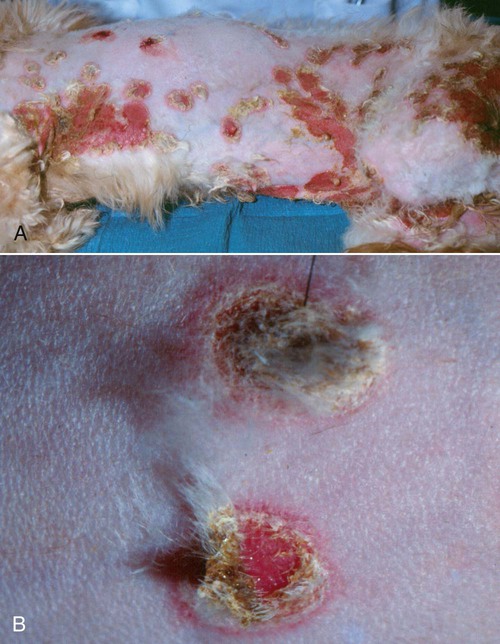
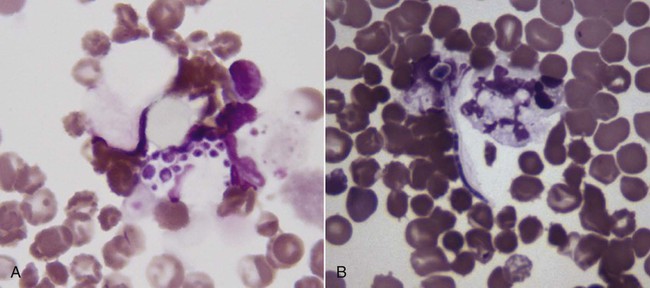
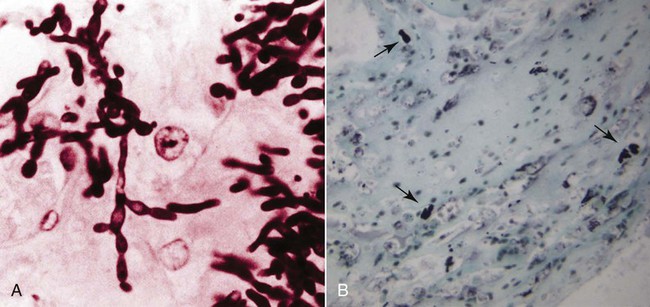
The genus Candida (phylum Ascomycota, class Saccharomycetes) is composed of more than 150 species of budding yeasts, including the historic genus Torulopsis.32,88 Candida spp. reproduce by asexual budding, with a parasexual stage that allows chromosomal reduction from a diploid to a haploid state without meiotic division, followed by recombination.66 Candida usually have a budding, ovate appearance, but may also appear filamentous, particularly after adherence to mucosal and catheter surfaces during biofilm formation or when tissue invasion has occurred.9,83 Switching between morphologic types occurs in response to various environmental factors, including changes in carbon dioxide concentration or pH, contact with a solid surface, and in response to biomolecules associated with oxidative stress.21,101 Candida albicans is the most common Candida sp. isolated from people and animals, although several other species have been identified in both asymptomatic and diseased dogs and cats.14,41,77,88 Candida spp., particularly C. albicans, are for the most part commensal organisms, with only occasional isolation from environmental sources such as soil, water, or plants.4 Organisms are acquired by neonates as they pass through the dam’s birth canal and become permanent, usually nonpathogenic, residents of the oral, gastrointestinal (GI), upper respiratory, and genital mucosa.32 Candida spp. do not appear to be host species specific, with few detectable genetic differences noted in human versus domestic or wild animal isolates.23 Multiple Candida species have been isolated from duodenal, mucocutaneous, or vaginal swab samples from healthy dogs, although results suggest that frequency of colonization and predominant species may vary with estrous cycle stage or in the presence of concurrent GI disease.14,18,18 Types, numbers, and location of Candida spp. and other yeasts inhabiting the skin of dogs varied between those that were clinically healthy or were suffering from seborrheic dermatitis.105 Candida spp. can also be isolated from the oropharyngeal mucosa and coats of approximately 20% of cats, a factor that is not associated with retroviral infection.84 A limited number of studies have investigated the relative prevalence of Candida spp. infections in ill dogs and cats. Fewer than 1% of urinary tract infections (UTIs) in dogs and cats and less than 2% of recurrent or resistant UTIs in dogs are due to Candida spp. However, in contrast to bacterial cystitis, Candida infections are commonly associated with clearly identifiable predisposing causes.67,77,77 Candida spp. are isolated at a high frequency from the foot pads, nail beds, noses, and perianal regions of bull terriers with lethal acrodermatitis,55 and from 5% to 10% of dogs with otitis externa,8,13,56,72 but colonization or infection has not been investigated in patients with more common, diffuse cutaneous diseases. C. albicans is the most common disease-causing species in people, dogs, and cats, but surveys and case reports have demonstrated an increase in non–C. albicans species isolation from both healthy and ill animals.41,77,77 This epidemiologic shift toward non–C. albicans species has also been documented in people and is hypothesized to be due to an increase in the number of chronically immunosuppressed patients.17 Widespread use of fluconazole (FCZ) may also be contributing to the increase in previously less-encountered species. C. albicans isolates are usually susceptible to FCZ, whereas other species are more likely to demonstrate resistance to azole drugs, and therefore overgrowth occurs after elimination of the predominant C. albicans resident microflora. The epidemiology of candidemia and candiduria in people varies slightly depending on the Candida species, but the overall pattern of disease is nevertheless relatively consistent. Nosocomial infections occur more frequently than spontaneous infections in outpatients, with more than 