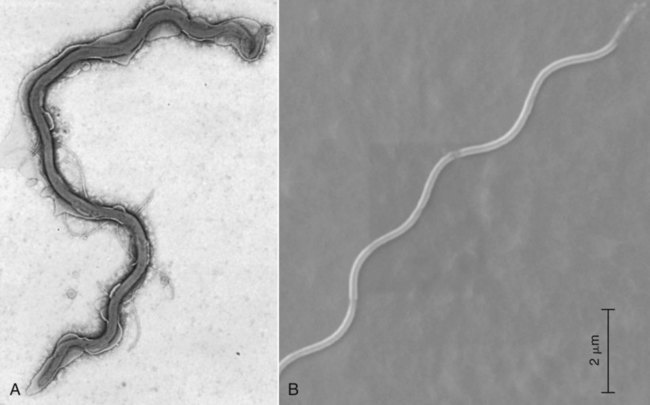
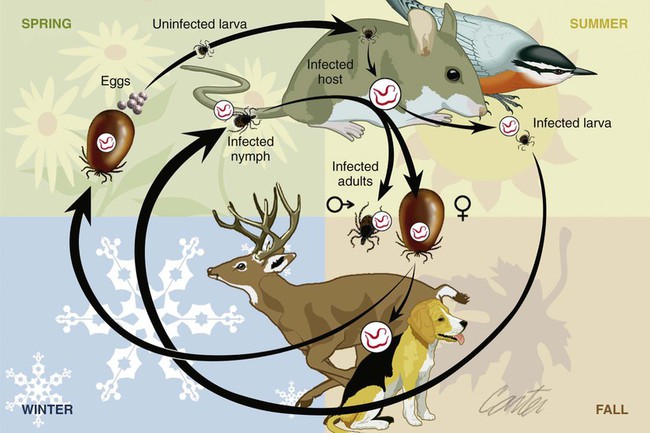
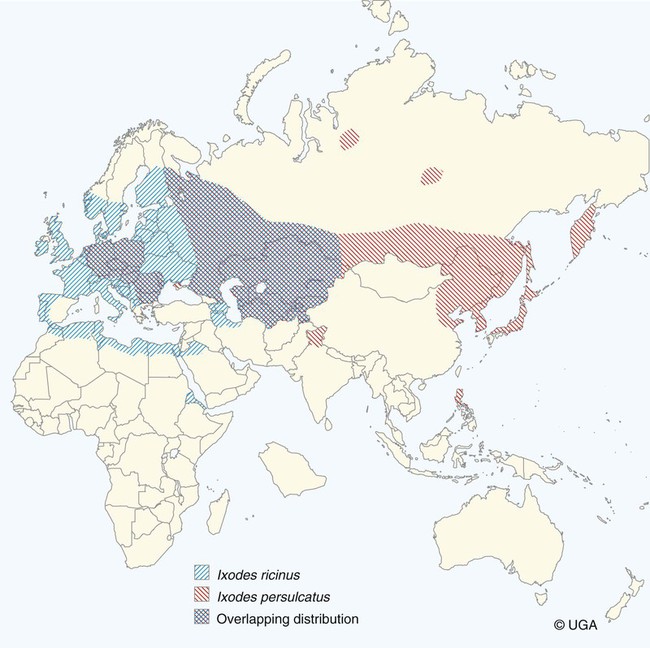
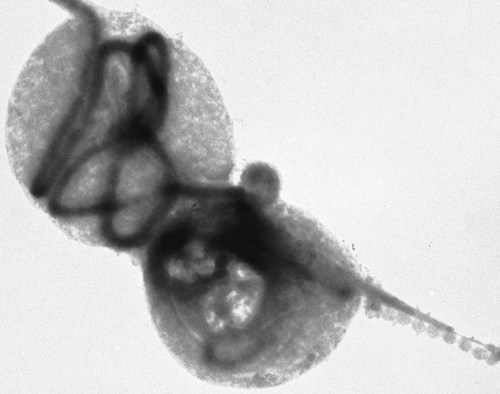
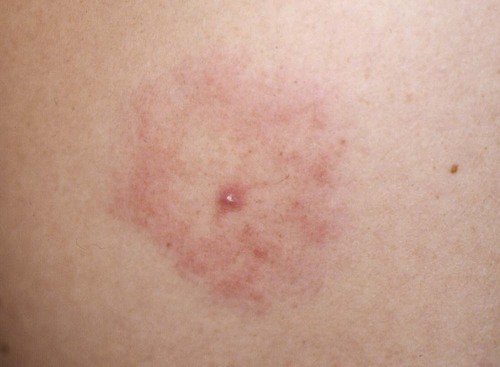
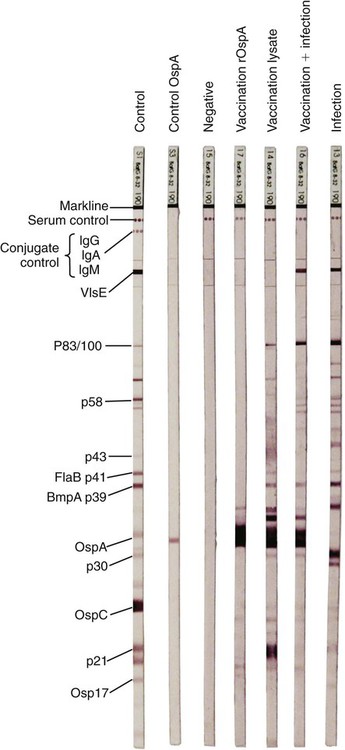
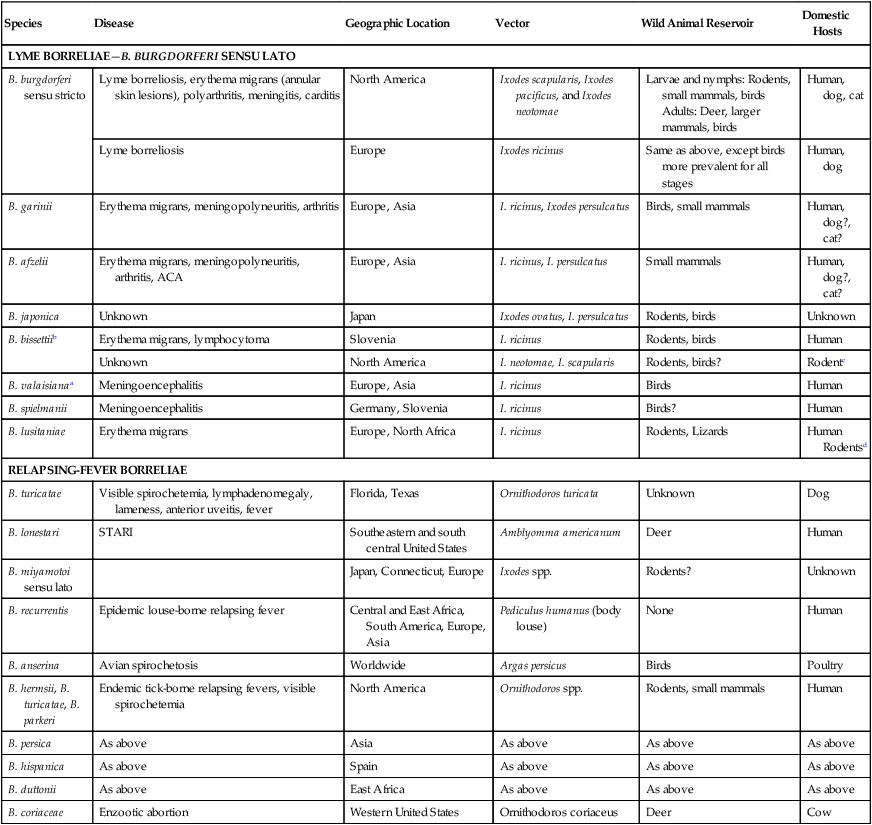
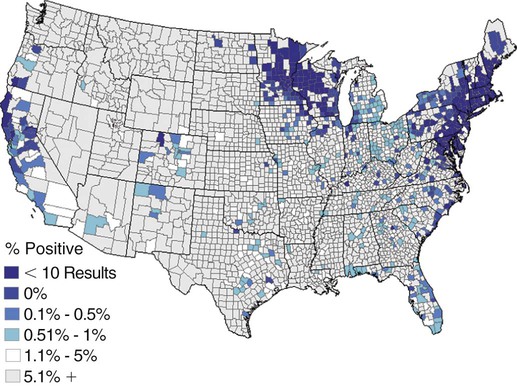
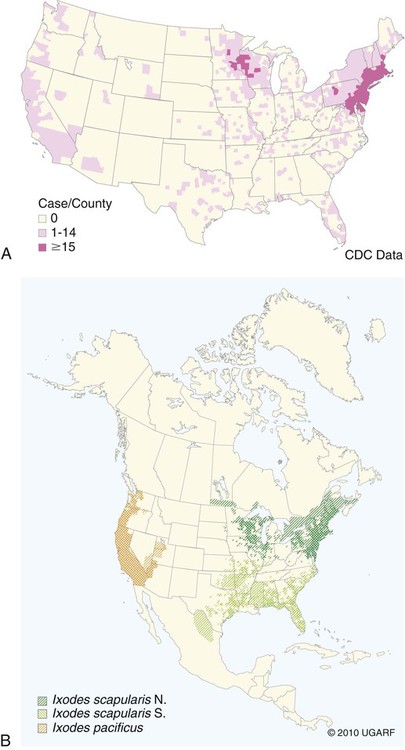
The borrelioses are vector-borne infections that affect mammalian and avian hosts. Members of the genus Borrelia, which contains at least 31 species, are usually categorized into one of two groups: the Lyme borreliosis borreliae or the relapsing-fever borreliae (Table 43-1). Both groups contain pathogenic species in addition to other borreliae that have been isolated only from ticks or asymptomatic animals. Lyme borreliosis is the most commonly diagnosed vector-borne disease in people. It has been reported from North America, Europe, and Asia. Unconfirmed accounts of the disease have come from Australia, South America, and Africa. Experimentally and spontaneously induced Lyme borreliosis has been described in dogs, cats, and other animals. Because of the difficulty in confirming a diagnosis and the diversity of borrelial species being isolated from ticks, controversy still exists regarding the exact prevalence and geographic distribution of infection. The relapsing-fever borreliae affect people and domestic animals. There are a few confirmed cases in which these organisms were isolated from clinically ill dogs. Unless otherwise indicated, the discussion that follows focuses primarily on Lyme borreliosis. TABLE 43-1 Borrelia Species of Medical and Veterinary Importancea ACA, Acrodermatitis chronica atrophicans; STARI, southern tick-associated rash illness; ?, uncertain involvement. aOther species isolated from ticks or their reservoir hosts, but not established to infect domestic hosts include: B. andersonii in I. dentatis, United States; B. californiensis sp nov., western United States; B. carolinensis sp nov. southeastern United States; B. americana sp nov., southeastern and western United States; B. tanukii in I. tanuki, Japan; B. turdi in I. turdis, Japan; B. sinica in I. ovatus and Niviventer confucianus, China. bIsolated reports exist for infection of humans with these Borrelia species. Like most spirochetes (e.g., leptospires), borreliae are thin and elongated spiral-shaped bacteria (0.2 µm × 30 µm), consisting of a protoplasmic cylinder wound around an axial filament composed of multiple periplasmatic endoflagella (see Fig 42-1). They are practically invisible with light microscopy so that dark-field or phase microscopy is needed for proper visualization of organisms (Fig. 43-1). Borrelia burgdorferi sensu lato is a group of at least 13 genospecies, of which the disease-relevant genospecies are listed in Table 43-1.47,88 B. burgdorferi sensu stricto is the primary isolate found in the United States. In people, it is associated with annular skin lesions, polyarthritis, meningitis and carditis. In the United States, Borrelia andersonii and Borrelia bissettii have been isolated from Ixodes ticks on rabbits or rodents and birds, respectively; their pathogenic significance is uncertain. The three main Borrelia genospecies in Eurasia (Borrelia garinii, Borrelia afzelii, B. burgdorferi sensu stricto) appear to have common rodent and avian hosts, and many of these hosts harbor multiple infectious agents.290,295 The greater diversity among species in Eurasia suggests that this complex of organisms originated there.360 However, other evidence suggests that B. burgdorferi sensu stricto may have been reintroduced into Eurasia. In Eurasia, meningopolyneuritis (Bannwarth’s syndrome) is the primary clinical sign in people infected with B. garinii, whereas seropositivity due to infection with B. afzelii has been associated with chronic arthritis and acute and chronic dermatitis (erythema migrans and acrodermatitis chronica atrophicans, respectively).76 Borrelia lusitaniae has been isolated from a person in Portugal with an erythema migrans and produced experimental infection in mice.378 Borrelia valaisiana has been isolated from I. ricinus ticks on vegetation and birds; its DNA was also found in a human with meningoencephalitis.70 B. afzelii has been isolated from a naturally infected dog,329 and mixed infections with this and other European species have been described.329 Borrelia japonica has been isolated from ticks found on dogs and people in Japan.12 In the same region two additional species, Borrelia tanukii and Borrelia turdi, have been isolated from ticks from small mammals; however, their pathogenic significance is uncertain. Numerous (at least 15) borreliae species comprise the relapsing-fever group of vector-borne agents that cause disease in domestic animals and people (see Table 43-1). Except for Borrelia recurrentis, which causes louse-borne relapsing fever, all are transmitted by ticks. A variant species of Borrelia has been associated with erythema migrans skin lesions in humans in southeastern and south central United States.229,230 It has been labeled Southern tick-associated rash illness (STARI), and the organism Borrelia lonestari has been cultured from, or detected by polymerase chain reaction (PCR) within, these individuals and from Amblyomma americanum ticks (the Lone Star tick).41,52,354,355 The differences in strains or species may account for the regional differences in clinical findings that have been reported. Inoculation studies with B. lonestari have shown that white-tailed deer (Odocoileus virginianus) become infected and develop spirochetemia, whereas infections of dogs were unsuccessful, although an increase in their antibody titers was observed.242 The occurrence of infections in dogs and cats, with the relapsing-fever spirochetes described earlier, has not been substantiated. However, visible spirochetemia accompanied by clinical illness has been observed in dogs in Texas and Florida.34,364 These pathogenic spirochetes have been genetically identified as Borrelia turicatae.309 Borrelia persica is a tick-borne relapsing fever spirochete that is found in southern countries of the former Union of Soviet Socialist Republics, Iran, Iraq, Syria, Jordan, Turkey, Israel, Egypt, and Cyprus and is transmitted by Ornithodoros tholozani. It is known to infect humans, dogs, and cats.11,136,136 Unlike Leptospira organisms, borreliae cannot survive as free-living organisms in the environment. They are host associated, being transmitted between vertebrate reservoir hosts (mammals, birds, and lizards) and hematophagous arthropod vectors. B. burgdorferi sensu lato infections are geographically dispersed (see Table 43-1). In general, Lyme borreliosis occurs through the Northern Hemisphere in temperate latitudes with cooler climatic conditions. In North America, the majority of canine and human cases have been reported in the mid-Atlantic to New England coastal states, northeastern states into southern Canada, and upper midwestern states. Ecology supporting Lyme disease exists in adjacent states and also on the West Coast down into northeastern Mexico, and the areas affected are expanding as tick