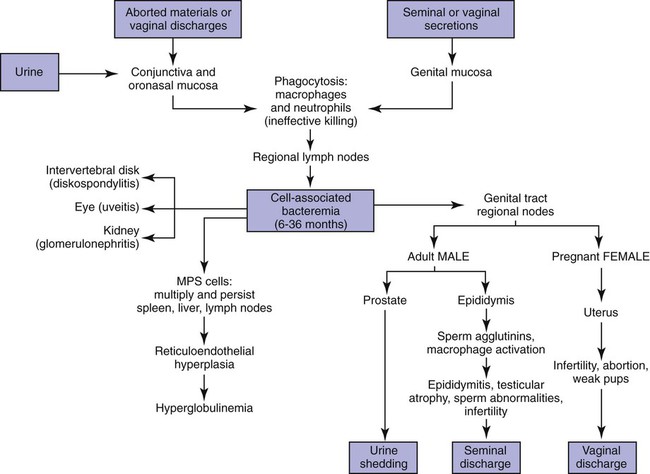
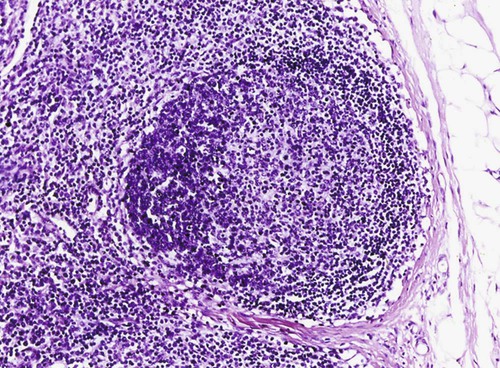
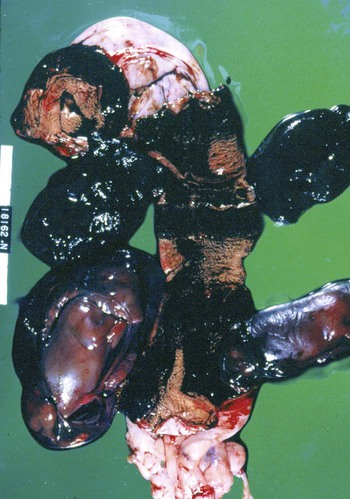
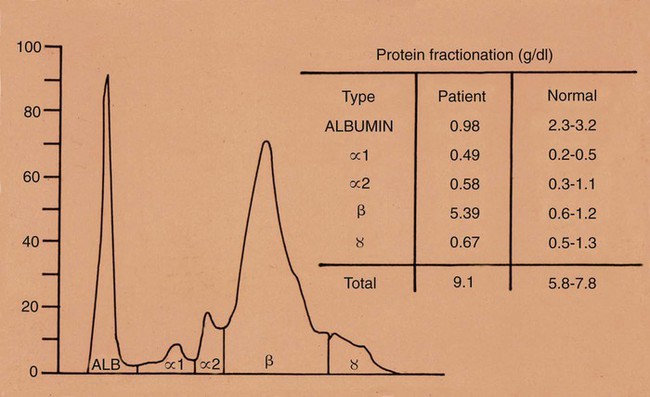
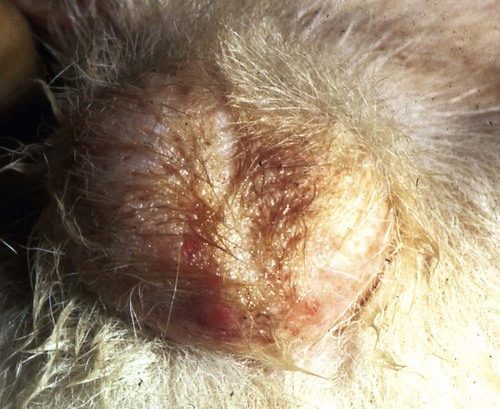
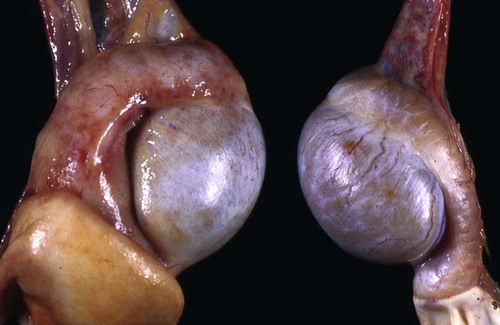
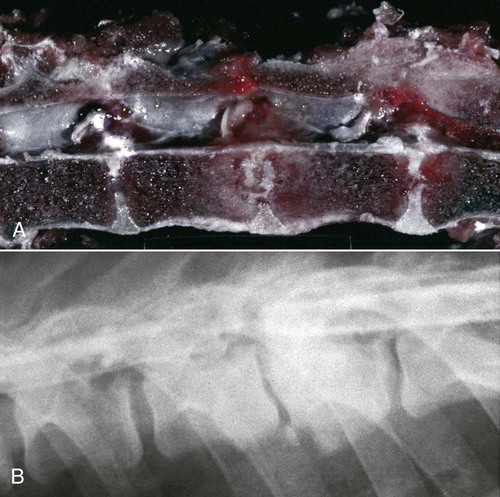
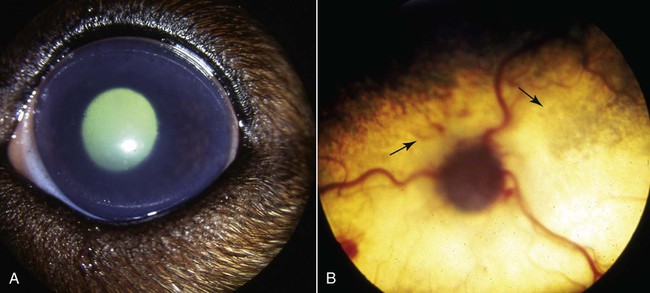
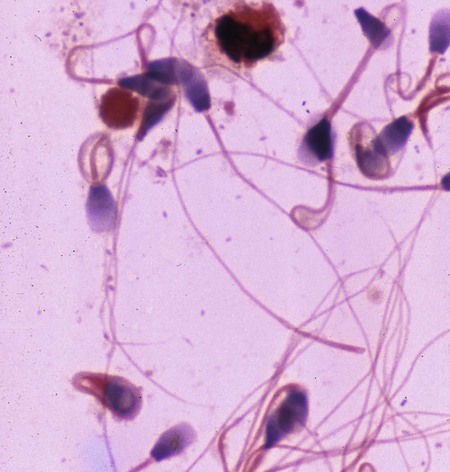
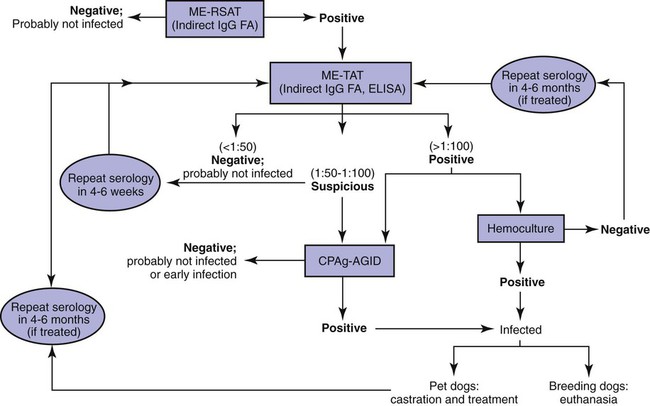
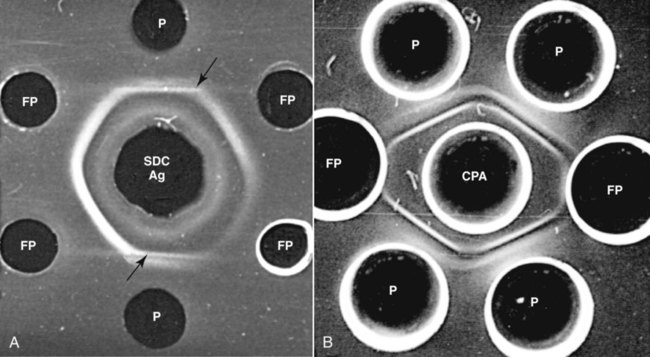
Brucella canis is a small (1.0 to 1.5 µm), gram-negative, aerobic, coccobacillary organism. Its rough colonial morphology, caused by lower levels of smooth lipopolysaccharide (LPS), and differences in biochemical and antigenic reactions distinguish it from other members of the genus Brucella. Unlike the smooth-morphology Brucella organisms that infect several domestic animal species, B. canis has a limited host range; only dogs and wild Canidae have been found to be susceptible. Cats can be infected experimentally, having a transient bacteremia, but are relatively resistant. Rabbits and nonhuman primates also have been found to be susceptible to experimental infections. No other animal species has developed significant agglutination titers. Human cases have been reported as a result of laboratory accidents and contact with infected dogs, but people appear to be relatively resistant (see Public Health Considerations). The Brucella genus is composed of six classical species based on host preference and genetic analysis42: Brucella abortus (cattle), Brucella canis (dogs), Brucella melitensis (goats, sheep), Brucella neotomae (rodents), Brucella ovis (sheep), and Brucella suis and its biovars (pigs, cattle, hares, rodents, and wild ungulates). Novel Brucella (Brucella ceti and Brucella pinnipedialis) have been isolated from cetaceans and pinnepeds19,77; however, these isolates appear to uniquely infect marine mammals. Other novel species have been isolated from people34 and mice.111 Although most Brucella, with the exception of the biovars of B. suis, show host preference, they can also infect other host species. Dogs are susceptible to infection with B. abortus9,41,41, and B. suis.13 Even attenuated vaccine strains of B. melitensis52 or B. abortus94 can infect dogs. Natural infection is thought to occur after ingestion of contaminated placentas and aborted fetuses from livestock. Dogs usually harbor organisms in the lymph nodes of their gastrointestinal tracts for extended periods. Dogs are not believed to be as important in the spread and maintenance of these non–B. canis infections as their respective farmanimal reservoirs. However, testing and removal of affected dogs from infected farms, in addition to affected livestock, are the optimal preventive measures for eradication. Seminal fluid and urine have been incriminated as sources of infection from males that harbor organisms in their prostates and epididymides. The rate of isolation of B. canis from the semen of infected dogs is usually high for the first 6 to 8 weeks postinoculation (PI). Intermittent shedding of the organism in low numbers has been noted for up to 60 weeks PI and may continue for at least 2 years. Urinary excretion begins between 4 to 8 weeks after the onset of bacteremia and continues for at least 3 months. Concentrations of 103 to 106 organisms/mL of urine have been found in