50% of candidal infections occurring in intensive care unit patients, and Candida spp. are among the top five organisms identified in results of blood cultures.57 Indwelling urinary catheters, central venous catheters, antibacterial administration, diabetes mellitus, immunosuppressive drugs, neoplasia and chemotherapy, and increased duration of hospitalization are consistently identified as predisposing factors for all Candida spp. infections (including Candida rugosa, Candida glabrata, and Candida parapsilosis).11,25,57,59,95 Similar epidemiologic studies have not been performed in dogs and cats, but results of retrospective studies and case reports have indicated similar predisposing factors as in people.41,77 Antibacterials with efficacy against anaerobic bacteria predispose mice to GI candidal overgrowth more so than antimicrobials with alternative spectra of activity, presumptively by critically altering the commensal enteric bacterial microflora to allow preferential adhesion and proliferation of yeasts.44 Pathogenicity of normally commensal Candida spp. is likely due to a combination of disruption of systemic or local host defenses and expression of virulence factors by the host’s previously nonpathogenic yeast microflora. As already mentioned, systemic immunocompromise due to glucocorticoid excess, diabetes mellitus, or cytotoxic chemotherapy is common in human and veterinary patients with candidiasis.41,77 Local immune compromise presumptively occurs after antibacterial- or concurrent disease-induced alterations in GI microflora and in patients with chronic dermatopathies after disruption of the stratum corneum, development of chronically moist lesions, and due to implantation of organisms during scratching.44,55 Compromised local immunity may be particularly important in establishment of Candida lower UTIs, with indwelling urinary catheters, permanent urinary tract stomata (e.g., perineal urethrostomies), bladder neoplasia, and diabetes mellitus present in approximately 50% of affected dogs and cats.41,77 Adherence and persistence of many Candida species is facilitated by biofilm formation. Candidal biofilms consist of extracellular polymeric polysaccharides and organism aggregates that form a dense network of budding, pseudohyphal, and hyphal yeast forms with increased expression of adhesion molecules.9,83 Biofilm formation is likely required for colonization of indwelling urinary and long-term intravenous catheters, and of the bladder mucosa in patients with persistent candiduria. Biofilm growth is enhanced by increased flow rate and availability of nutrients over the adherent surface (e.g., in polyuric or glucosuric patients), and in vitro, latex urinary catheters induce more extensive biofilm production than catheters made of alternative materials.40,83 Biofilm formation also increases adherent Candida resistance to antifungal drugs when compared to nonadherent Candida because of the slower growth rate of yeast cells at the center of the biofilm matrix, making them less susceptible to growth-cycle dependent antifungal agents; the physical barrier provided by the extracellular polysaccharides; and the upregulation of drug efflux pumps by biofilm yeast cells.83 Candida spp. adhere to epithelia via integrin-like and carbohydrate adhesion molecules.31,96 Their superficial penetration into epithelium leads to deeper tissue invasion via denuding of adjacent endothelium and hyphal extension.10,31 It is unclear whether adhesion-associated changes in Candida spp. gene expression noted in vitro are true virulence factors or if they are nonspecific genus-wide responses. However, proteins known to be critical for establishment and persistence of Candida infection include secreted hydrolases, which ensure nutrient availability and promote adhesion and penetration; stress genes, which reduce injury by reactive oxygen species; and heat shock proteins.15 Resistance to Candida spp. infection is via TH1-induced cell-mediated immunity.10 Recruitment and activation of monocytes and neutrophils occurs via tumor necrosis factor-α, interleukin-6, and granulocyte-macrophage colony-stimulating factor release by cells of the innate immune system and resident tissue macrophages; complement activation and appropriate TH1-directed modulation of the immune response is highly dependent on yeast binding by host mannan-binding lectin.10,16 Although anti–Candida spp. antibodies occur in infected people and laboratory animals, they are insufficient to confer protection alone.10 Infection may persist via Candida spp. binding to Toll-like receptor-2 and subsequent release of interleukin-10, a TH2 phenotype-inducing cytokine, or by generation of inhibitory regulatory T cells rather than activated effector T cells.10,65 Candidiasis in dogs and cats most commonly results in cutaneous or lower UTIs, with GI, ocular, and systemic infections occurring less frequently (Box 63-1). Cutaneous candidiasis may be diagnosed in systemically immunosuppressed animals or patients with cutaneous diseases such as pemphigus foliaceus, acrodermatitis, or hypersensitivity reactions that disrupt local dermal or mucosal immunity; however, cutaneous overgrowth has also been reported without identifiable predisposing causes.* Infections may be localized, particularly to the inguinal region or the external ear,8,13,56,63,72 but most infections reported in dogs have diffuse lesions. Typical clinical signs include erythema, scales, and crusts with underlying eroded skin and nonhealing ulcers, with variable pruritis (Fig. 63-1).62,63 Dogs and cats with candidal UTIs may show typical signs of lower urinary tract disease (i.e., dysuria, pollakiuria, micro- or macroscopic hematuria), or show no signs of urinary dysfunctions.† On rare occasions flocculent, yellow-white debris and mucous may be grossly visible in urine. Animals with diabetes mellitus or with chronic exogenous or endogenous glucocorticoid excess appear to be more likely to have no clinical signs referable to urinary infections, as are dogs and cats with previously diagnosed but unsuccessfully treated Candida spp. UTIs. GI overgrowth with Candida spp. may occur secondarily to chronic antibacterial administration or concurrent enteropathies (i.e., Parvovirus infection or food hypersensitivity), resulting in small- or large-bowel diarrhea, and in severe cases eventual dissemination and systemic illness may occur.2,58,68,81,102 C. albicans has been isolated from the oral cavities of slightly more than 10% of dogs with clinical signs of stomatitis, including halitosis, oral bleeding, and gingivitis; however, whether isolates played an etiologic role in development of clinical signs is unknown.39 Disseminated Candida spp. infections have been reported after unsuccessfully treated pulmonary, GI, or UTIs, and after intestinal perforation and candidal peritonitis.‡ Clinical signs in these patients reflect the organs involved, but may include neurologic signs secondary to central nervous system infection, general lymphadenomegaly with fistulous tracts, or signs referable to osteomyelitis, renal failure, myocarditis or pericarditis, or disseminated intravascular coagulation. In addition, fever, leukocytosis, or fungemic shock (erythemic mucous membranes, hypotension, bradycardia) may also be noted.19,34,35,46,61 Isolated reports of ocular involvement including candidal conjunctivitis, panuveitis, or endophthalmitis are presumptive sequelae to infected local wounds such as corneal ulcers, or after systemic dissemination.29,49 Typically, Candida spp. infections are first suspected after visualization of fungal elements in skin scrapings, fecal smears, or in urine sediment.34,41,58,63,77 Organisms isolated from skin or feces are usually budding, whereas yeasts shed in urine may be budding or filamentous.9 Fungal elements may be noted on routine urine sediment examination, but in some animals, particularly after partial resolution of infection with antifungal treatment, modified Wright’s staining of standard or Cytospin urine sediment preparations may be required because of the lower number of organisms (Fig. 63-2). Calcofluor white or lactophenol cotton blue staining of urine sediment may be used by some laboratories when fungal elements are suspected. Specific fungal culture is required to confirm that the microscopically visualized fungal elements are in fact Candida spp. and to identify the exact species. Candida spp. readily grow on blood agar within 48 hours, which is the standard time that aerobic bacterial cultures are monitored, and thus candidal infections are often detected even when specific fungal culture was not performed.32,77 However, in those cases when candiduria is suspected or has been previously documented, a yeast or fungal culture should be requested. Fungal culture specimens are inoculated onto Sabouraud’s medium, which allows morphologic identification of genus and species based on growth characteristics. Candida spp. colonies are typically white to cream-colored and composed of 5- to 7-µm diameter rounded yeast cells, pseudohyphae, and 3- to 5-µm-wide septate hyphae. However, because Candida spp. are normal inhabitants of dog and cat mucosa, positive cultures should be interpreted in the context of clinical signs and the concentration of yeast elements noted on cytologic examination. Additionally, evidence of tissue invasion should be present within histopathologic specimens. In patients where low growth of Candida spp. is noted on urine culture even when fungal elements were not seen on sediment examination, infections can be confirmed by positive results on repeat testing, because transient candiduria is very rare. Alternative diagnostic tests have been investigated as more rapid methods for diagnosis of candidal infections. Candida spp.–specific polymerase chain reaction of urine, blood, or skin samples has been evaluated in dogs and people and may be faster and more accurate than traditional microbiologic techniques or commercial test kits; however, the ease of diagnosis by conventional diagnostic methods and limited availability of laboratories offering Candida-specific polymerase chain reaction have not allowed its routine use.* Serologic testing for C. albicans mannan protein antigen and antibodies and urine D-/L-arabinitol ratios have also been investigated as possible diagnostic tests in people with candiduria, but similar studies are not available in veterinary patients.33,36,91,92,100 Gross necropsy findings from dogs and cats with systemic candidiasis typically include scattered white to yellow caseous plaques on the mucosal surfaces of the GI tract and serosal surfaces of other organs, particularly the spleen, kidneys, pancreas, lymph nodes, and heart; similarly colored plaques or nodules may be noted on cut surfaces.† The mucosal surfaces of bladders from animals with candidal cystitis may have visible biofilms, with occasional fungal bezoars within the bladder lumen or renal pelves if infection has extended proximally. Hematoxylin and eosin staining findings are variable degrees of predominantly granulomatous inflammation with associated necrosis in affected tissues.7,12,17,18 Budding yeast, pseudohyphae, and true hyphae are often noted within these foci of inflammation, but alternative stains (e.g., periodic acid–Schiff [PAS]) may be required when low numbers of organisms are present (Fig. 63-3). Ocular infections may result in hyphae within the vitreous or extending across the corneal stroma with associated retinal inflammation.29,49 However, regardless of the site or number of yeastlike organisms noted, definitive identification as Candida spp. requires culture, as the cytologic and histologic appearance of this genus is not sufficiently unique to differentiate it from other opportunistic or pathogenic fungi. In some cases, the negatively staining exterior fungal wall has resulted in incorrect preliminary diagnoses as disparate as systemic Histoplasma capsulatum or Prototheca spp. infection.
Candidiasis and Rhodotorulosis
Candidiasis
Etiology
Epidemiology
Pathogenesis
Clinical Findings
Diagnosis
Pathologic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Candidiasis and Rhodotorulosis