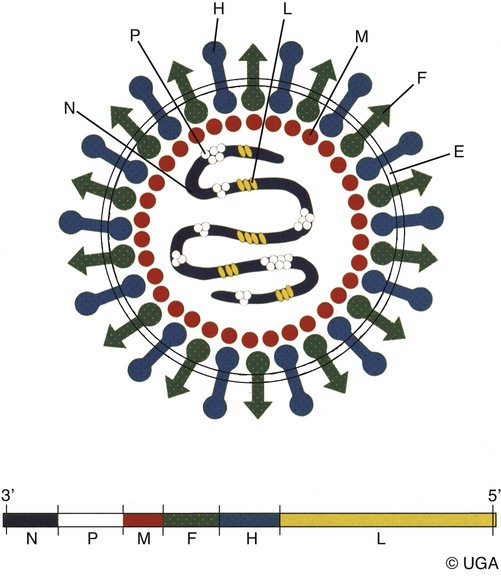
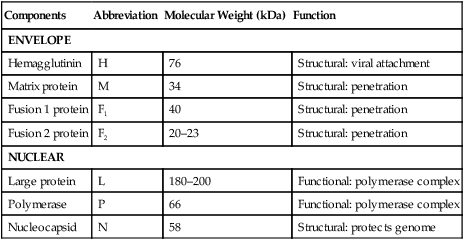
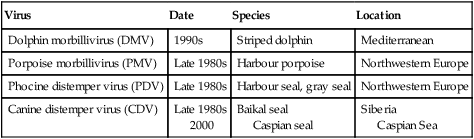
Canine distemper virus (CDV) is a member of the genus Morbillivirus of the family Paramyxoviridae and is closely related to other viruses (Web Table 3-1). CDV has a relatively large, variable diameter (150 to 250 nm) with single negative-stranded RNA enclosed in a nucleocapsid of helical symmetry. It is surrounded by a lipoprotein envelope derived from the cell membrane incorporating viral glycoproteins H (attachment protein) and F (fusion protein) (Fig. 3-1 and Web Table 3-2). Viruses such as CDV that code for proteins capable of integrating in the cell membrane make infected cells susceptible to damage by immune-mediated cytolysis. CDV also can induce cellular fusion (syncytial formation). Syncytial induction involves a complex interplay of viral proteins and the host cell316 and occurs with CDV strains less likely to produce cellular apoptosis.211 WEB TABLE 3-1 Host Susceptibility to Morbilliviruses PDV-2, Phocine distemper virus-2. Modified from Osterhaus ADME, de Swart RL, Vos HW, et al. 1995. Morbillivirus infections of aquatic mammals: newly identified members of the genus. Vet Microbiol 44:219–227. WEB TABLE 3-2 Structure of Canine Distemper Virus CDV and cellular interactions have been elucidated to explain the variety of outcomes of CDV infection. Signaling lymphocyte activation molecule (SLAM or CD 150) is a membrane glycoprotein and cellular receptor for morbilliviruses that is expressed on the surface of cells of the immune system including immature thymocytes, activated lymphocytes and monocytes, and mature dendritic cells. Virulent CDV selectively binds to SLAM on these immune cells via its H and F proteins, enabling rapid spread in lymphoid tissues.312 Immunosuppression occurs not only because of virally induced cytolysis but also because virulent CDV inhibits interferon (IFN) and cytokine responses of lymphoid cells via the P gene expression of virulence proteins V and C.312 In the brain, CDV binds to a hitherto-undefined receptor305 inducing a noncytolytic persistent infection.229,230 In response to acute CDV infection, upregulation of SLAM occurs in the dog’s immune cells infiltrating the central nervous system (CNS), which can further amplify viral replication in the brain.315 In contrast to virulent CDV, Vero-cell adapted CDV vaccine strains have lower pathogenicity and bind to heparin-like cell receptors found in nonimmune cells such as epithelial tissue culture cell lines.98 Because these cells lack its appropriate receptor, virulent CDV does not grow well in continuous cell lines, such as Vero cells, making laboratory isolation more difficult.163,165 In addition, virulent CDV strains can cause chronic persistent intracellular infections of the CNS by producing reduced cell-cell fusion and cytolysis.229,230 See the discussion of subacute to chronic CDV encephalitis under Pathogenesis. In contrast, Onderstepoort vaccine strains, which are easily propagated in vitro, have different F-protein structure and produce extensive cell-cell fusion leading to cytolysis. Increased replication and viral release from cells results in host antibody response and elimination of the vaccine virus with a self-limiting or attenuated infection. In contrast, reduced F-gene expression and viral release with virulent CDV strains leads to viral persistence and neuroinvasion with delayed onset of CNS manifestations.229 Synthetic recombinant modification of the genome to increase F-gene expression leads to attenuation in CDV virulence in ferrets and may be the future of development of vaccine strains.15 Despite minor genetic variation, CDV isolates are serologically homogeneous. However, various strains differ in their pathogenicity, which may affect the severity and extent or type of clinical disease. Certain isolates, such as Snyder Hill, A75/17, and R252 strains, are highly virulent and neurotropic. The Snyder Hill strain causes polioencephalomyelitis, whereas the latter two cause demyelination. Other strains vary in their ability to cause CNS lesions. In addition to the F protein influences described previously, properties of proteins coded by the N and M-genes also affect viral persistence282 and the ability to cause CNS disease. CDV is susceptible to ultraviolet light, although surrounding protein or antioxidants in its environment help protect it from inactivation. Extremely susceptible to heat and drying, CDV is destroyed by temperatures of 50° C to 60° C for 30 minutes. In excised tissues or secretions, it survives for at least an hour at 37° C and for 3 hours at 20° C (room temperature). In warm climates, CDV does not persist in kennels after infected dogs have been removed. Storage and survival times of CDV are longer at colder temperatures. At near-freezing (0° C to 4° C), it survives in the environment for weeks. Below freezing the virus is stable, surviving at −65° C for at least 7 years. Lyophilization reduces the lability of the virus and is an excellent means of preserving it for commercial vaccine and laboratory use. CDV remains viable between pH 4.5 and 9.0. As an enveloped virus, it is susceptible to ether and chloroform, dilute (less than 0.5%) formalin solution, phenol (0.75%), and quaternary ammonium disinfectants (0.3%). Routine disinfection procedures are usually effective in destroying CDV in a kennel or hospital (see Chapter 93). The disease and natural host ranges of CDV include certain species of terrestrial carnivores (see Web Table 3-1 and Table 3-1), and other species can be infected experimentally with varying degrees of susceptibility.20 CNS signs have been produced in mice and hamsters by intracerebral inoculation. Rabbits and rats are resistant to parenteral inoculation. Inapparent, self-limiting infections, produced in domestic cats, nonhuman primates, and humans by parenteral inoculation of virulent CDV, resemble those in dogs that have been given modified live virus (MLV) vaccines. Nonhuman old-world primates (Macaca fuscata and Macaca mulatta) have been naturally infected.285,328 With passing years the host range of this disease appears to have widened as interspecies transmissions and viral recombination events have occurred, leading to epizootics with high mortality.68,127,129,292 Molecular changes in the hemagglutinin gene may be responsible for the spread of CDV to nondog hosts in the wild.187,330 Despite the wide host range, dogs are the principal reservoir host for CDV, and they likely act as reservoirs of infection for wildlife.62,129 Certain wildlife species such as raccoons (Procyon lotor) and martens (Martes spp.) can serve as a reservoir of infection for susceptible dog populations.146,205 CNS infections in captive and wild, large, exotic Felidae have been attributed to infection with CDV* (see Feline Paramyxovirus Infections, Chapter 16). Pigs (Sus domestica) are subclinically infected, and peccaries (Tayassu tajacu) that have been naturally infected develop encephalitis.21 Encephalitis was documented in a naturally infected monkey.328 Subclinical infections have been documented in the Asian elephant (Elephas maximus).217 Phocine distemper virus, a Morbillivirus most closely related to CDV, and some wild strains of CDV have caused severe morbidity in Baikal (Phoca sibirica) and Caspian (Phoca caspica) seals (see Web Tables 3-1 and 3-3).93,151,151 It may have spread to the seals from dogs or other susceptible terrestrial carnivores. Genetic analysis of strains causing outbreaks shows that CDV does not become more virulent and spread to new host species in a region, but the same strain circulates among susceptible animals of several host species in a given geographic area.42,52,52 Other closely related but distinct morbilliviruses cause illness in other aquatic mammals (dolphins and porpoises), and they are more closely related to ruminant morbilliviruses. TABLE 3-1 Terrestrial Carnivores Susceptible to Canine Distempera,b aSubclinical infections occur in noncarnivores including the Asian elephant and Tayassuidae (peccaries). Information from Ref. 20 and 217. bSee also Table 100-10 for vaccination recommendation for these animals. WEB TABLE 3-3 Aquatic Morbillivirus Infections Data from Osterhaus ADME, De Swart RL, Vos HW, et al. 1995. Morbillivirus infections of aquatic mammals: newly identified members of the genus. Vet Microbiol 44:219–227, and Kuiken