populations, reservoir hosts, and white-tailed deer populations expand (Figs. 43-2 and 43-3, A). Canine cases are often detected before and in higher numbers than human cases in areas of Lyme emergence. Although 1% of the reports were from other states, the organism has never been cultured from humans or dogs outside the mentioned endemic areas. In a serosurvey of dogs in the United States, overall positive prevalence rates were highest in the Northeast (11.6%), followed by the Midwest (4.0%), West (1.4%), and Southeast (1%) in decreasing order.31 These data mirror the human prevalence data for disease prevalence. In Canada, human Lyme borreliosis is endemic in southeastern Ontario. Less is known about the seroprevalence of canine infection or exposure in Canada. The principal vectors of B. burgdorferi sensu lato are various species of slow-feeding hard ticks of the Ixodes complex, whose distribution is associated with the prevalence of disease (Table 43-2). In the United States and Canada, closely related black-legged ticks, Ixodes scapularis (in the northeastern, midwestern, southeastern states down into northeastern Mexico, and up into southern Canada) and Ixodes pacificus together with Ixodes neotomae (in the western states)36 appear to be involved (Fig. 43-3, B). Ticks in the north central United States prefer deciduous, dry to mesic forests and sandy or loamy soils overlying sedimentary rock.131 Ticks are not associated with grasslands, conifer forests, or acidic soils with low fertility or a claylike consistency. In the coastal states, tick infection and dog seroprevalence are greatest in the coastal counties,336 presumably reflecting the tick’s preference for moisture.142b TABLE 43-2 Selected Vectors of Borrelia burgdorferia aB. burgdorferi or related organisms have been recovered from numerous other ticks and arthropods in nature, but the significance is uncertain. Many sylvan cycles exist in nature in which tick vectors do not feed on large mammals. Only the established vectors for humans or domestic animals are listed here. These small (less than 3 mm) ixodid ticks generally feed on more than one host during their life cycle (Fig. 43-4). The number of hosts varies depending on the tick species. In North America, where approximately 50 to 80 vertebrate species serve as hosts, larvae and nymphs of I. scapularis in the north generally feed on small mammals, whereas immature stages of I. scapularis in the South often feed on a variety of hosts including lizards.263,264 Adults of these ticks feed on deer or larger mammals. Reptiles and other preferential hosts of I. scapularis in the southern region are not competent reservoir hosts, and differences in the timing of feeding on these hosts do not allow for efficient transfer of infection for its overwintering in ticks. Therefore, the infection rate of southern I. scapularis ticks (less than 1%) is much lower than that of I. scapularis ticks in the northeastern United States (10% to 50%).262 At least one lizard possesses a borreliacidal substance in its blood similar to complement that reduces or prevents infection with B. burgdorferi.181 Furthermore, because the southern I. scapularis ticks do not always feed on mammals, the prevalence of Lyme borreliosis in humans and domestic animals is low in the southern regions.372 Infected Ixodes ticks may be dispersed to new areas by feeding on migratory birds.152,168b,177,312,324 In Europe, B. garinii, B. afzelii, and B. burgdorferi sensu stricto are responsible for the majority of infections, and most cases have been documented in the Scandinavian countries and in central Europe in areas with moderate temperature and moderate humidity (Austria, Belgium, Croatia, Czech Republic, parts of France, Germany, Hungary, Netherlands, Portugal, Slovenia, Switzerland, etc.) in association with I. ricinus (Fig. 43-5). I. ricinus ticks, across the Mediterranean ocean in northern Africa, are also infected with B. burgdorferi sensu lato.298 I. ricinus ticks have also been infected with other genospecies of Borrelia, including B. bissettii, B. valaisiana, and B. lusitaniae.65,292 In these cases, the infection is being maintained in nature; however, the clinical significance of such infections in humans or domestic animals is unknown. The distribution of infection stretches eastward across Eurasia, corresponding to the habitat of the tick Ixodes persulcatus, which transmits only B. garinii and B. afzelii. Whereas B. burgdorferi sensu stricto and B. afzelii are associated with rodent reservoirs, B. garinii and B. valaisiana are associated with bird reservoirs.103 I. ricinus and I. persulcatus parasitize more than 200 vertebrate species. Several species of mice, voles, and rats are important reservoir hosts. Squirrels, hedgehogs, shrews, and birds are also involved in the maintenance of the infection cycle. On the North American and Eurasian continents, larger ungulates such as deer and moose are important in the life cycle because adult Ixodes ticks feed on them. However, the level of infection in these large mammals is insufficient to transmit to feeding ticks, making them unsuitable reservoir hosts.159,250,250 The Ixodes ticks that transmit Lyme borreliosis have a 2-year life cycle and maintain infection in nature by harboring the organism over the winter period (see Fig. 43-4). In the northeastern and upper midwestern United States, I. scapularis primarily becomes infected when immature stages (especially larvae) feed on infected white-footed mice (Peromyscus leucopus). Direct transmission of borreliae between reservoir hosts is unlikely, and transovarial transmission in ticks is practically nonexistent.275 Larvae, which have become infected by feeding in the fall, harbor the organism over the winter and molt to infected nymphs.42 When these nymphs feed in the spring, they transmit organisms to competent reservoir hosts. Nymphs, which feed on a wide variety of animals, are thought to be primarily responsible for the transmission of infection to domestic animals and humans, as well as infecting new rodent reservoir hosts. However, nymphs are more likely to feed on cats than on dogs.191 Larvae and other stages of ticks can also become infected from previously uninfected hosts by cofeeding with infected ticks that have attached in close temporal and spatial proximity.259,287 However, in other studies, cofeeding of infected and noninfected I. ricinus ticks was not as efficient in transmitting B. afzelii infection as was the transmission of the organism from previously infected mice to uninfected larvae.290,291 Adult female ticks feed on larger mammals such as white-tailed deer, dogs, and livestock. Although feeding on deer is necessary to maintain the tick population, deer are not effective reservoir hosts for infection.159 Nymphs are more likely to cause human infections because they remain undetected owing to their small size (Fig. 43-6). This allows them to remain attached for sufficient periods for successful transmission of spirochetes. Despite the zoonotic risk from nymphal ticks, adult ticks appear to have the highest rate of infectivity among stages, presumably because of repeated