less toxic substances. Discontinuous (sinusoidal) capillaries are also found in the spleen and bone marrow. Most of the factors that affect the rate of diffusional exchange between capillary blood and interstitial fluid have been mentioned. These factors include the distance involved, the size of the capillary pores (or fenestrae, when present), and the properties of the diffusing substance (i.e., lipid-soluble vs. lipid insoluble). The German physiologist Adolph Fick incorporated all these factors into an equation: Fick’s law of diffusion. Figure 23-3 shows how Fick’s law applies to the diffusional exchange between capillary fluid and interstitial fluid. The rate of diffusion of any substance (S) depends, first, on the concentration difference, that is, the difference between the concentration of the substance in capillary fluid and its concentration in interstitial fluid. Diffusion is driven by this concentration difference, and diffusion always proceeds from the area of higher concentration toward the area of lower concentration. Next, the rate of diffusion is determined by the area available for diffusion, the term A in the equation. For lipid-soluble substances, this area is equivalent to the total surface area of the capillaries. For lipid-insoluble substances, this area is much smaller, being equal to the area of the pores (or clefts) between capillary endothelial cells (plus the area of fenestrae, when present). The term Δx in the equation represents the distance over which diffusion must occur. Functionally, Δx equals the distance from a tissue cell to the nearest capillary that is carrying blood by bulk flow (see Figure 23-3). The greater the distance from the tissue cells to the capillaries, the slower is the rate of diffusional exchange of substances between that cell and the capillary blood; therefore, Δx appears in the denominator in the equation. The tendency for water to move by diffusion is quantified as osmotic pressure (see Chapter 1). The normal osmotic pressure created by the proteins in the plasma is 25 mm Hg; that is, the osmotic effect of the plasma proteins is equivalent to a pressure of 25 mm Hg driving water into the capillaries. The osmotic pressure created by the plasma proteins is also called plasma oncotic pressure or colloid osmotic pressure. (The term colloid is used because the plasma proteins are not in a true solution but rather in a colloidal suspension.) In addition to being affected by diffusional (osmotic) forces, water responds to hydrostatic pressure differences across the capillary wall. Hydrostatic pressure differences cause water to move by bulk flow; in this case the bulk flow occurs through the capillary pores. The hydrostatic pressure within the capillaries (capillary blood pressure) is higher at the arteriolar end of capillaries than at the venous end (see Figure 22-1). However, a representative average capillary hydrostatic pressure would be about 18 mm Hg. In contrast, interstitial fluid hydrostatic pressure is normally about −7 mm Hg. The negative sign simply means that interstitial fluid pressure is less, (although only slightly less) than atmospheric pressure. The negative interstitial fluid pressure (–7 mm Hg) together with the positive capillary hydrostatic pressure (18 mm Hg) creates a hydrostatic pressure difference of 25 mm Hg across the wall of a typical capillary. This hydrostatic pressure difference tends to force water out of the capillaries and into the interstitial spaces; that is, the hydrostatic pressure difference favors filtration. In most capillaries of the systemic circulation, the hydrostatic pressure difference (which favors filtration) almost balances the oncotic pressure difference (which favors reabsorption). However, the balance is rarely perfect. Usually, the hydrostatic pressure difference slightly exceeds the oncotic pressure difference, so there is a small, net filtration of water out of the capillaries. This water would simply accumulate in the interstitial spaces and cause swelling there if not for the lymph vessels, which collect excess interstitial fluid and return it to the bloodstream through the subclavian veins (Figure 23-4).
Capillaries and Fluid Exchange
Capillaries, the Smallest Blood Vessels, Are the Sites for the Exchange of Water and Solutes Between the Bloodstream and the Interstitial Fluid
Lipid-Soluble Substances Diffuse Readily Through Capillary Walls, Whereas Lipid-Insoluble Substances Must Pass Through Capillary Pores
Fick’s Law of Diffusion Is a Simple Mathematical Accounting of the Physical Factors That Affect the Rate of Diffusion
Water Moves Across Capillary Walls Both by Diffusion (Osmosis) and by Bulk Flow
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Capillaries and Fluid Exchange
Only gold members can continue reading. Log In or Register to continue