T, Kennedy S, Barrett T, et al. 2006. The 2000 canine distemper epidemic in Caspian Seals (Phoca caspica): pathology and analysis of contributory factors. Vet Pathol 43:321–338. Viral shedding occurs by 7 days following experimental inoculation (postinoculation [PI]). CDV, most abundant in respiratory exudates, is commonly spread by aerosol or droplet exposure; however, it can be isolated from most other body tissues and secretions, including urine. Transplacental infection can occur from viremic dams. Virus can be excreted up to 60 to 90 days after infection, although shorter periods of shedding are more typical. Contact among recently infected (subclinical or diseased) animals maintains the virus in a population, and a constant supply of puppies helps provide a susceptible population for infection. Although immunity to virulent canine distemper is prolonged or lifelong, it is not as absolute after vaccination. Dogs that do not receive periodic immunizations can lose their protection and become infected after stress, immunosuppression, or contact with diseased individuals. Based on results of serosurveys, the infection rate is considered to be higher than the disease rate,66 which reflects a certain degree of natural and vaccine-induced immunity in the general dog population. Many susceptible dogs can become subclinically infected but clear the virus from the body without showing signs of illness. Although most recovered dogs clear the virus completely, some may harbor virus in their CNS. The prevalence rate of spontaneous distemper in cosmopolitan dogs is greatest between 3 and 6 months of age, correlating with the loss of maternal-derived antibodies (MDAs) in puppies after weaning. In contrast, in susceptible, isolated populations of dogs, the disease is severe and widespread, affecting all ages. Increased susceptibility among breeds has been suspected but not proved. Brachiocephalic dogs have been reported to have a lower prevalence of disease, mortality, and sequelae compared with dolichocephalic breeds. Concurrent infections, such as with CDV and canine adenovirus (CAV)-2, can cause severe fatal pneumonia in pups.245 During natural exposure, CDV spreads by aerosol droplets and contacts epithelium of the upper respiratory tract (Web Fig. 3-1, Fig. 3-2A,B). Within 24 hours PI, it multiplies in tissue macrophages and spreads in these cells via local lymphatics to tonsils and bronchial lymph nodes. Original studies in dogs16 have been repeated in ferrets, in which virulent CDV takes advantage of mucosal surfaces for host invasion and lymphocytes for swift dissemination via recognition of the SLAM receptors on lymphocytes.312 By 2 to 4 days PI, viral numbers increase in tonsils and retropharyngeal and bronchial lymph nodes, but low numbers of CDV-infected mononuclear cells are found in other lymphoid organs.16 By days 4 to 6 PI, virus multiplication occurs within lymphoid follicles in the spleen, the gut-associated lymphatic tissue of the lamina propria of the stomach and small intestines, the mesenteric lymph nodes, and the Kupffer’s cells in the liver. Widespread virus proliferation in lymphoid organs corresponds to an initial rise in body temperature and leukopenia between days 3 and 6 PI. The leukopenia is primarily a lymphopenia caused by viral damage to lymphoid cells, affecting both T and B cells. Further spread of CDV to epithelial and CNS tissues on days 8 to 9 PI probably occurs hematogenously as a cell-associated and plasma-phase viremia and depends on the dog’s humoral and cell-mediated immune status (see Fig. 3-2A). Shedding of virus from all body excretions begins at the time of epithelial colonization, even in dogs with subclinical infections. By day 14 PI, animals with adequate CDV antibody titers and cell-mediated cytotoxicity clear the virus from most tissues and show no clinical signs of illness. Specific IgG-CDV antibody is effective in neutralizing extracellular CDV and inhibiting its intercellular spread. Dogs with intermediate levels of cell-mediated immune responsiveness with delayed antibody titers by days 9 to 14 postinfection have viral spread to their epidielial tissues. Clinical signs that develop may eventually resolve as antibody titer increases and virus is cleared from most body tissues. However, complete virus can persist for extended periods in uveal tissues and neurons and in integument such as footpads.118,119,119 The pathogenesis of replication and persistence of CDV in footpads has been well investigated. Microscopic lesions consist of hyperkeratosis and parakeratosis with vesicle, pustule, and inclusion body formation.221 Virus entering the footpad epithelium during the viremic period causes proliferation of basal keratinocytes, resulting in the observed hyperkeratosis; however, neither the virus nor its nucleic acid appears to persist indefinitely.88,120,120 This epidermal localization has been associated with certain wild-type CDV strains that produce noncytocidal infection in footpad keratinocytes in vitro and experimentally infected dogs.88,120,120 Cytokine expression is upregulated in virus-infected footpad epidermal cells.118,119 Alteration in the viral H protein gene sequence has been associated with this viral adaptation.243 By days 9 to 14 PI, dogs with poor immune status undergo viral spread to many tissues, including skin, exocrine and endocrine glands, and epithelium of the gastrointestinal (GI), respiratory, and genitourinary tracts. Clinical signs of disease in these dogs are usually dramatic and severe, and virus usually persists in their tissues until death. The sequence of pathogenic events depends on the virus strain and may be delayed by 1 to 2 weeks.284 Secondary bacterial infections increase the severity of clinical illness. Studies on serologic response to CDV in gnotobiotic dogs confirm that serum antibody titers vary inversely with the severity of the disease. Antibody response in dogs has been separated into envelope and core determinants of the virus. Only dogs producing anti-envelope antibodies appear to be able to prevent persistent viral infection of the CNS. The outcome of CNS infection seems to depend on the appearance of circulating IgG antibodies to the H glycoprotein.242 Mortality in gnotobiotic dogs approaches that of naturally infected animals, deemphasizing the role of secondary bacterial infection in influencing the severity of disease. However, bacteria are probably important in complicating the signs of disease in the respiratory and GI tracts. Acute CDV infection causes lymphocytic apoptosis, T-cell depletion, and immunosuppression.162,312 Studies have documented the occurrence of cell-mediated immunosuppression after CDV infection. Lymphocyte transformation testing of experimentally infected neonates has shown profound depression of lymphocyte response to phytomitogens at a time corresponding to acute viremia and lymphopenia. This depressed response persisted for more than 10 weeks in convalescing puppies and never returned to baseline values in those that died of acute causes.157 Prenatal and neonatal distemper infections are causes of immunodeficiency in surviving puppies and can make concurrent infections with other viruses such as parvovirus, bacteria such as Clostridium piliforme, or protozoa such as Neospora caninum more severe.131,132 Neuroinvasion occurs when viremia is of sufficient magnitude, which depends on the degree of systemic immune responses mounted by the host. If virus enters the nervous system of CDV-infected dogs, many may develop microscopic CNS lesions. In ferrets, the development of clinical neurologic signs in CNS infection may relate more to the disease duration once virus enters the CNS, rather than particular virulence of the infecting strain.43 Viral entry and spread in the nervous system have been studied in ferrets, using a CDV bearing a green fluorescent protein, and occur via hematogenous and neural routes (see Fig. 3-2A).249,250 With hematogenous spread, virus (free, platelet- or mononuclear cell-associated) enters the brain parenchyma via fine blood vessels throughout and deposits in perivascular (Virchow Robin) spaces (see Fig. 3-2A). Virus entering in this manner is first detected in CNS perivascular astrocytic foot processes and then neurons. In addition, via the systemic circulation, virus enters the choroid plexus choroid of the fourth ventricle and replicates in the choroid plexus epithelial cells. In