exposure to infected mammals and birds. In the western United States, transmission of infection by I. pacificus involves two sylvan cycles. The maintenance cycle involves Ixodes spinipalpis feeding on dusky-footed wood rats (Neotoma fuscipes) and California kangaroo rats (Dipodomys californicus). Immature I. pacificus become infected by feeding on these rodent reservoirs, and the adult ticks transmit the infection to larger mammals during subsequent feedings. As occurs in Eurasia, birds may be more important in the sylvatic cycle of infection in the western United States.373 Ixodes trianguliceps may also play an analogous but lesser role in Eurasia of maintaining infection in nature, similar to that of I. spinipalpis in the western United States, by feeding exclusively on rodent reservoirs.114 Ixodes ticks can be simultaneously infected with additional animal and human pathogens, including Rickettsia spp., Anaplasma phagocytophilum (see Chapter 26 and Table 26-1), Babesia species (Babesia microti, Babesia odocoilei, and Babesia gibsoni [see Chapter 76]), and multiple Borrelia species.145,148,148 Diagnosis and interpretation of serologic tests, and correlating them with specific signs of clinical illness, are problematic when a co-infection exists (see Diagnosis). Numerous hematophagous arthropods, including other tick species, fleas, flies, mites, and mosquitoes, have been found to carry Borrelia organisms in nature. Whether these findings indicate vector competency or accidental contamination is uncertain, but their role in transmission of infection relative to ticks is insignificant. Contamination from feeding on infected vertebrates is suspected; accordingly, these other arthropods have not been documented to transfer infection to new hosts.280 Only I. scapularis—not A. americanum or Dermacentor variabilis—has effectively transmitted B. burgdorferi in North America.280 Although Lyme borreliosis is generally associated with forests, it may be acquired in parks in major metropolitan centers.161 In these areas, rats appear to be the effective reservoir host for feeding ticks.233,325 In the northern midwestern United States, seropositivity among dogs was positively associated with numerous risk factors including increased tick exposure, time spent outdoors, and living in forested or urban wooded habitats and on sandy, fertile soils.133 Among various host species, and sometimes under experimental situations, direct transmission has occurred. However, most contact studies in which transmission occurred in dogs have been performed by exposure that coincided with the time of parenteral inoculation.54 Pregnant bitches were inoculated parenterally every 2 weeks during pregnancy that was induced by artificial insemination of semen from infected male dogs.134 Infection in the offspring was determined by PCR detection of DNA sequences. A majority of the females had at least one infected neonate when the pups were tested up to 6 weeks of age. The presence of IgM antibodies in a pup that did not nurse suggested that the infection occurred in utero. However, infection by parenteral inoculation may have superseded the uterine-placental barrier. In a more natural infection model study with ticks, control dogs that were in direct contact with infected dogs for up to 1 year did not undergo seroconversion, and organisms could not be isolated from the urine or urinary bladder of infected dogs.8 Canine urine is an unlikely source of infection. Furthermore, results of natural infection studies were not consistent with in utero spread despite seroconversion of the dam. B. burgdorferi can survive freezing and storage, making semen intended for artificial insemination a potential source of infection.176 Likewise, blood transfusions could be considered as another source of infection. B. burgdorferi organisms can survive in blood processed for transfusion and stored under blood-bank conditions.163,248 However, not a single case of B. burgdorferi infection caused by blood transfusion has been documented in humans29,102 or animals, and no animal model exists in which Lyme borreliosis can be induced by artificial insemination or by intravenous injection of B. burgdorferi organisms. Relapsing fever is a typical zoonotic disease in which the pathogen is maintained in nature between susceptible reservoir hosts and competent soft ticks. Soft (argasid) ticks, which are more susceptible to environmental desiccation than the hard (ixodid) ticks, prefer a variety of burrow-inhabiting mammals as hosts. Thus, the borreliae causing relapsing fevers have more restricted geographic ranges and niche vectors than those causing Lyme borreliosis (see Table 43-1). Borrelia hermsii is the most common cause of tick-borne relapsing fever in North America and involves at least three main vectors. In the western United States and Mexico, Ornithodoros turicata infests burrows of rodents, terrapins, and snakes, as well as domestic habitats of people and animals. Also in the western United States, Ornithodoros hermsi and Ornithodoros parkeri feed on rodents and squirrels and live in dead trees, fallen logs, and in cabins and homes. Tick-borne relapsing fever in the United States has not been reported in states east of Texas and Oklahoma; however, sporadic isolations of these spirochetes have been made from ticks and humans outside this area. In the southern United States, A. americanum has been found to harbor B. lonestari, a presumptive species that causes an erythema migrans–like rash in humans and may be associated with seropositivity to Borrelia spp. in humans and animals in areas where Ixodes ticks are not found.314 Experimentally, deer but not mice or dogs (see Clinical Findings, later) were infected with B. lonestari, and organisms were observed in Giemsa-stained blood smears.207 Deer may be reservoir species for this organism. In addition to B. lonestari, A. americanum can be co-infected with Ehrlichia ewingii and Ehrlichia chaffeensis (see Chapter 26). All three of these organisms are pathogenic for humans. In the Southern Hemisphere, a Lyme-like disease (Baggio-Yoshinari syndrome), has been discovered in people in Brazil.330a The clinical signs are similar to those of Lyme borreliosis in the Northern Hemisphere with fever, erythema migrans, shifting intermittent lameness, and joint swelling. Visible spirochetemia, more typical of the relapsing fever spirochetoses, is apparent in the peripheral blood of affected humans. Dogs in the disease area are seropositive to Borrelia spp. assays; however, the incriminated spirochetes have not been isolated by cultivation or genetically identified in affected humans or seropositive dogs. A relapsing fever-like spirochete (Borrelia brasiliensis) has been isolated from the soft tick, Ornithodoros brasiliensis) which predominantly infests dogs. Further study is needed to determine if it is the cause of this infection. A large body of information has been accumulated to explain the pathogenic features of borrelial spirochetes based on their genetic makeup. Most genes on the chromosome of B. burgdorferi are typical of other bacteria. Besides, B. burgdorferi carry up to 12 linear and 9 circular plasmids. The plasmid genome does not contain so far identifiable virulence factors, but codes largely for outer surface proteins responsible for survival and persistence in vertebrate hosts and successful transmission by tick vectors.345 Borreliae grown in vitro can lose plasmids and hence their pathogenic potential. The onset of spirochete transmission requires 1 to 2 days of tick attachment, during which time organisms multiply and cross the gut epithelium into the hemolymph, disseminate to the salivary glands, and infect the host through tick saliva.68,131,131 Outer surface protein (Osp)A, the predominant surface protein, is expressed on the outer surface of borreliae in the midgut of infected ticks and helps the borreliae adhere to the midgut. In unfed ticks, virtually all spirochetes reside in the midgut. During feeding and because of the warmth of the new environment (skin surface temperature of the host), spirochetes downregulate their expression of OspA protein and express OspC, which correlates with the migration of the spirochetes from the midgut into the hemolymph and finally to the salivary glands of the feeding tick.273 After dermal inoculation into the mammalian host, OspC in combination with saliva protein 15 (Salp15) from the ixodid tick blocks clearance of spirochetes and allows for their dissemination, presumably by evasion of host immunity.147,284,284 OspC production allows the organism to become established in the host; however, it is not required for persistence beyond several weeks.350 Levels of the immunodominant variable surface lipoprotein component (VlsE), increase during the last 2 days of tick engorgement; the increase continues after OspC production ceases.281 VlsE and other proteins are involved in chronic persistence of the parasite and its avoidance of the host immune response. A genetically conserved, invariable region of VlsE protein is strongly immunodominant and important in serodiagnosis (see the later section under Diagnosis, Antibody to Specific Outer Surface Proteins). Despite the numerous humans and animals that are bitten by infected ticks, only a few develop clinical disease. Host immune reactions are likely involved in preventing many of these infections. Studies show that the percentage of dogs developing severe clinical signs of the disease is low compared with the frequency of exposure based on seropositivity (3% to 10% in central Europe; 75% in endemic areas in the United States) and the rate of infection demonstrated in ticks.173,197 Part of this seropositivity in dogs may indicate infection with low numbers of B. burgdorferi organisms in a host with an efficient immune system or represent an exposure to infectious but nonpathogenic isolates of Borrelia.3 Even among genetically similar B. burgdorferi sensu stricto strains, pathogenicity may vary because of their plasmid content. From the site of the tick bite, the organisms replicate and migrate through skin and connective tissues. Later, they colonize many tissues, including the joints. Active migration in tissues is more common than passive dissemination via the blood. Their requirement for N-acetyl-glucosamine, a component of collagen synthesis, may explain this tissue tropism.173a Once in the body, B. burgdorferi acts as a persistent pathogen. Even without antibacterial treatment, the host immune response is capable of reducing spirochete numbers. Although the spirochete is not eliminated from the host after a few weeks of infection, B. burgdorferi is virtually not detectable in body fluids or internal organs, and exceedingly few are found in other tissues.8,61,61 The organism can evade host antibody and exist extracellularly by surviving in protected tissues, by varying or masking its immunoreactive proteins,284,374 and by changing its entire shape. Spiral, motile borreliae can convert into spherical life forms within minutes after they have encountered unfavorable environmental conditions (Fig. 43-7).2,35 In this form they survive for days without nutrition and without metabolic activity and revert back into the well-known spiral form when conditions improve. These spherical borreliae are able to infect mice.130 This may explain the reason B. burgdorferi can still persist and be detected in tissue samples by PCR or occasionally by culture even after months of antibacterial treatment. Clinical illness results from the host’s own inflammatory response. Some of the immunopathologic events in nervous tissue may be related to immune responses generated against specific borrelial antigens. Flagellin, one of the most immunogenic proteins, can elicit an antibody that binds to host neuroaxonal proteins.319 This sequence may in turn stimulate the inflammatory response in nervous tissue.320 Clonal analysis indicates the immune response in nervous tissue may be directed at the pathogen and neural self-antigens.174 Co-infection with A. phagocytophilum may increase spirochetal proliferation and penetration of vascular endothelium and the blood-brain barrier.115,257 Cytokine release regulates the inflammatory response in order to reduce the organism burden, but also results in damage to surrounding tissue. In the joints, upregulation of interleukin-8, which recruits neutrophils to inflammatory sites, has been found in the synovial membranes of experimentally infected dogs.340 This may be an important mechanism in producing suppurative polyarthritis. In a few animals, the development of arthritis is probably related to pathologic perpetual host immune reactions. Humans with certain haplotypes of the major histocompatibility complex are prone to more severe, treatment-resistant clinical manifestations of the disease in which B. burgdorferi cannot be detected.331 The tissues of preferential localization may vary by Borrelia species and determine the pathogenicity and, therefore, the difference in clinical manifestations seen in various geographic regions. B. garinii was found in the liver of dogs having elevated liver enzyme activities, a finding not associated with B. burgdorferi sensu stricto.150 Despite the presence of glomerulonephritis in seropositive dogs, spirochetes or their subcomponents are rarely, if ever, found in renal lesions.57,153,153 The glomerular injury may relate to deposition of circulating immune complexes secondary to infection rather than primary spirochetal localization. B. burgdorferi–specific circulating immune complexes have been found in the serum of infected dogs.109 In the mammalian host, B. hermsii has developed highly adapted mechanisms to evade the immune system. Each inoculated spirochete may produce 30 unique antigenic variants, each expressing a major immunodominant protein that defines a specific serotype. In the mammalian host, episodes of cyclic spirochetemia are caused by waves of replicating spirochetes with relatively homogenous serotypes. As each new wave of replication becomes recognized by the host defenses, a new protein is expressed, allowing the subsequent generation to proliferate—hence the relapsing nature of infection. Analyses of the surface proteins of B. hermsii have shown that the organism expresses variable major proteins, such as Vsp 33; however, after ingestion by the tick, a new major protein is upregulated on the cell surface and confers the unique specific serotype. The Vsp 33 protein is homologous to the OspC protein of B. burgdorferi and as a result allows the organism to maintain its infectivity within the nonfeeding tick. Similar to the VlsE in B. burgdorferi organisms, relapsing-fever spirochetes rely on antigen diversity by rearrangement of silent genetic cassettes in the vmp-gene in order to change their outer surface antigen profile within a few days and thus evade the host’s immune system.288 Besides asymptomatic seroconversion, clinical features of Lyme borreliosis in humans have included dermatitis, arthritis, meningoencephalitis, and myocarditis. Some of these manifestations have also been observed in dogs and cats. Co-infections are common, especially in tick-borne diseases where multiple pathogens can be transmitted simultaneously. Clinical illness in infected animals may be precipitated or exacerbated by other infecting pathogens such as Anaplasma phagocytophilum (see Chapter 26).25 Initial attempts to recreate experimental Lyme borreliosis in dogs using laboratory-derived strains were unsuccessful, as they were in cats.43,125 However, fever and polyarthritis have been experimentally produced after inoculation or natural exposure to tick-derived B. burgdorferi.8,54,118,361 Inoculation of spirochetes by allowing feeding of field-collected ticks from other animals has been most successful.8 Clinical illness in experimentally infected dogs occurred 2 to 6 months after tick exposure, and the severity and propensity to develop signs of illness seem to vary inversely with the animal’s age and immune status. The onset of clinical illness in experimental infection usually correlates with the initial increase in serum antibody titer. Signs of experimental disease are generally milder than those of the natural disease described next. Acute signs of fever (39.5° C to 40.5° C [103.1° F to 104.9° F]), shifting leg lameness, articular swelling, lymphadenomegaly, anorexia, and general malaise, all of which are responsive to antimicrobials, most commonly develop in experimentally and naturally exposed seropositive dogs.193,197,205,236 The accuracy of the diagnosis in many spontaneously diseased dogs is difficult to determine because limb and joint signs (swelling, lameness, and pain) with fever and inappetence have been observed with equal frequency in dogs with and without B. burgdorferi–specific antibodies.59,219,219 Polyarthritis is the most overt experimentally and clinically documented syndrome caused by acute B. burgdorferi infection in dogs (Fig. 43-8). Spread of the organism in skin, connective, joint, and muscle tissues is most likely responsible for some of the observed lameness. The first limb affected is usually closest to the site of tick attachment. The onset of lameness can correspond to an increased body temperature. Lameness in a particular limb often lasts for a few days and then may shift to another limb or disappear. Despite the transient nature of the arthritis, inflammatory changes can persist in the joint as evidenced by abnormal synovial fluid analysis.118 Lesions have been most consistently found in skin, lymphatic tissues, and joints, even though the organism can be isolated from other tissues. Dogs with naturally occurring cranial cruciate ligament arthropathy in a Lyme borreliosis endemic area were found to have a high prevalence of B. burgdorferi and other bacterial gene sequences within synovial biopsy tissue as compared to dogs with clinically healthy stifle joints and those with experimental cranial cruciate ligament rupture.243,244 These bacteria may be important in the causation and pathogenesis of the inflammatory arthritis. Protein-losing glomerulopathy has been described in naturally infected dogs.97,98,117,239 An acute progressive renal failure associated with azotemia, uremia, proteinuria, peripheral edema, thromboembolism, body cavity effusions, and occasional neurologic signs has been characterized in dogs from Borrelia-endemic areas.62,209 A preponderance of Labrador and golden retrievers was affected. The duration of clinical illness has varied from 24 hours to 8 weeks, with a sudden onset of anorexia, vomiting, and lethargy. Dogs with more chronic progression had weight loss. Some dogs had recent or concurrent lameness. Laboratory abnormalities include nonregenerative anemia, stress leukogram, thrombocytopenia, hypoalbuminemia, azotemia, hypercholesterolemia, hyperphosphatemia, and variable hyperkalemia and hyperalbuminemia. Urinalysis abnormalities were proteinuria, with variable loss of concentrating ability, hemoglobinuria, hematuria, glucosuria, bilirubinuria, and active sediment.62 All dogs died or were euthanized as a result of renal failure. The dogs had specific antibodies to B. burgdorferi. Natural exposure to B. burgdorferi, and not vaccination against B. burgdorferi, was likely involved based on the reported data. A similar association between increased prevalence of antibodies against B. burgdorferi sensu lato and glomerulonephritis has been observed in Bernese mountain dogs.97,98a,99 In rapidly progressing glomerular disease, the role, if any, of vaccination in the development of the lesion is undetermined. Often later manifestations of Lyme borreliosis infection in humans include neurologic signs, which are more prevalent in infections caused by B. garinii.269 Experimental infections of nonhuman primates, the only animal model to exhibit this predominant clinical form, have produced similar features.293 Experimentally infected dogs developed mild focal meningitis, encephalitis, and perineuritis; however, neurologic signs were not observed.56 In dogs presumed to be naturally infected based on positive serologic test results, clinical signs of neurologic dysfunction were not correlated with positive serologic reactivity to B. burgdorferi.158 Nucleic acids of B. burgdorferi were not found in the brain tissue or central nervous systems (CNS) of dogs with a variety of natural CNS diseases.18 A small, reddish lesion develops in the skin of dogs at the site of tick attachment but disappears within the first week (Fig. 43-9). The organism can be isolated from the skin for extended periods. This lesion is not like the dramatic lesion associated with erythema migrans in humans. Other syndromes reported in a few spontaneously diseased dogs include rheumatoid arthritis294 and myocarditis-induced cardiac arrhythmia.195 These other signs are similar to those that have been reported in humans. Unfortunately, the diagnoses in these naturally diseased dogs were often circumstantial, based on serologic data or microscopic evidence but without organism isolation. In humans, conjunctivitis, vitreitis, choroiditis, hepatitis, and myositis or fasciitis have been reported as rare syndromes and have not been reported in dogs. The strain used or the relatively short observation period in experimental studies may be the reason that the nonarthritic syndromes have not been reproduced in experimentally inoculated animals. Despite evidence for seropositivity for B. burgdorferi in cats, natural disease has not been described as a distinct clinical entity. Cats in endemic areas of the northeastern United States become infested with Ixodes ticks. Reported prevalence rates of exposure to B. burgdorferi have ranged from 13% to 47% based on seropositive results by indirect fluorescent antibody (FA) testing, enzyme-linked immunosorbent assay (ELISA), or immunoblotting confirmation; however, no difference in positive results was found between cats with or without lameness.218,221 Coincidentally, cats in these studies also had seropositive results to A. phagocytophilum, suggesting simultaneous exposure via Ixodes ticks.221 Similarly, cats that were screened as clinical patients in the United Kingdom had a low rate (4.2%) of seroreactivity.237 The seropositive cats in the United Kingdom study had no symptoms of lameness. Cats may be more resistant than dogs to the development of clinical signs of Lyme borreliosis. Cats inoculated by various nonnatural routes (i.e., parenteral, oral, conjunctival) with cultivated spirochetes developed IgG seropositivity; however, organisms were found only in the blood of the intravenously and ocularly infected cats.44 The spirochetemia was transient, clearing by day 24, and only one cat had organisms in its tissues at necropsy. In contrast, when cats were inoculated with organisms directly from arthropods, they developed multiple-limb lameness and had joint, pulmonary, lymphoid, and CNS inflammation at necropsy.104,105 Arthritis or meningitis seems to be the predominant manifestation that would warrant investigation of Lyme borreliosis in cats. Erythematous rashes in humans, once thought to be characteristic for early Lyme borreliosis, may not be that specific. In the southern United States, focal rashes (Fig. 43-10) and transient fever and malaise associated with the bite of A. americanum ticks were attributed to infection with B. lonestari, a relapsing-fever Borrelia.46,160 Although dogs seroconverted, they did not become infected after inoculation with this organism.242 Borreliosis caused by B. turicatae was reported in two dogs from Florida34 and in three dogs from Texas.364 Clinical signs in the dogs included elevated rectal temperature, shifting leg lameness, hepatosplenomegaly, visible spirochetemia, and anterior uveitis, all similar to the signs of endemic tick-borne relapsing fever. In the infected dogs from Texas, the only signs were elevated rectal temperature, lethargy, and lameness. Many of the other relapsing-fever spirochetes may infect dogs and cats as incidental hosts in the same way they are known to infect humans (see Table 43-1). Additional studies are needed to determine the clinical significance of these infections. There is no pathognomonic test for Lyme borreliosis; however, dogs with compatible clinical signs in endemic areas that have been exposed to ticks bear the highest consideration. Reports of Lyme borreliosis often exceed the geographic boundaries of known endemic foci; however, travel accounts for a minor number of disparities. Frequently, the overestimation of numbers of cases results from cross-reactivity to other infectious agents, as a result of subclinical infections, from the inaccuracies of serologic procedures, or insufficient experience of laboratories. Dogs with seropositive test results can have other illnesses or infections that cause signs similar to those of borreliosis.89,328 Furthermore, the interpretation of serologic results may be confused by transmission of multiple organisms by the Ixodes spp. ticks. In a survey of dogs in Baxter, MN, where I. scapularis is endemic, those with clinical illness were more likely to have positive antibody and PCR test results for both A. phagocytophilum and B. burgdorferi (than to either alone), compared to clinically healthy dogs in the same clinic population.25 These findings suggest that co-infections may be important in determining whether infected animals develop clinical illness. Diagnosis of borreliosis should be based on known tick exposure in the endemic region; compatible clinical signs and laboratory findings with exclusion of other diseases; and favorable response to selective antimicrobial administration. Serologic screening of clinically healthy dogs for infection with B. burgdorferi is controversial. From a positive standpoint, infection or exposure might be found at a preclinical stage of illness before immunopathologic consequences such as renal dysfunction occur. Conversely, should proteinuria be detected first, then a work-up to determine its cause might include screening for antibodies to B. burgdorferi in dogs from endemic areas.97,98 Finding seropositive dogs would add more convincing support for the use of tick prevention measures, vaccination measures, and recognition by owners of their own potential exposure to ticks in areas frequented by their pets. On the negative side, routine testing may lead to overdiagnosis with the potential result of overtreatment or unnecessary owner concerns. No specific hematologic or biochemical changes are pathognomonic of borreliosis, although cerebrospinal fluid (CSF), joint fluid, and urine may show evidence of inflammatory changes. If a dog in an endemic area for borreliosis has leukopenia or thrombocytopenia, these hematologic abnormalities are likely caused by infection or co-infection with a rickettsial pathogen (e.g., A. phagocytophilum; see Chapter 26). The synovial fluid changes in dogs with borreliosis have been best substantiated by increased cell counts of 5000 to 100,000 cells/µL with neutrophils predominating (up to 95%). Protein concentration and turbidity are increased. CSF from human patients with neuroborreliosis demonstrates lymphocytic pleocytosis and a mild protein increase. Consistent CSF values have not been found in dogs with suspected neurologic dysfunction. Laboratory findings for dogs with renal dysfunction are described under Clinical Findings. The presence of an elevated antibody titer to B. burgdorferi signifies exposure to the spirochete but does not prove that a current clinical illness is caused by the organism. In endemic areas, asymptomatic animals are often seropositive, possibly a result of adequate host immune responses or, with less specific assays, from exposure to nonpathogenic forms of B. burgdorferi or other closely related spirochetes.9 In addition to seropositive results with a validated assay, the animal should have a history of tick exposure with compatible clinical signs and a rapid response to antimicrobial therapy. The diagnosis of Lyme borreliosis has become a serologic diagnosis because culture and microscopic or genetic detection of the organism from specimens of tissue samples or body fluids are uncommon. Serologic studies should be viewed as determining “seroreactivity to B. burgdorferi”319 rather than definitive evidence of the disease being caused by the spirochete. In the past, whole borrelial cell serologic tests have had inaccuracies that have decreased with the use of immunoblotting, recombinant protein ELISA, and genetic detection methods. Serial measurement of antibody titers and corresponding responses to therapy can help determine the active nature or specificity of infection. The first available immunodiagnostic tests were done with antigens from whole-spirochete preparations. These methods were not standardized and had a high level of cross-reactivity with other bacteria.* For example, the whole-cell ELISA and indirect FA methods had positive results for B. burgdorferi reactivity with Leptospira-positive sera. Furthermore, antibodies to the relapsing-fever–like organism B. turicatae, described in dogs from Florida and Texas, cross-reacted with B. burgdorferi–derived antigens.34,364 Experimentally infected dogs developed IgG-ELISA-positive (whole bacterial cell) titers by 4 to 6 weeks after exposure.8,118 Titers were at their highest levels by 3 months after exposure and lasted for at least 2 years.342 Antibody titers often decline after treatment, and patients may become clinically healthy, but measurable whole-organism-based ELISA IgG titers often remain for many years. High serum antibody levels seem to decline or disappear with antibacterial treatment, but increases that occurred 6 months or later after termination of therapy have been interpreted as proliferation of surviving spirochetes.338,340 Although protective antibody levels may decline after vaccination, vaccinated dogs show seroreactivity with whole-spirochete assays for months to years, making a diagnosis of B. burgdorferi infection based only on ELISA with whole-cell preparations difficult.156 Because of the shortcomings of antibody measurements with whole-cell antigens, serologic tests of this type have been largely discontinued. Instead, separated or recombinant proteins are used as test antigens. Rarely used for initial screening, immunoblotting, where the spirochete proteins are separated, has been employed as a second phase of diagnosis to help confirm positive results from other serologic tests. It is a helpful tool to differentiate infected from vaccinated animals and to detect reactivity in some B. afzelii infections that may be missed by recombinant protein-based ELISA methods. The pattern of antibody reactivity after natural tick infection differs from that produced by vaccination. Sera from some dogs in endemic areas that are vaccinated with any product (lysate or recombinant antigens derived from B. burgdorferi organisms) exhibit reactivity predominantly to OspA that is expressed only on the surface of B. burgdorferi organisms at ambient temperatures (in ticks or on borreliae in culture).95,119,146,223,317 The actual measured size of the bands (in kilodaltons [kDa]) described in the following section varies in different investigations because of varied techniques and strain differences and should only be used as a guideline. After natural exposure to B. burgdorferi, antibodies develop to proteins in the range of 100/83, 75, 66, 60, 58, 43, 41 (flagellin), 39, 30, 23 (OspC), and 21 kDa.337 A reactivity against the VlsE surface protein can be observed, when recombinant VlsE antigen is incorporated on Western blot strips (Fig. 43-11). Reactivity, particularly to 31 kDa (OspA), occurs in vaccinated dogs but is absent or occurs (rarely) very late in naturally infected dogs.8,126,128,196 In one European study, immunoblotting results between natural infection and vaccinated animals were not easily differentiated.187 For a further discussion on immunoblotting and the interpretation of specific bands, consult other references.173a Variation in the genes that encode for the immunodominant VlsE surface lipoprotein of B. burgdorferi helps the spirochete to escape the host immune response when it enters the host. One immunodominant region of VlsE, known as IR6, is genetically, structurally, and antigenically highly conserved among many B. burgdorferi strains and genospecies. The genes for IR6 are expressed only during infection of and replication in the mammalian host and not during ex vivo cultivation or in the arthropod vector. A synthetically produced peptide known as C6 is encoded by the IR6 gene sequence. Host antibody response to C6 has been used in serodiagnostic testing of humans and animals as a marker for host invasion and infection. Testing for C6 has been substituted for conventional whole-cell ELISA and subsequent immunoblotting in clinical diagnosis of human borreliosis.14,226,226 A commercially available point-of-care test SNAP 4DX (IDEXX Laboratories, Westbrook, ME) is available that detects the presence of serum antibody to the C6 peptide. The C6 commercial test can be used to more accurately differentiate between vaccinated and infected dogs compared to the whole-spirochete and immunoblot test methods (Table 43-3 and Web Appendices 5 and 6). In studies with dogs, C6 protein has been used in an ELISA evaluating experimentally202,279 and naturally100,189 infected animals. The C6 antibody response increased 3 to 5 weeks after experimental infection in dogs and before the antibody increases with conventional assays and before the onset of clinical lameness. C6 antibody titers decreased more quickly after treatment than the antibody response measured by ELISA using whole-spirochete antigen extracts of B. burgdorferi.279 C6 antibody levels were not increased in sera from healthy, uninfected dogs, those with other infections (e.g., dirofilariasis, babesiosis, ehrlichiosis, Rocky Mountain spotted fever, leptospirosis), or those vaccinated with either OspA, whole-spirochete vaccines, or commonly used vaccines for other diseases.202,258 Use of this protein in serotesting helps determine the presence of infection. In a comparison when immunoblotting was used as the reference standard for infection in dogs, the sensitivity and specificity of the C6 point-of-care test were 98.8 and 100%, respectively.55a Because not all dogs infected with spirochetes become ill, and because species and strains within B. burgdorferi sensu lato complex vary in pathogenicity, the C6 antibody response does not always correlate with clinical illness in dogs. Caution in overinterpreting the significance of a positive test result in a clinically healthy dog has been addressed by the American College of Veterinary Internal Medicine consensus statement.209 False-positive results may be observed in young pups as a result of in utero or colostral antibody transfer.84 These results will become negative on subsequent evaluation through the postnatal period. TABLE 43-3 Comparative Serologic Test Results for Borreliosis in Dogs
Borreliosis
Species
Disease
Geographic Location
Vector
Wild Animal Reservoir
Domestic Hosts
LYME BORRELIAE—B. BURGDORFERI SENSU LATO
B. burgdorferi sensu stricto
Lyme borreliosis, erythema migrans (annular skin lesions), polyarthritis, meningitis, carditis
North America
Ixodes scapularis, Ixodes pacificus, and Ixodes neotomae
Larvae and nymphs: Rodents, small mammals, birds
Adults: Deer, larger mammals, birds
Human, dog, cat
Lyme borreliosis
Europe
Ixodes ricinus
Same as above, except birds more prevalent for all stages
Human, dog
B. garinii
Erythema migrans, meningopolyneuritis, arthritis
Europe, Asia
I. ricinus, Ixodes persulcatus
Birds, small mammals
Human, dog?, cat?
B. afzelii
Erythema migrans, meningopolyneuritis, arthritis, ACA
Europe, Asia
I. ricinus, I. persulcatus
Small mammals
Human, dog?, cat?
B. japonica
Unknown
Japan
Ixodes ovatus, I. persulcatus
Rodents, birds
Unknown
B. bissettiib
Erythema migrans, lymphocytoma
Slovenia
I. ricinus
Rodents, birds
Human
Unknown
North America
I. neotomae, I. scapularis
Rodents, birds?
Rodentc
B. valaisianaa
Meningoencephalitis
Europe, Asia
I. ricinus
Birds
Human
B. spielmanii
Meningoencephalitis
Germany, Slovenia
I. ricinus
Birds?
Human
B. lusitaniae
Erythema migrans
Europe, North Africa
I. ricinus
Rodents, Lizards
Human
Rodentsd
RELAPSING-FEVER BORRELIAE
B. turicatae
Visible spirochetemia, lymphadenomegaly, lameness, anterior uveitis, fever
Florida, Texas
Ornithodoros turicata
Unknown
Dog
B. lonestari
STARI
Southeastern and south central United States
Amblyomma americanum
Deer
Human
B. miyamotoi sensu lato
Japan, Connecticut, Europe
Ixodes spp.
Rodents?
Unknown
B. recurrentis
Epidemic louse-borne relapsing fever
Central and East Africa, South America, Europe, Asia
Pediculus humanus (body louse)
None
Human
B. anserina
Avian spirochetosis
Worldwide
Argas persicus
Birds
Poultry
B. hermsii, B. turicatae, B. parkeri
Endemic tick-borne relapsing fevers, visible spirochetemia
North America
Ornithodoros spp.
Rodents, small mammals
Human
B. persica
As above
Asia
As above
As above
As above
B. hispanica
As above
Spain
As above
As above
As above
B. duttonii
As above
East Africa
As above
As above
As above
B. coriaceae
Enzootic abortion
Western United States
Ornithodoros coriaceus
Deer
Cow
Etiology
Lyme Borreliosis
Relapsing-Fever Borreliosis
Epidemiology
Lyme Borreliosis
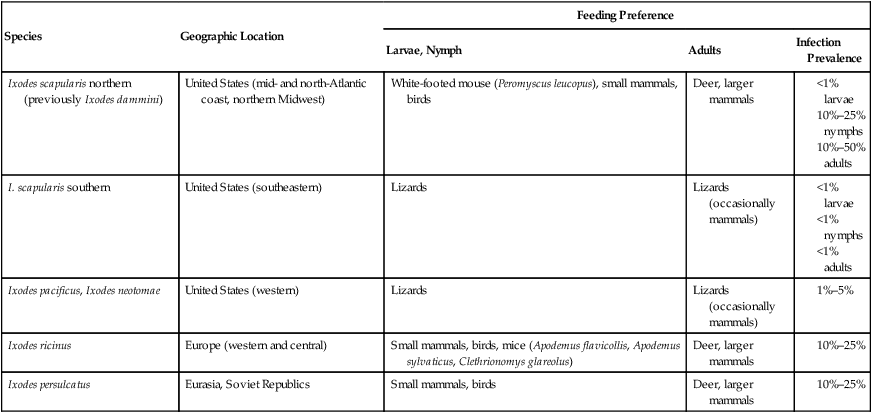
Species
Geographic Location
Feeding Preference
Larvae, Nymph
Adults
Infection Prevalence
Ixodes scapularis northern (previously Ixodes dammini)
United States (mid- and north-Atlantic coast, northern Midwest)
White-footed mouse (Peromyscus leucopus), small mammals, birds
Deer, larger mammals
I. scapularis southern
United States (southeastern)
Lizards
Lizards (occasionally mammals)
Ixodes pacificus, Ixodes neotomae
United States (western)
Lizards
Lizards (occasionally mammals)
Ixodes ricinus
Europe (western and central)
Small mammals, birds, mice (Apodemus flavicollis, Apodemus sylvaticus, Clethrionomys glareolus)
Deer, larger mammals
Ixodes persulcatus
Eurasia, Soviet Republics
Small mammals, birds
Deer, larger mammals
Relapsing-Fever Borreliosis
Pathogenesis
Lyme Borreliosis
Relapsing-Fever Borreliosis
Clinical FINDINGS
Lyme Borreliosis
Dogs
Systemic Signs
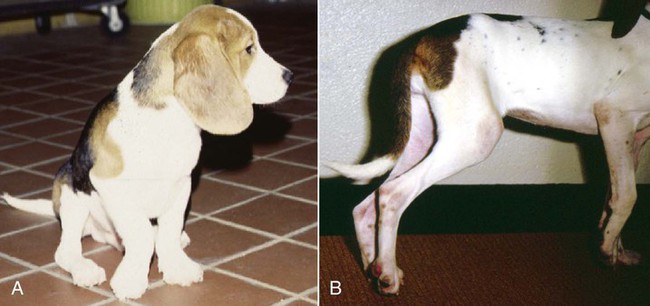
Arthritis
Renal Disease
Meningitis
Other Manifestations
Cats
Relapsing-Fever Borreliosis
Diagnosis
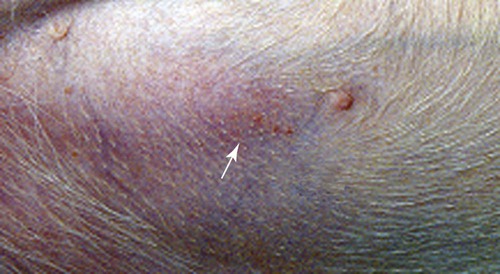
Clinical Laboratory Findings
Serologic Testing for Lyme Borreliosis
Antibody Measurements Using Whole-Spirochete Preparations
Immunoblotting
Antibody to Specific Outer Surface Proteins—VlsE and C6
Serologic Test
Naturally Infected via Tick
Vaccinated Whole Cell
Vaccinated OspA
IgM whole-cell ELISA or indirect FAa
Positive within first 60–90 days for a transient duration
Positive within 2–4 weeks, can last for months to years
Positive within 2–4 weeks, can last for months to years
IgG whole-cell ELISA or indirect FAb
After 60–90 days, positive for months or years
Positive beginning 4–6 weeks, will last for months to years
Positive beginning 4–6 weeks, will last for months to years
Immunoblotc
Numerous bands but not OspA or OspB
Predominant OspA and some produce OspC bands, also numerous other bands
OspA and few bands in the lower molecular range
C6 ELISA assayd
Positive
Negative
Negative
Multiplex Antibody Testinge
Positive OspC (>2–3 wks) OspF (>6–8 wks)
Positive (OspA)
Positive (OspA) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree