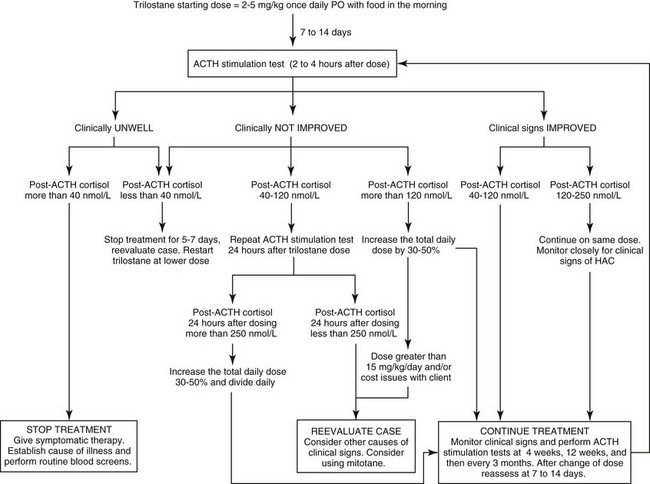
Chapter 51 Mitotane was once the mainstay of medical management of canine hyperadrenocorticism in many countries and is reviewed in detail elsewhere (Kintzer and Peterson, 1991). It is a cytotoxic agent that principally causes necrosis of the zona fasciculata and zona reticularis of the adrenal glands. It is slightly more efficacious than trilostane (see next section) but is reported to have a higher incidence of side effects (Kintzer and Peterson, 1991). Because it can be absorbed through the skin and is cytotoxic to humans, it should be handled carefully with gloves. Splitting tablets should be avoided when possible. Occasionally there may be evidence of hyperkalemia and hyponatremia. If these occur, an ACTH stimulation test should be performed; post-ACTH cortisol would be expected to be less than 20 nmol/L (<0.7 µg/dl). Mineralocorticoids should be given if hyperkalemia has been documented and will likely be needed for the rest of the animal’s life (see Chapter 53). Pancreatitis and hemorrhagic gastroenteritis are potential complications of the acute iatrogenic hypoadrenocorticism. Oral prednisolone (0.2 to 0.4 mg/kg PO every 24 hours) is given once the vomiting has subsided. Some dogs require this for life, but others may revert to their original state of hyperadrenocorticism. Trilostane has proven to be well tolerated by almost all dogs with pituitary-dependent hyperadrenocorticism in several published trials (totaling more than 120 dogs) summarized elsewhere (Ramsey, 2010). Few dogs develop signs of hypoadrenocorticism when treated with trilostane, although mild asymptomatic hyperkalemia is common. When hypoadrenocorticism does occur, dogs usually rapidly recover with appropriate therapy. The low prevalence of side effects compares favorably with those reported with mitotane. Although few pharmacokinetic studies have been performed, trilostane is known to be short acting. The recommended starting dose is 2 to 5 mg/kg orally once daily, using the lower dosage range in small dogs. (Figure 51-1 is an algorithm of trilostane therapy.) Trilostane is better absorbed if given with food. It is effective in resolving the signs of pituitary-dependent hyperadrenocorticism in about 75% of cases (Neiger et al, 2002; Ruckstuhl, Nett, and Reusch, 2002). Polyuria, polydipsia, and polyphagia should dissipate within 4 weeks after starting trilostane. Skin changes should resolve within 4 months of starting treatment. All these improvements should be maintained as long as the dogs remain on adequate doses of trilostane.
Canine Hyperadrenocorticism Therapy
Treatment of Pituitary-Dependent Hyperadrenocorticism
Medical Options
Mitotane
Trilostane
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Canine Hyperadrenocorticism Therapy
Only gold members can continue reading. Log In or Register to continue