dogs, free or lymphocyte-associated virus can enter the cerebrospinal fluid (CSF) from infected choroid plexus, where it spreads to periventricular and subpial structures. Spread of virus through CSF pathways might explain the early distribution of lesions in subependymal and subpial areas such as optic tracts and nerves, rostral medullary velum, cerebral peduncles, and spinal cord.307 In the neural route of dissemination in ferrets, virus spreads to olfactory neurons during the period of high level viremia and simultaneous massive replication in the respiratory mucosal epithelium rather than as a consequence of intranasal exposure to virus.250 During this epithelial proliferation within the ethmoid region, virus passes through the cribiform plate, enters the olfactory receptor neurons and subsequently spreads in an anterograde fashion into the olfactory nerve fibers (see Fig. 3-2A). From there it spreads caudally in the olfactory cortex and enters regions of the limbic system. It is not clear whether this means of spread also occurs in dogs. It could explain why some rare dogs develop selective polioencephalomalacia of rhinencephalic structures including the pyriform and temporal lobes.177 (See the discussion of “chewing gum” seizures under Neurologic Signs, later.) Acute CDV encephalitis, which occurs early in the course of infection in young or immunosuppressed animals, is characterized by direct viral replication and injury. CDV antigen and messenger RNA (mRNA) are detected in lesions CDV causes multifocal lesions in the gray and white matter. Gray matter lesions are the result of neuronal infection and necrosis and can lead to polioencephalomalacia. However, neuronal infection can also occur with minimal evidence of cytolysis. In the acute stage of infection animals have lymphoid depletion, from viremia-induced apoptosis, that essentially targets CD4+ cells.297,321,321 Therefore it is not surprising that early in clinical illness, perivascular cuffing is minimal. Despite lack of classical signs of inflammation, there is a marked increase in CD8+ T cells throughout the CNS in dogs with acute distemper in response to the presence of CDV nucleocapsid protein and increased interleukin (IL)-8 activity.294,297 This can be in part explained by the fact that the depletion of CD4+ cells is earlier and longer than that of CD8+ cells.297 In a study of acute CDV encephalitis in dogs, virus was present in a diffuse or multifocal distribution, and class II major histocompatibility complex (MHC), which normally has a very low expression in the CNS, was upregulated throughout the white matter and in CDV-infected foci.8 MHCII upregulation is also observed in multple sclerosis (MS) and experimental allergic encephalitis and can result from elevated levels of IFN-γ, which are frequently seen in viral infections including distemper.299 Besides upregulation of MHC molecules, microglial cells in distemper exhibit other changes such as enhanced secretion of reactive oxygen radicals.278 Upregulation of proinflammatory cytokines also occurs in distemper lesions, whereas anti-inflammatory cytokines remain unchanged.32,95 Light and electron microscopy findings with acute noninflammatory demyelination in white matter are associated with active viral nucleocapsid replication in microglial and astroglial cells (see Figs. 3-2B and 3-2C) rather than oligodendroglial cells, the myelin-producing cells.49,100,201,307 Despite the lack of productive viral replication in oligodendroglial cells, their function has been impaired when observed in primary canine brain cell cultures, where CDV causes a slow-spreading, noncytolytic infection. CDV viral proteins and nucleocapsids are difficult to detect in oligodendroglia by immunocytochemical or ultrastructural methods. However, the use of in situ hybridization shows that the complete viral genome is present in these cells.336 The restricted infection of viral transcription without translation likely leads to metabolic dysfunction and morphologic degeneration of oligodendroglial cells108 and results in demyelination via a downregulation of myelin gene expression.333 In vitro studies have elucidated additional means by which CDV can cause CNS injury. In primary rat brain cell cultures, CDV infects both neurons and astrocytes.48 Glutamatergic stimulation was implicated in virus-mediated cytopathogenic effect as demonstrated by attenuation of necrosis by receptor blocking. Dramatic increases in glutamine concentrations were observed in culture media. Neurochemical changes have also been described in the hippocampus of CDV-infected dogs with intractable seizures; however, these alterations may relate to either a cause or effect of the status epilepticus.81 Subacute to chronic CDV encephalitis, in contrast to the acute form, is characterized by reduced expression of CDV antigen and mRNA and a strong upregulation of the inflammatory response (see Figs. 3-2D and 3-2E). This results in perivascular mononuclear cell infiltrations and a virus-independent immunopathologic process. These animals are recovering from lymphoid depletion throughout the body, and they have a significant increase in T- and B-lymphocyte populations compared with dogs with acute CDV encephalitis. Virus is found predominantly in follicular dendritic cells of the lymphoid system, suggesting a change in cell tropism for viral persistence.322 There is also upregulation of proinflammatory cytokines and metalloproteinases.32 A predominance of CD8+ lymphocytes and lesser numbers of CD4+ lymphocytes dominate the perivascular infiltrates during all states of distemper encephalitis.321 These cells induce a strong humoral immune response, and increased intrathecal virus-neutralizing antibodies are present.196,321 Antimyelin antibodies and myelin-sensitized T cells are thought to be a secondary reaction to the inflammatory process and do not correlate with the course of the disease.307 Antibodies to CDV appear to interact with infected macrophages in CNS lesions, causing their activation with the release of reactive oxygen radicals. This activity can lead to further destruction of oligodendroglial cells and myelin through an “innocent bystander” mechanism.44,49 The reaction of the immune system, not viral interference, is the pathogenic mechanism for demyelination in this phase. In addition to the humoral response, a CD4+-mediated delayed hypersensitivity response and the presence of cytotoxic CD8+ T cells may facilitate the myelin injury.32 If viral spread through the CNS has been extensive by the time the host immune system responds to the virus, widespread damage occurs with a fatal outcome. Alternatively, in surviving animals, CDV is cleared from the inflammatory lesions but can persist in brain tissue in unaffected sites.200 Apparently, virally infected brain tissue can be spared the inflammatory process because of impaired or delayed immune recognition associated with noncytolytic infections produced by virulent CDV (see Etiology).306,331,331 Lack of cytolysis prevents extracellular release of virus, thus shielding it from humoral immune responses. Reduced expression of CDV proteins on the surface of inflammatory cells has also been implicated as a means of immune avoidance.6,7,7 Abundant expression of all viral protein mRNAs and reduced or lacking protein expression (transcription without translation) have been found not only in oligodendrocytes as discussed previously but also in neurons in distemper.200,206,206 Thus, low viral protein antigen levels may also be a means by which CDV persists through evading immune recognition. Old dog encephalitis (ODE) is an extremely rare, chronic, active progressive inflammatory disease of the gray matter of the cerebral hemispheres and brainstem of the CNS, associated with CDV infection.131,132 This form of infection occurs in immunocompetent animals with persistent virus in their neurons in a replication-defective form.23 Transmission of infection using brain homogenates of dogs with ODE was unsuccessful; however, reisolation of infectious virus required prolonged cocultivation of brain explant cells with susceptible Vero cells.23 Similar observations have been made with persistent measles virus in humans with subacute sclerosing panencephalitis. Inclusion body polioencephalitis is a variant form of CDV encephalitis. It can occur after vaccination206 or in dogs with a sudden onset of only neurologic manifestations