males, with lesser numbers of bacteria in the urine of females. At one time, urine of infected males was thought to contain too few organisms to be infectious by the oronasal route; however, studies have demonstrated that B. canis can be transmitted from infected to uninfected mature male dogs after several weeks or months of close contact with infected dogs.25,113 The propensity of males to shed the organism in the urine is probably related to its localization in the prostate and epididymis, which are in close association with the urinary bladder. Neutering helps reduce this risk to a large extent. Alternative means of transmission occur less frequently under natural circumstances. Low numbers of organisms have been found in feces and nasal and ocular secretions. Transmission via fomites has been reported after vaginoscopy, blood transfusion, artificial insemination, and use of contaminated syringes. Brucellae are stable in the environment for up to 2 months in the presence of cooler temperatures and organic debris. Otherwise, B. canis is relatively short-lived outside the body and is readily inactivated by common disinfectants such as iodophors or quaternary ammonia compounds, and sunlight.46 The prevalence of infection varies according to the animal’s age, housing conditions, breed, and geographic location. Pet dogs in suburban environments have a lower prevalence compared with stray dogs in economically depressed areas, which may reflect increased population density and uncontrolled breeding of dogs. However, infection has also been maintained in breeding kennels producing pups for the pet trade and has been spread after the transport of infected dogs to new locations.20 A relatively low prevalence has been reported in the United States and Japan (range, 1% to 18%) compared with rates as high as 28% in Mexico and Peru.22 The southern United States appears to have a relatively higher (approximately 8%) prevalence of infection. Among breeds in this region, beagles and Labrador retrievers have a higher prevalence of infection, as do feral dogs. Determination of seroprevalence is strongly influenced by the method of testing used and its interpretation. Cases have also been identified in other countries in Central and South America and in Germany, Spain, Czechoslovakia, and Tunisia.27 The disease also appears to be prevalent in regions of the People’s Republic of China.55 Given the insidious nature of B. canis in dogs, isolated infections have been found in imported dogs, even entering countries such as the United Kingdom, where quarantine standards are very stringent.37 Dogs that are imported from endemic regions of the world should always be serologically screened for this infection, because outbreaks have occurred in countries that do not require testing for this disease for entry.3 The general sequence of events after infection by Brucella is summarized in Fig. 38-1. Brucellae lack classical virulence factors (exotoxins, capsules, cytolysins, plasmids, etc.), and they have an LPS that is less toxic than that of other gram-negative bacteria.112 These organisms are able to survive in macrophages by avoiding or suppressing host bactericidal mechanisms. Brucellae are phagocytized at contaminated mucosal sites by tissue macrophages and other phagocytic cells and transported to lymphatic and