of distemper.207 Multifocal gray matter necrosis, perivascular lymphocytic inflammation, and cytoplasmic and intranuclear inclusions are observed (see Pathologic Findings). Neuronal infection may also be of the restricted type, especially of the matrix and fusion proteins. The immune response is dominated by T-cell infiltration and class II MHC upregulation. Clinical signs of canine distemper vary depending on virulence of the virus strain, environmental conditions, and host age and immune status. More than 50% of CDV infections are probably subclinical. Mild forms of clinical illness are also common, and signs include listlessness, decreased appetite, fever, and upper respiratory tract infection. Bilateral serous oculonasal discharge can become mucopurulent with coughing and dyspnea. Many mildly infected dogs develop clinical signs that are indistinguishable from those of other causes of “kennel cough” (see Chapter 6). In contrast, severe fatal respiratory failure from pneumonia, without other signs, has been reported in neonatal223 or copathogen-infected pups.61 Keratoconjunctivitis sicca can develop after systemic or subclinical infections in dogs. Persistent anosmia was reported as a sequela in dogs that had recovered from canine distemper.114 Vesicular and pustular dermatitis in puppies is rarely associated with CNS disease (Fig. 3-3A and B), whereas dogs developing nasal and digital hyperkeratosis (Figs. 3-4 and 3-5) usually have various neurologic complications. Viral invasion of cutaneous tissues has been demonstrated.178 (See Pathogenesis.) Neurologic manifestations usually begin 1 to 3 weeks after recovery from systemic illness; however, no way is known to predict which dogs will develop neurologic disorders. Neurologic signs can also coincide with multisystemic illness, or less commonly, they can occur weeks to months later. Neurologic signs frequently develop in the presence of nonexistent or very mild extraneural signs.296 Empirically, certain features of systemic disease can be predictive of the occurrence of neurologic sequelae. Mature or partially immune dogs that have been previously vaccinated and have no history of systemic disease can suddenly develop neurologic signs.236 Neurologic signs, whether acute or chronic, are typically progressive. Chronic relapsing neurologic deterioration with an intermittent recovery and a later, superimposed acute episode of neurologic dysfunction can occur. ODE is characterized by this type of progressive history. Neurologic complications of canine distemper are the most significant factors affecting prognosis and recovery from infection. Neurologic signs vary according to the area of the CNS involved. Hyperesthesia and cervical or paraspinal rigidity can be found in some dogs as a result of meningeal inflammation, although parenchymal rather than meningeal signs usually predominate. Seizures, cerebellar and vestibular signs, paraparesis or tetraparesis with sensory ataxia, and myoclonus are common. Seizures can be of any type, depending on the region of the forebrain that is damaged by the virus. The “chewing-gum” type of seizures, classically associated with CDV infection, often occurs in dogs developing polioencephalomalacia of the temporal lobes. However, lesions in these lobes from other causes can produce similar seizures. Hippocampal alterations have been observed in dogs that develop generalized tonic-clonic seizures that may progress to status epilepticus.81 Myoclonus, the involuntary twitching of muscles in a forceful simultaneous contraction, can be present without other neurologic signs. With more extensive spinal cord damage, the dog may have upper motor neuron paresis of the affected limb associated with myoclonus. The rhythmic contractions can be present while the dog is awake, although they more commonly occur while it is sleeping. The neural mechanisms for myoclonus originate with local irritation of the lower motor neurons of the spinal cord or cranial nerve nuclei. Although considered specific for CDV infection, myoclonus can also be seen in other paramyxovirus infections of dogs and cats (see Chapters 7 and 16) and, less commonly, in other inflammatory conditions of the CNS.293 Young puppies infected transplacentally may develop neurologic signs during the first 4 to 6 weeks of life.156 Mild or inapparent infections are seen in the bitch. Depending on the stage of gestation at which infection occurred, abortions, stillbirths, or the births of weak puppies can occur. Puppies infected in utero that survive such infections may suffer from permanent immunodeficiencies because of damage to primordial lymphoid elements. Young puppies infected with CDV before the eruption of permanent dentition can have severe damage to the enamel, dentin, or roots of their teeth.36 Enamel or dentin can show an irregular appearance (Fig. 3-6), in addition to partial eruption, oligodontia, or impaction of teeth. Enamel hypoplasia with or without neurologic signs may be an incidental finding in an older dog and is relatively pathognomonic for prior infection with CDV. Neonatal (less than 7 days old) gnotobiotic puppies have developed virus-induced cardiomyopathy after experimental infection with CDV. Clinical signs including dyspnea, depression, anorexia, collapse, and prostration develop from 14 to 18 days PI. Lesions are characterized by multifocal myocardial degeneration, necrosis, and mineralization, with minimal inflammatory cell infiltration. The clinical significance of this process after natural infection is uncertain, and whether it has a relationship with onset of adult cardiomyopathy in dogs remains to be determined. Other viruses such as canine parvovirus (CPV)-1 and CPV-2 can produce similar lesions (see Canine Enteric Viral Infections, Chapter 8). Young, growing dogs with experimentally and naturally induced CDV infection develop metaphyseal osteosclerosis of the long bones.3,30,94,176,190 Large-breed dogs between 3 and 6 months of age are most commonly affected. Studies have not shown animals with systemic distemper to develop overt clinical signs related to these long bone lesions. However, CDV RNA transcripts have been seen in the bone cells of young dogs with hypertrophic osteodystrophy (HOD), a metaphyseal bone disease that can be similar to and confused with metaphyseal osteomyelitis (see Musculoskeletal Infections, Chapter 85).192,194 Juvenile cellulitis, HOD, or both have developed in some puppies in association with MLV distemper vaccination (see Postvaccinal Complications, Chapter 100 and Modified Live Virus Vaccines in this chapter).181 Morbilliviral RNA transcripts have also been detected in the bony lesions of people with Paget’s disease (see Public Health Considerations in this chapter). Dogs with rheumatoid arthritis had high levels of antibodies to CDV in sera and synovial fluid compared with dogs with inflammatory and degenerative arthritis.33 CDV antigens were found in immune complexes from synovial fluid of dogs with rheumatoid arthritis but were not found in synovial fluid from dogs with inflammatory or degenerative arthropathies. Dogs with CDV encephalomyelitis often have a mild anterior uveitis that is clinically asymptomatic. More obvious ophthalmologic lesions in canine distemper have been attributed to an effect of the virus on the optic nerve and the retina (see Canine Distemper, Chapter 92). Optic neuritis can be characterized by a sudden onset of blindness, with dilated unresponsive pupils. Degeneration and necrosis of the retina produce gray-to-pink irregular densities on the tapetal or nontapetal fundus or both. Bullous or complete retinal detachment can occur where exudates dissect between the retina and choroid. Chronic, inactive fundic lesions are associated with retinal atrophy and scarring. These circumscribed, hyperreflective areas are called gold medallion lesions and are considered characteristic of previous canine distemper infection. Immunosuppression caused by or responsible for systemic CDV infection can be associated with combined opportunistic infections. Salmonellosis has been a common complication, causing protracted or fatal hemorrhagic diarrhea or sepsis in affected dogs. Combined infections with Toxoplasma gondii or Neosporum caninum have produced lower motor neuron dysfunction from myositis and radiculoneuritis (see Chapter 79). Pneumocystis jirovecii pneumonia has also been associated with CDV infection (see Chapter 66).283 Practical diagnosis of canine distemper is primarily based on clinical suspicion. A characteristic history of a 3- to 6-month-old unvaccinated puppy with a compatible illness supports the diagnosis. Most dogs with severe disease have clinical signs distinctive enough to make a presumptive diagnosis, but upper respiratory infections in older dogs are often incorrectly diagnosed as infectious tracheobronchitis (see Chapter 6). Specific laboratory tests are not always available to confirm the suspicion of CDV infections, so the practicing veterinarian must rely on nonspecific findings of routine laboratory procedures. Abnormal hematologic findings include an absolute lymphopenia caused by lymphoid depletion. This frequently persists in very young dogs with rapidly progressive systemic or neurologic signs. Thrombocytopenia (as low as 30,000 cells/µL) and regenerative anemia have been found in experimentally infected neonates (aged less than 3 weeks) but have not been consistently recognized in older or spontaneously infected dogs. Intracytoplasmic distemper inclusions can be detected in the early phase of disease by examination of stained peripheral blood films, in low numbers in circulating lymphocytes, and with even less frequency in monocytes, neutrophils, and erythrocytes. Wright-Leishman-stained inclusions in lymphocytes are large (up to 3 µm), single, oval, gray structures, whereas erythrocytic inclusions (which are most numerous in polychromatophilic cells) are round and eccentrically placed and appear light blue (Fig. 3-7). The sizes of erythrocytic inclusions are between those of metarubricyte nuclei and Howell-Jolly bodies. Buffy coat and bone marrow examination and use of phloxinophilic stains can improve the chances of detecting inclusions. Electron microscopy has confirmed that these inclusions consist of paramyxovirus-like nucleocapsids. Hyperintense foci and loss of contrast between gray and white matter was found in T2-weighted images in the brain (Fig. 3-9).29,116 Histopathologic changes in these corresponding regions were demyelination. Post contrast T1-weighted dural enhancement was observed in a chronically infected dog.116 Abnormalities are detectable in dogs with neurologic signs of distemper; however, false-negative results can be anticipated. Dogs with acute noninflammatory demyelinating encephalomyelitis may have normal CSF analysis results. Increases in protein (more than 25 mg/dL) and cell count (more than 10 cells/µL with a predominance of lymphocytes) are characteristic of subacute to more chronic, inflammatory forms of CDV encephalomyelitis.11 Intracytoplasmic inclusions can be found in CSF cells.1 Protein increase in the CSF is due to leakage from inflammation of the blood-CSF barrier and from increased production of immunoglobulins.35,70,101,274 IgG has specific anti-CDV activity. IFN levels are also increased in the CSF of dogs with acute and chronic distemper encephalitis.299 Differences in the humoral immune response in CSF and sera to the H and F envelope proteins (see Web Table 3-2) have been noted between some dogs with chronic progressive encephalitis and those with other forms of distemper encephalitis.242 Increased anti-CDV antibody in CSF offers definitive evidence of distemper encephalitis because antibody is locally produced; these increases have not been found in vaccinated dogs or those with systemic distemper without CNS disease. CSF antibody can be increased from traumatic collection procedures causing contamination by whole blood. An antibody ratio can help identify the effect of nonspecific leakage of distemper-specific IgG into the CSF from serum. Divide the concentration of distemper-specific IgG in CSF by that of IgG in serum. Compare the result with a corresponding CSF-serum antibody ratio for another infectious agent for which serum antibody titers are expected, such as CAV or CPV. If the ratio for CDV is higher than that for CAV or CPV, then de novo local production of CSF antibody caused by CNS infection with distemper is expected. Ideally the titer determination for both diseases should use the same methodology (e.g., neutralization, enzyme-linked immunosorbent assay [ELISA], indirect fluorescent antibody [FA]). Alternatives are to compare the CDV-specific CSF-serum ratio with the ratio of IgG or albumin in CSF and serum,295 but this approach is less accurate because of the differences in methodology used in their determinations. The CSF IgG antibody concentration is more likely to be increased in dogs with inflammatory demyelinating encephalitis than in younger or immunosuppressed dogs with acute polioencephalitis and noninflammatory virus-induced cellular injury.274,296 Although the test for CSF antibodies is sensitive and specific for CDV, it can be performed only by properly equipped diagnostic or research laboratory personnel (see Web Appendix 5). In acute CNS infections, some mononuclear cells may contain large (15 to 10 µm), oval homogenous eosinophilic intracytoplasmic inclusions.9 Immunofluorescent techniques can facilitate a specific diagnosis of canine distemper; however, these tests also require special equipment and are usually handled by regional diagnostic laboratories (see Web Appendix 5). In clinically affected dogs, immunofluorescence is usually performed on cytologic smears prepared from conjunctival, tonsillar, genital, and respiratory epithelium. The technique also can be performed on cells in CSF, blood (buffy coat), urine sediment, and bone marrow (Fig. 3-10, Web Fig. 3-2). Smears should be made on precleaned slides, air-dried thoroughly, and preferably fixed in acetone for 5 minutes before transport to the laboratory. At the laboratory, they are stained directly or indirectly with fluorescein-conjugated CDV antibody and examined by fluorescent microscopy. Antigen, first detected in buffy coat smears from 2 to 5 days PI, decreases as antibody titer increases by 8 to 9 days PI. Clinical signs become apparent shortly after this time (day 14), and positive results are recognized only in dogs that do not mount a sufficient immune response and succumb to infection. Positive fluorescence in conjunctival and genital epithelium is usually detected only within the first 3 weeks PI, when systemic illness is apparent. Virus also disappears in these tissues after the first 1 to 2 weeks of clinical illness (21 to 28 days PI) as antibody titers rise in association with clinical recovery. Beginning with the recovery stage, antibody can bind and mask antigen in infected cells, resulting in false-negative results. Virus can be detected for longer periods in epithelial cells and macrophages from the lower respiratory tract, and transtracheal washings can be obtained for diagnosis. Virus also persists for at least 60 days in the skin, uveal tissue, footpad, and CNS. Direct FA examination of cells in conjunctival scrapings, CSF, or blood films is helpful in acute phases of illness. In chronic cases, it is usually unrewarding because antibody coating or elimination of viral antigen yields negative results with diagnostic immunofluorescence. ELISA has been used to detect viral antigen in serum and CSF of naturally and experimentally infected dogs.106,140,140 A test based on this methodology would be extremely valuable to the practitioner. In one study, MLV vaccination produced a false-positive result in testing for serum antigen.273 Viral antigen detected by fluorescent antibody (FA) or ELISA methods is difficult to find in body fluid specimens from dogs with neurologic distemper that lack or have recovered from systemic signs. More sensitive tests such as the polymerase chain reaction (PCR) might be more valuable in such instances. Immunochemical staining techniques, using fluorescent or peroxidase conjugates, can be performed on frozen sections of biopsy or necropsy specimens. CDV antigen detected in biopsy specimens of nasal mucosa, footpad epithelium, and haired skin of the dorsal neck region has been used consistently for the antemortem diagnosis of infection (Fig. 3-11).125 Tissues collected from dogs that died from distemper should include spleen, tonsils, lymph nodes, stomach, lung, duodenum, bladder, and brain. Animals dying of generalized infection frequently have abundant quantities of virus in these tissues. Immunochemical techniques can also be adapted to paraffin-embedded sections if special cold (4° C) ethanol (95%) fixation is used.
Canine Distemper
Etiology
Disease (Virus Abbreviation)
Natural Hosts
Experimental Infection
Canine distemper (CDV)
Seal (previously PDV-2)
Canidae (e.g., dog, fox, wolf, coyote)
Mustelidae (e.g., weasel, mink, skunk, badger, ferret)
Procyonidae (e.g., kinkajou, coati, red panda, raccoon)
Felidae (e.g., cat, lion, leopard, tiger)
Tayassuidae (peccary)
Nonhuman primates
Dog, mouse, rat, hamster, mink, pig, cat, nonhuman primate, ferret
Measles (MV)
Domestic: human
Wild: nonhuman primate
Macaque, marmoset, mouse, hamster, rat
Rinderpest (RPV)
Domestic: cattle, pig, goat, sheep
Rabbit, mouse, hamster, dog, ferret, rat, suslik
Wild: buffalo, eland, giraffe, kudu, warthog, wildebeest, banteng, black buck, gaur, nilgai, sambhar
Peste des petits ruminants (PPRV)
Domestic: goat, sheep
Wild: gazelle, ibex, gemsbok
Goat, cattle, pig, deer
Phocine distemper (PDV)
Seal
Dog, mink, seal
Dolphin morbillivirus (DMV)
Dolphin
Cattle, sheep, goat, dog
Porpoise morbillivirus (PMV)
Porpoise
Cattle, sheep, goat, dog
Equine morbillivirus (EMV; Hendra virus)
Domestic: horse, human
Wild: Pteropus bat
Cat
Porcine morbillivirus (Menangle virus)
Domestic: pig, human
Wild: Pteropus bat
No data
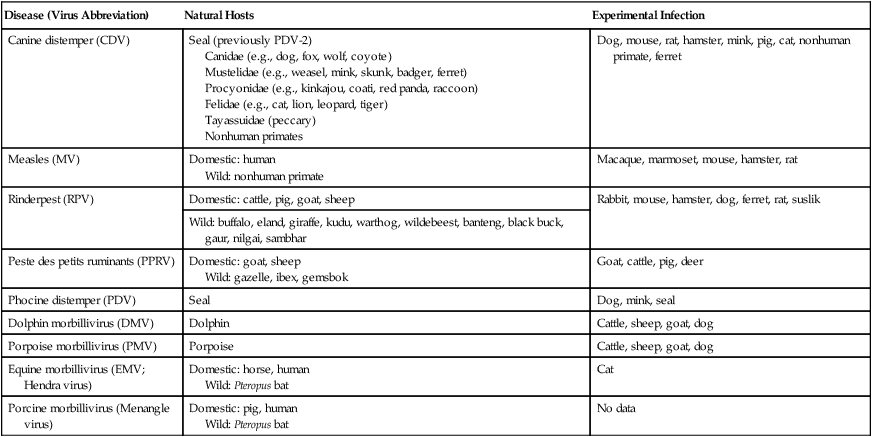
Components
Abbreviation
Molecular Weight (kDa)
Function
ENVELOPE
Hemagglutinin
H
76
Structural: viral attachment
Matrix protein
M
34
Structural: penetration
Fusion 1 protein
F1
40
Structural: penetration
Fusion 2 protein
F2
20–23
Structural: penetration
NUCLEAR
Large protein
L
180–200
Functional: polymerase complex
Polymerase
P
66
Functional: polymerase complex
Nucleocapsid
N
58
Structural: protects genome
Order
Description
Ailuridae
Lesser panda
Canidae
Coyote, dingo, raccoon dog, wolf, fox
Mustelidae
Ferret, marten, mink, otter, wolverine, badger
Mephitidae
Skunk
Procyonidae
Coati, kinkajou, raccoon
Ursidae
Bear, giant panda
Viverridae
Binturong, fossa, linsang, civet
Herpestidae
Mongoose, meerkat
Felidae (large species)
Cheetah, lion, jaguar, margay, ocelot
Virus
Date
Species
Location
Dolphin morbillivirus (DMV)
1990s
Striped dolphin
Mediterranean
Porpoise morbillivirus (PMV)
Late 1980s
Harbour porpoise
Northwestern Europe
Phocine distemper virus (PDV)
Late 1980s
Harbour seal, gray seal
Northwestern Europe
Canine distemper virus (CDV)
Late 1980s
2000
Baikal seal
Caspian seal
Siberia
Caspian Sea
Epidemiology
Pathogenesis
Systemic Infection
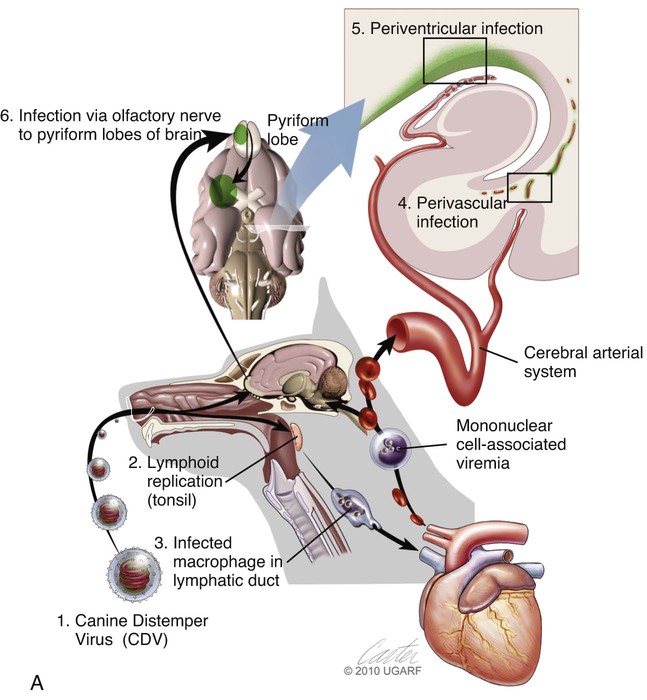
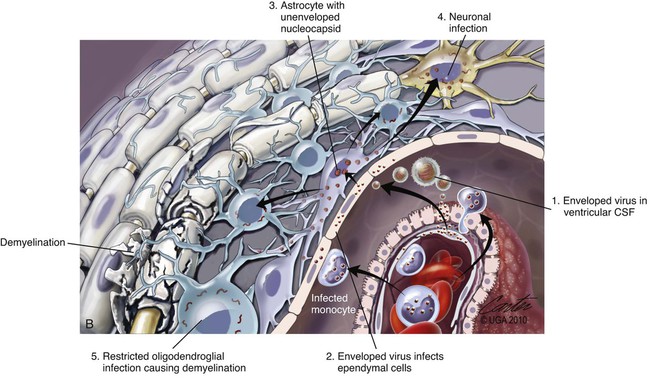
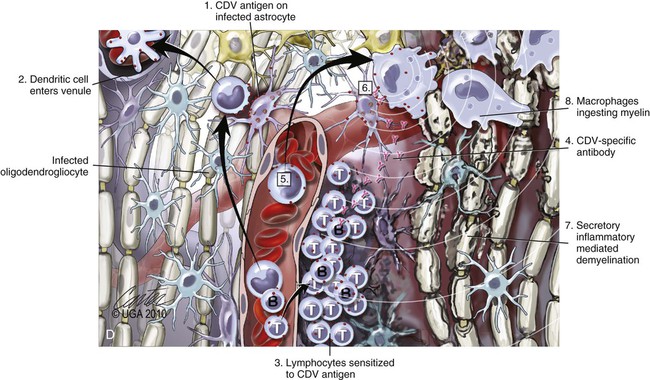
(A, B, and D Art by Kip Carter © 2010 University of Georgia Research Foundation Inc.)
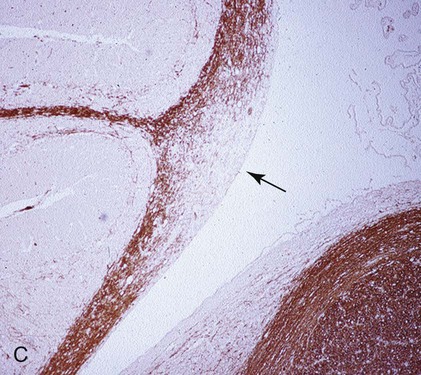
B, Acute noninflammatory demyelination. This lesion is typical of dogs with poor anti-CDV immunity. Large amounts of virus are present with minimal cellular infiltration. 1, Virus is shown entering the CNS by mononuclear cells in the choroid plexus where deposited virus replicates intracellularly in choroid plexus epithelial cells. After cytolysis, free virus enters the CSF, where it can spread to infect ependymal cells. 2, Alternatively, infected mononuclear cells enter the CSF through damaged vascular tissue and fuse with the ependymal cell surfaces. Once in ependymal cells, virus enters astrocytes by directly contacting astrocytic foot processes. 3, Viral replication in astrocytes results in the production of large numbers of naked (unenveloped) intracytoplasmic nucleocapsids. CDV nucleocapsids spread through astrocyte processes to adjacent astrocytes, oligodendroglial cells, and neurons. 4 and 5, In oligodendroglial cells, and sometimes in neurons, a restricted infection (transcription without translation) occurs and intracytoplasmic viral RNA accumulates, in relatively moderate amounts. Restricted infection in oligodendroglial cells leads to primary demyelination. C, Microphotograph of the type of lesion described in B with an acute noninflammatory demyelinating area (arrow). (Myelin-basic protein immunostain ×20).
D, Chronic perivascular cuffing with demyelination. For periods after viral infection, low quantities of unenveloped viral nucleocapsids remain in astrocytes with minimal cellular inflammatory response. Virus may also be found in oligodendrogliocytes and some neurons as untranslated viral RNA. 1, CDV antigens are expressed on the surface of astrocytes. Signaling lymphocyte activation molecule (SLAM) on the surface of immune cells such as dendritic cells interacts with this viral antigen. 2, Macrophages activated by the viral interaction transform to dendritic cells and emigrate from the CNS via venules and enter the systemic circulation, traveling to lymph nodes There they present antigen to T cells and cause CDV-specific activation of other immunocytes. 3, Lymphocytes, now sensitized to CDV antigens, enter the CNS via the blood vessel as a result of immune recruitment. Perivascular cuffing occurs with antigen-directed B cells and CD8+ and CD4+ T cells. 4, B cells synthesize and release CDV-specific antibody. 5, Similarly, sensitized mononuclear cells enter the perivascular space of the CNS with increased expression of cytokines (interleukin [IL]-6, IL-8, IL-12, and tumor necrosis factor-α) as a heightened immune response against the virus. 6, CDV-specific antibody from the B cells that has become bound to surface antigen of infected astrocytes, attracts activated monocyte/macrophages, displaying increased major histocompatibility complex class II and SLAM molecule expression. These bind to the Fc portion of this bound antibody, triggering antibody-dependent, cell-mediated cytotoxicity. In this process, activated macrophages secrete “highly active molecules” (e.g., matrix metalloproteinases, their associated inhibitors, and reactive oxygen radicals). These radiate into surrounding tissue, further damaging the blood-brain barrier, myelin, and other cellular elements in a “bystander” mechanism. 7, Myelin unraveling and fragmentation occur as a result of the inflammation and necrosis. 8, Macrophages ingest degenerating myelin and necrotic debris as a part of the clean-up process.
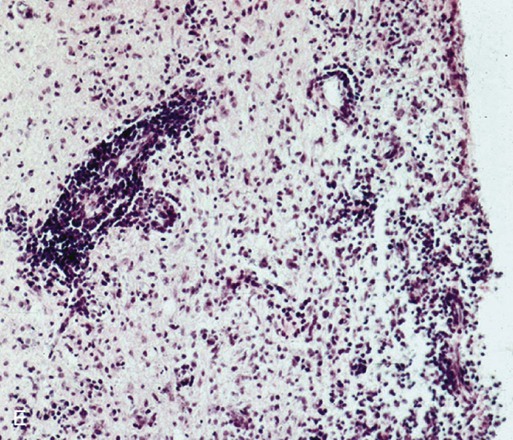
E, Microphotograph of chronic inflammatory demyelination, as described in D, in the periventricular area of the medulla. A perivascular cuff and surrounding tissue damage is present in the CNS of a CDV-infected dog (H&E stain, ×100).
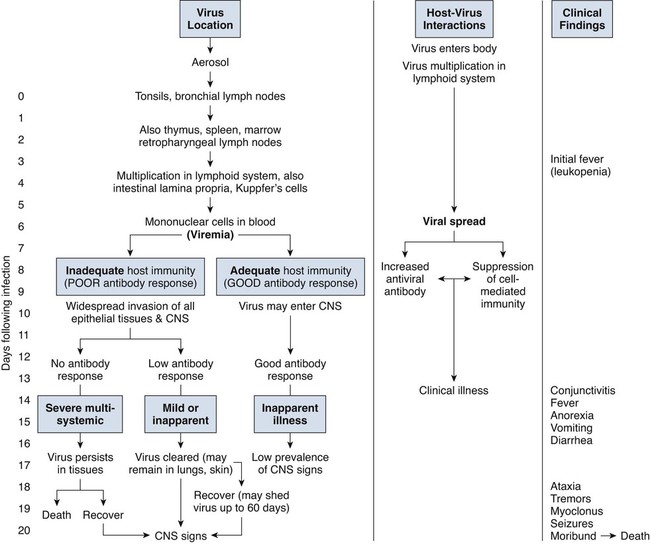
Central Nervous System Infection
Clinical Findings
Systemic Signs
Skin Lesions
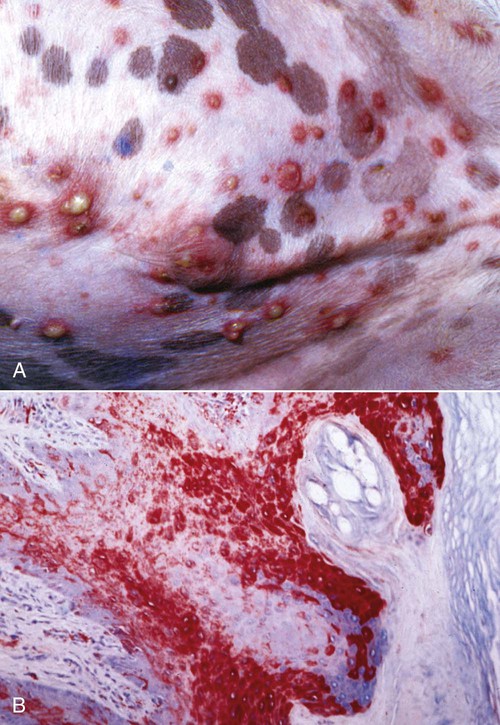
B, Microscopic view of skin from a dog with distemper. Large amounts of viral antigen in epidermal cells. Immunocytochemical stain for CDV nucleoprotein (red color) ×100.
Neurologic Signs
Transplacental Infection
Neonatal Infections
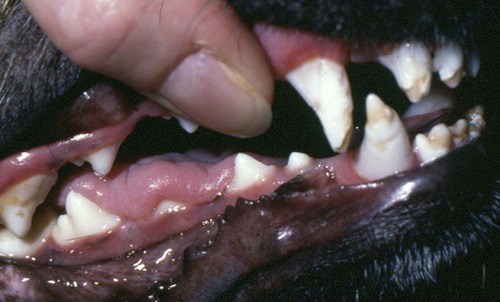
Bone Lesions
Rheumatoid Arthritis
Ocular Signs
Combined Infections
Diagnosis
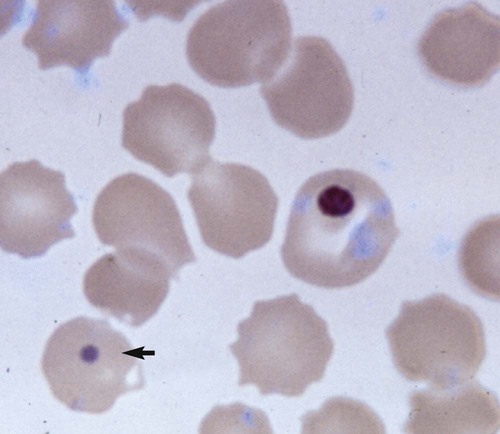
Clinical Laboratory Findings
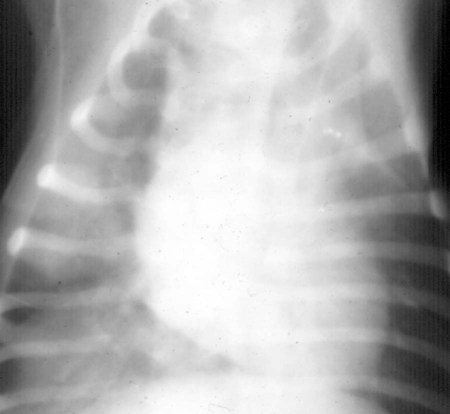
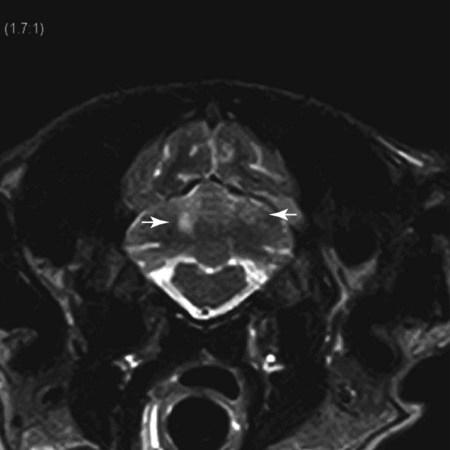
Magnetic Resonance
Cerebrospinal Fluid Analysis
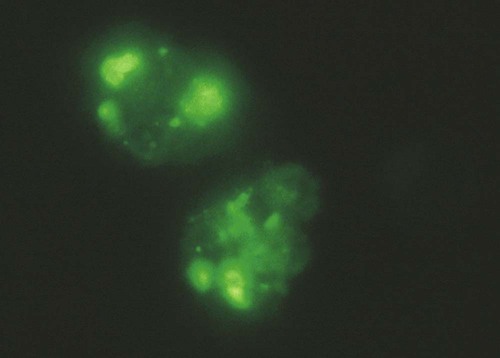
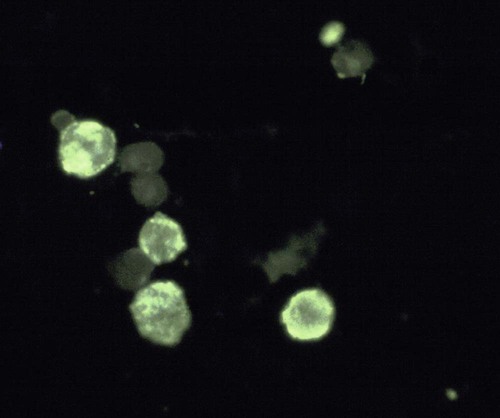
Immunocytology
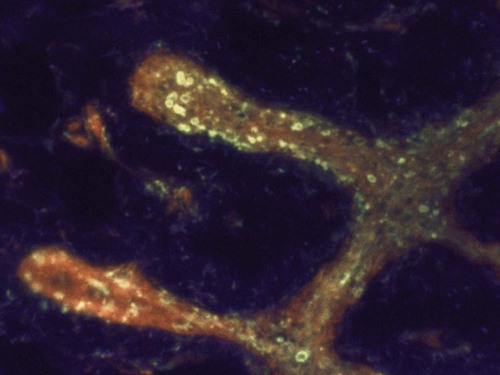
Immunohistochemistry
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Canine Distemper