genital tract tissues where they multiply. Some of the organisms are able to persist intracellularly within mononuclear phagocytes.106 The organism inhibits tumor necrosis factor-α, disrupting the bactericidal activity of natural killer cells and macrophages.96 Brucellae evade phagolysosome fusion and replicate in a compartment within the cell’s endoplasmic reticulum.35,48 A leukocyte-associated bacteremia occurs beginning 1 to 4 weeks PI and can persist for 6 to 64 months. Generalized lymphoreticular hyperplasia (Fig. 38-2) and development of hyperglobulinemia occur during the course of infection. As with other intracellular parasites, cell-mediated immunity is probably the most important defense mechanism against B. canis. Studies in people have indicated decreased T-lymphocyte responsiveness to Brucella cytoplasmic proteins in more chronic infections.45 Persistent, nonprotective antibody titers are characteristic of such infections and appear to have little influence on the level of bacteremia or number of organisms in tissues. The greatest numbers of B. canis organisms are found in the lymph nodes, spleen, and tissues of gonadal steroid dependency. Although usually confined to mononuclear phagocytes, they may enter other cells such as placental epithelium. In fact, the uterus is not a favored site of growth in the nongravid or diestrual female. Inflammation of the epididymides and testes in males causes sperm leakage, which provokes the immune system to produce a complex of anti-sperm agglutinating antibodies and delayed-type hypersensitivity reactions against sperm that are unrelated to the antibodies against B. canis. The immune responses produced against spermatozoa contribute to the epididymitis, infertility, and eventual spermatogenic arrest seen in most infected males. B. canis, similar to other bloodborne bacteria, may localize in nonreproductive tissues such as the endarterial circulation of the intervertebral disk, causing diskospondylitis (see Chapter 85). Other tissues that filter bloodborne organisms or immune complexes may become involved, including the eye (anterior uveitis), kidney (glomerulopathy), and meninges (meningoencephalitis). Dogs that recover naturally have low or negative agglutination titers and yet are immune to reinfection, suggesting that protective immunity is cell mediated. Recovered dogs that were challenged orally or intravenously as long as 4 years after spontaneous recovery from experimental infection were completely immune.86 However, chronically infected dogs that were successfully treated with antibacterials were found to be fully susceptible to oronasal challenge 12 weeks after treatment had been halted. Immunosuppression with glucocorticoids and antilymphocyte serum appears to increase the susceptibility of dogs to initial infection, but it does not augment the severity of the disease or alter the course of infection in experimentally infected dogs. Overt clinical signs usually involve reproductive disturbances in sexually mature animals. Bitches usually abort dead pups between 45 and 60 days of gestation but show no other clinical signs. Pups are usually partially autolyzed, with subcutaneous edema and congestion and hemorrhage of the abdominal subcutaneous region (Fig. 38-3). Moderate quantities of serosanguineous peritoneal effusions are found. Their appearance suggests fetal death in utero some time before abortion. Decomposed fetuses are not usually found because the bitch ingests them. Abortion is characterized by a brown or greenish-gray vaginal discharge that lasts for 1 to 6 weeks. Brucellosis should be suspected under any circumstance when apparently healthy bitches abort 2 weeks before term. Although abortion of dead puppies is the primary clinical sign reported with brucellosis, conception failures could occur at any time after breeding. In utero death with fetal resorption or abortion and ingestion of fetuses may be suspected if a bitch fails to conceive after an apparently successful mating. Embryonic death may occur as early as 20 days after mating, although most conception failures are actually undetected abortions. Less commonly, bitches may carry infected puppies to term and whelp both live and dead puppies within a single litter. Most pups that are born alive will die within a few hours or days, but those that survive or that are infected as neonates usually have generalized peripheral lymphadenomegaly as the primary clinical manifestation of disease until they reach sexual maturity. Such puppies usually have persistent hyperglobulinemia (Fig. 38-4), and some may have transient fever, leukocytosis, or seizures as the systemic manifestations of their infections. Because of the prominent testicular abnormalities, male dogs are presented for examination more often than are females, even though impairment of male reproductive performance often is less noticed. Microscopic epididymal inflammation usually develops beginning 5 weeks after infection with a gradual progression of signs. Males appear to be in good health but may have an enlarged scrotum because of accumulation of serosanguineous fluid in the tunica. Scrotal dermatitis is the result of constant licking and secondary infection with nonhemolytic staphylococci (Fig. 38-5). A major cause of testicular swelling is enlargement of the tail of the epididymis (Fig. 38-6); orchitis and primary testicular enlargement are rarely apparent. In fact, chronically infected males usually develop unilateral or bilateral testicular atrophy. A decreased volume of ejaculate without loss of libido is usually present. Acute pain is not usually evident on scrotal or testicular palpation, but discomfort may be seen at the time of ejaculation. There is a variable degree of male infertility in all cases. Nonreproductive abnormalities can also occur. Splenomegaly may accompany the diffuse lymphadenomegaly in some dogs. Dogs with diskospondylitis initially experience spinal pain and later paresis and ataxia if spinal cord compression develops (Fig. 38-7). Osteomyelitis or polyarthritis of the appendicular skeleton causes lameness of the affected limb. Meningoencephalitis has been reported after experimental and natural infections; however, neurobrucellosis, as often occurs in people, is uncommon in dogs.84 One of the authors (CEG) observed a male with confirmed B. canis infection and suppurative meningoencephalitis that had behavioral changes, anisocoria, ataxia, hyperesthesia, head tilt, and circling. Neurologic signs began within 3 weeks after the dog’s first breeding. Chronic multifocal pyogranulomatous dermatitis that resembled lick granuloma lesions has also been reported in an infected dog, but a direct causal relationship was not established. Ocular lesions include anterior uveitis, secondary glaucoma, hyphema, retinal detachment, chorioretinitis, optic neuritis vitreal haze, endophthalmitis with secondary glaucoma or phthisis bulbi, and corneal edema with opacification (Fig. 38-8).122 Residual chorioretinal hyperreflective scarring may be observed after treatment of dogs with uveitis.72,122 See Chapter 92 for a review of ocular brucellosis. Semen abnormalities, evident by 5 weeks PI, become pronounced by 8 weeks PI. Abnormalities include immature sperm, deformed acrosomes, swollen midpieces, and retained protoplasmic droplets. By week 15 PI, bent tails, detached heads, and head-to-head agglutination are seen. Large aggregates of inflammatory cells, usually consisting of neutrophils, surround adherent macrophages containing phagocytized sperm (Fig. 38-9). More than 90% of the sperm are abnormal by 20 weeks PI. Aspermia without inflammatory cells corresponds with the development of bilateral testicular atrophy. Semen morphology should always be evaluated in dogs with infertility because of the obvious abnormalities that occur with brucellosis. Serologic testing is the most frequently used diagnostic method for detecting canine brucellosis. The tests incorporating cell-wall antigens are subject to considerable interpretive error because LPS antigens of several bacterial species cross-react with B. canis.80,82 The problem of false-positive cross-reactions, therefore, is more common than that of false-negative reactions. All sera should be free of hemolysis because hemoglobin causes false-positive agglutination of the tube test antigen. Single titer testing may be prone to false-positive or false-negative results. Serologic test results, using any method, are often negative during the first 3 to 4 weeks PI despite the presence of bacteremia by 2 weeks PI. For this reason, newly acquired animals should be tested sequentially at least twice at 30-day intervals before introduction into a breeding kennel. Titers by any method are usually positive by 8 to 12 weeks after infection. Low or intermediate titers may mean previous disease or very recent infection, and testing should be repeated or attempts made to isolate the organism by hemoculture. Males may harbor the organism in the prostate glands and epididymides for extended periods after bacteremia ceases. In such instances, agglutination titers may decline naturally or as a result of treatment, yielding false-negative test results.125,130 However, false-positive results also occur because serologic titers may remain positive for up to 36 months in some dogs after they become abacteremic. Chronically infected bitches may have diagnostically equivocal antibody titers and negative blood cultures. In females, recrudescence of bacteremia and increased antibody titers develop during proestrus, estrus, pregnancy, or abortion. These are the most reliable times to screen bitches for infection. Antibacterial therapy may suppress bacteremia and the associated serologic response, possibly contributing to false-negative serology and failure to isolate the organism from infected dogs. Antibacterials should not be given until diagnostic tests have been completed. Tetracycline drugs cause a reduction in bacteremia and a corresponding decrease in antibody titer that may rebound after treatment is discontinued because tetracyclines are not bactericidal. Table 38-1 compares the serologic tests described next. Consult Web Appendices 5 and 6 for information concerning commercially available testing. Serologic tests should be evaluated in light of clinical findings in the dog being evaluated (Fig. 38-10). A single high agglutination titer to B. canis usually indicates active infection, but this should be substantiated by additional tests. Dogs that are asymptomatic but have positive results on agglutination procedures should never be condemned as infected until blood culture results or the more specific cytoplasmic protein antigen (CPAg)–agar gel immunodiffusion (AGID) test results confirm the positive findings. Antibodies to cell wall LPS antigens of Brucella cross-react with those of the same and some other species of Brucella and of other genera of bacteria such as those in the α-proteobacteria. Therefore, all commercially available tests, with exception of the CPAg-AGID method, measure antibodies to LPS antigens and may give nonspecific test results. If polymerase chain reaction (PCR) is done with precision and proper controls, positive results may be considered for confirmation as this method can be highly specific; however, the sensitivity varies according to the specimen tested (see Genetic Detection). TABLE 38-1 Comparison of Serologic Procedures for Canine Brucellosis ELISA, Enzyme-linked immunosorbent assay; FA, fluorescent antibody; LPS, lipopolysaccharide; PCR, polymerase chain reaction; PI, postinoculation. aFirst significant titer to appear. Data based on adult dog. The 2-mercaptoethanol (ME) rapid slide agglutination test (RSAT; ME-RSAT) is preferred as an in-office screening procedure because it is inexpensive, rapid, and sensitive; it also detects antibodies early (D-Tec CB, Synbiotics, San Diego). A 99% correlation exits between a negative test and lack of infection. The test kit uses rose Bengal–stained B. ovis because its growth is less mucoid than B. canis. B. canis cross-reacts with all rough Brucella and with certain other bacterial species, such as mucoid strains of Pseudomonas aeruginosa and Staphylococcus spp., Bordetella bronchiseptica, and Actinobacillus equuli, to which serum antibodies may be present. Most causes of cross-reactions, however, have not been identified. Some breeds (e.g., Irish wolfhounds and Old English sheepdogs) have an exceptionally high false-positive test result rate for unknown reasons.27 Tests such as the RSAT using the rough B. ovis strain may not detect antibody from dogs infected with smooth strains such as B. suis.39a The ME-RSAT substantially reduces false-positive reactions by eliminating less specifically reacting IgM antibodies. 2-ME is labile and must be kept in a dark, tightly stoppered bottle at 4° C; use of inactivated ME gives false-positive results. A modification of the RSAT, using a less mucoid variant of B. canis, further reduces the rate of false-positive results.80,82,82 The ME-RSAT with the B. canis M-antigen therefore can be employed for confirmation of positive results of screening tests. Lack of standardized reagents or methods makes absolute ME-TAT titer comparisons difficult. Nevertheless, a titer of 50 may indicate either very early (less than 3 weeks) or recovering infections. Titers of 50 to 100 should be considered suspect for infection; titers of 200 or greater are highly presumptive of active infection because they often correlate with positive hemocultures. However, sera from noninfected dogs have been found to have titers from 50 to 100 and occasionally higher. As a semiquantitative test, the ME-TAT is best in control programs to quantitate serologic responses of dogs over months to determine whether infection has been eliminated with chemotherapy (see Therapy). The TAT has been adapted for a microtiter plate system that reduces the amount of reagents and increases the number of specimens that can be processed within a given time period.66 This test, using selected antigens, has been developed as both a sensitive and specific procedure for the serodiagnosis of canine brucellosis. With regard to sensitivity, cell wall AGID tests reveal precipitins in the sera of infected dogs only after 5 to 10 weeks PI; however, antibodies persist for several weeks or months after the bacteremia has ceased. The AGID test, using cell wall (somatic) LPS antigen, has the same problems of cross-reactions as do agglutination tests, but positive sera may be distinguished from false-positive sera by a distinct precipitin band for B. canis (Fig. 38-11, A). However, the somatic LPS antigen is seldom employed in the diagnostic regimen because of the lack of standardization and difficulty of interpreting test results. The CPAg-AGID test, which is highly specific for Brucella spp., uses internal cytoplasmic antigens liberated by sonication of B. canis (Fig. 38-11, B) or B. abortus.11 Cytoplasmic antigens are shared only among Brucella organisms and will not react with antibodies to other genera of bacteria as seen with agglutination testing. The CPAg test specifically detects precipitins in the sera from dogs after other tests have become equivocal or even negative. It should be used as a confirmatory test after other serologic methods. A high proportion of sera sent to a reference laboratory for CPAg-AGID testing that are positive or suspect in the commercial ME-RSAT or the ME-TAT are found to be false-positive reactions.27 A disadvantage of tests with CPAg is the high proportion of false-negative results.59 There is a longer period (8 to 12 weeks) than with other AGID antigen tests between infection and the presence of detectable precipitins26 (see Table 38-1). False-positive results can occur as one or more precipitin lines may persist up to 12 months, and occasionally up to 36 months, after bacteremia has ceased.82 In contrast to LPS antigens, both rough and smooth Brucella share the internal protein antigens. Thus the possibility of infection with other Brucella (e.g., B. suis or B. abortus) must be considered when CPAg is used. False-positive reactions have not been observed with non-Brucella species. False-negative AGID results can occur in some dogs, presumably with early infections, whose sera are RSAT positive and AGID negative. Rarely, in chronic infections, B. canis may replicate in sequestered sites hidden from the immune system, and antibody levels decline, leading to false-negative AGID results.130 Hemoculture or PCR testing, if positive, is the only definitive way to resolve these differences (see Bacterial Isolation and Genetic Detection, later).
Canine Brucellosis
Etiology and Epidemiology
Pathogenesis
Clinical Findings
Diagnosis
Clinical Laboratory Findings
Semen Examination
Serologic Testing
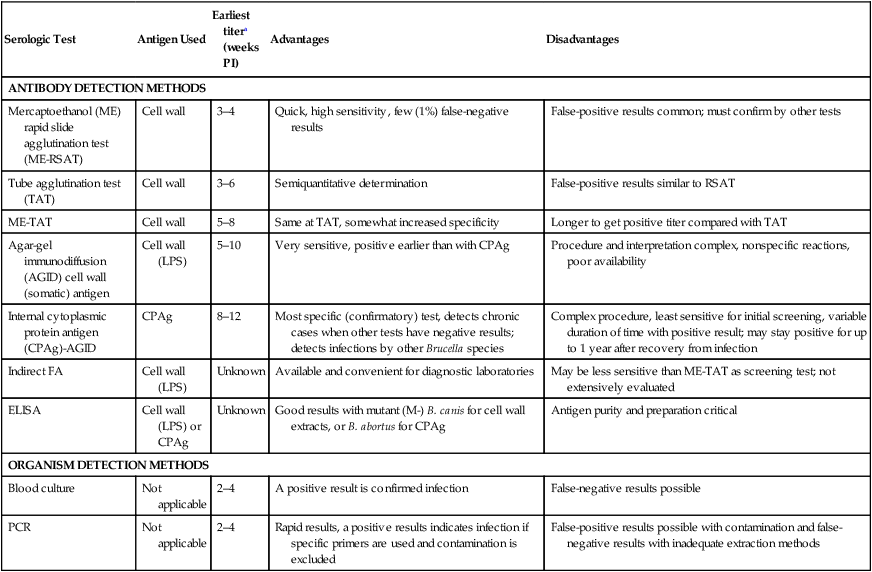
Serologic Test
Antigen Used
Earliest titera (weeks PI)
Advantages
Disadvantages
ANTIBODY DETECTION METHODS
Mercaptoethanol (ME) rapid slide agglutination test (ME-RSAT)
Cell wall
3–4
Quick, high sensitivity, few (1%) false-negative results
False-positive results common; must confirm by other tests
Tube agglutination test (TAT)
Cell wall
3–6
Semiquantitative determination
False-positive results similar to RSAT
ME-TAT
Cell wall
5–8
Same at TAT, somewhat increased specificity
Longer to get positive titer compared with TAT
Agar-gel immunodiffusion (AGID) cell wall (somatic) antigen
Cell wall (LPS)
5–10
Very sensitive, positive earlier than with CPAg
Procedure and interpretation complex, nonspecific reactions, poor availability
Internal cytoplasmic protein antigen (CPAg)-AGID
CPAg
8–12
Most specific (confirmatory) test, detects chronic cases when other tests have negative results; detects infections by other Brucella species
Complex procedure, least sensitive for initial screening, variable duration of time with positive result; may stay positive for up to 1 year after recovery from infection
Indirect FA
Cell wall (LPS)
Unknown
Available and convenient for diagnostic laboratories
May be less sensitive than ME-TAT as screening test; not extensively evaluated
ELISA
Cell wall (LPS) or CPAg
Unknown
Good results with mutant (M-) B. canis for cell wall extracts, or B. abortus for CPAg
Antigen purity and preparation critical
ORGANISM DETECTION METHODS
Blood culture
Not applicable
2–4
A positive result is confirmed infection
False-negative results possible
PCR
Not applicable
2–4
Rapid results, a positive results indicates infection if specific primers are used and contamination is excluded
False-positive results possible with contamination and false-negative results with inadequate extraction methods
Rapid Slide Agglutination Test
Tube Agglutination Test
Agar Gel Immunodiffusion Test
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine