CHAPTER 16 The skeleton consists of bones and joints and their supporting structures and is responsible for supporting and protecting the body and enabling movement initiated by the nervous system and facilitated by muscles. The skeleton can be divided into axial skeleton (head, vertebrae, ribs, and sternum) and appendicular skeleton (thoracic and pelvic limbs). Information on the methods for postmortem examination and evaluation of the skeleton is available in Web Appendix 16-1, which can be found at evolve.elsevier.com/Zachary/McGavin/. Structure and function can be discussed at the organ, tissue, and cellular levels. In this section, normal structure and function are briefly reviewed, beginning at the cellular level and including bone matrix and mineral. Cells directly involved with the structural integrity of bone include osteoblasts, osteocytes, and osteoclasts (Box 16-1). Osteoblasts are cells on any bone surface (periosteal, endosteal, trabecular, intracortical) that produce bone matrix (osteoid), initiate the mineralization of this matrix (deposition of hydroxyapatite), and seemingly paradoxically initiate the resorption of this matrix by osteoclasts. Osteoblasts are derived from mesenchymal stem cells. Active osteoblasts are plump (Fig. 16-1), with abundant basophilic cytoplasm that is rich in rough endoplasmic reticulum and contains prominent Golgi apparatus and numerous mitochondria (Web Fig. 16-1). Inactive osteoblasts are disk-shaped with little cytoplasm because fewer organelles are needed for matrix synthesis and secretion (Fig. 16-2 and Web Fig. 16-2). Osteoblasts likely interact with osteocytes to assist in fine control of calcium homeostasis and detection of mechanical use and microscopic damage to bone, as discussed later. Indirect measurements of osteoblast activity are reflected in blood concentrations of the bone-specific isoform of alkaline phosphatase, an enzyme on the cell membrane of osteoblasts, and the noncollagenous protein, osteocalcin, that is secreted by osteoblasts and is present in bone matrix. Both are thought to have roles in mineralization and calcium ion homeostasis. Fig. 16-1 Osteoblasts and osteocytes, long bone, young dog. Fig. 16-2 Osteoblasts, vertebra, adult dog. Web Fig. 16-1 Osteoblast, bone, tibia, rodent. Web Fig. 16-2 Inactive osteoblast, bone, tibia, rodent. Osteocytes are osteoblasts that have been surrounded by mineralized bone matrix (Web Fig. 16-3) and are the most numerous and longest living of the bone cell types. They occupy small spaces in the bone called lacunae (singular: lacuna) and make contact with osteoblasts and other osteocytes by means of long cytoplasmic processes that pass through thin tunnels in the bone called canaliculi (singular: canaliculus). Under conditions of extreme stress to calcium homeostasis, osteocytes might have the ability to resorb perilacunar mineral and matrix, thus enlarging the lacuna (osteocytic osteolysis). This process apparently is rare and likely does not contribute significantly to development of osseous lesions. Osteocytes also retain a limited capacity to form bone and are exquisitely sensitive to mechanical strain in the form of shear stress. Other functions of osteocytes are somewhat speculative and are presented later in osteoblast-osteocyte interactions. Web Fig. 16-3 Osteocyte, bone, tibia, rodent. Osteoclasts are multinucleated cells that are derived from hematopoietic stem cells of the granulocyte-monocyte series and are responsible for bone resorption (Fig. 16-3). They have abundant eosinophilic cytoplasm and a specialized brush border along the margin of the cell that is adjacent to the bone surface that is being resorbed (Web Fig. 16-4). For osteoclasts to resorb bone, they must gain access to the bone surface that is usually covered by osteoblasts, and they must attach to the mineralized surface by transmembrane receptors in their sealing zones. These receptors bind to specific ligands in the matrix, likely residing in noncollagenous proteins. Osteoclasts are not able to bind to unmineralized bone matrix even though it contains these same ligands. Once bound to the matrix, the osteoclast resorbs bone in two stages. First, the mineral is dissolved by secretion of hydrogen ions through a proton pump located in the brush border. These hydrogen ions are derived from carbonic acid produced within the osteoclast from water and carbon dioxide by the enzyme carbonic anhydrase. Second, the collagen of the matrix is cleaved into polypeptide fragments by cysteine and metalloproteinases and cathepsins, particularly cathepsin K, released from the numerous lysosomes in the osteoclast and secreted through the brush border. The concavity in the bone created by the resorbed bone matrix is called a Howship’s lacuna, or a resorption lacuna. Physiologically, osteoclast activation is controlled by osteoblasts and bone marrow stromal cells (see later discussion of interactions). Calcitonin is a systemic inhibitor of osteoclasts. Osteoclasts have receptors for calcitonin and respond to this hormone by involuting their brush border and detaching from the bone surface. The activity of osteoclasts can be indirectly measured by determining serum concentrations of collagen degradation products (pyridinoline and deoxypyridinoline cross-links) or tartrate-resistant acid phosphatase (TRAP) activity. TRAP is a glycosylated monomeric metalloenzyme that is highly expressed by osteoclasts. TRAP staining also may be used as a cytochemical marker of osteoclasts in histologic sections (Web Fig. 16-5). Fig. 16-3 Osteoclasts, long bone, young dog. Web Fig. 16-4 Osteoclasts, brush border, bone, tibia, rodent. Web Fig. 16-5 Osteoclast, bone, pig. Osteoblast-osteocyte interactions are apparent from their connections to each other by thin, tortuous, cytoplasmic processes. This network of osteoblasts and osteocytes forms a functional membrane that separates the extracellular fluid bathing bone surfaces from the general extracellular fluid and can regulate the flow of calcium and phosphate ions to and from the bone fluid compartment (Fig. 16-4). Because of the large surface area of perilacunar and canalicular bone available for rapid ion exchange, significant amounts of calcium can be shifted from the bone fluid compartment to the extracellular fluid compartment without structural changes within the bone. In addition, this network allows osteocytes to detect alternations in the fluid flow within the bone extracellular fluid compartment. It is thought that such flow contributes to electric currents called streaming potentials. Changes in these streaming potentials caused by altered stress and strain on the bone or disruption of these potentials by microcracks (minuscule fractures within the bone visible only microscopically) might be detected by osteocytes with subsequent signaling to the overlying osteoblasts to initiate bone formation or resorption. Fig. 16-4 Schematic diagram of the movement of calcium. Osteoclast-osteoblast/stromal cell interactions are required for physiologic resorption of bone. The bone surface is protected from osteoclastic resorption by a continuous layer of osteoblasts and also by a very thin layer of unmineralized bone matrix normally present beneath resting osteoblasts (lamina limitans) (see Web Fig. 16-2). Osteoclasts are not able to bind to unmineralized bone matrix. For parathyroid hormone (PTH) to initiate bone resorption, receptors on the osteoblast bind PTH (osteoclasts do not usually express receptors for PTH). The binding of PTH to osteoblasts signals them to retract and secrete collagenases, which erode the unmineralized layer of matrix and allow osteoclasts access to a mineralized bone surface. In addition, osteoblasts and stromal cells that are activated by binding PTH and in response to a variety of other bone-resorbing stimuli (1, 25 dihydroxyvitamin D3; interleukin [IL]-1, IL-6, and IL-11; tumor necrosis factor-α [TNF-α]; prostaglandin E2 [PGE2]; and glucocorticoids) express or secrete receptor activator for nuclear factor κ B ligand (RANKL), also called osteoclast differentiation factor (ODF). RANKL binds to the RANK receptor on osteoclasts and activates the resorption process. Osteoblasts and bone marrow stromal cells also can secrete osteoprotegerin (OPG), a RANK homolog that works by binding to RANKL, thus blocking the RANK-RANKL interaction and inhibiting the differentiation of osteoclast precursors into mature osteoclasts. OPG expression can be stimulated by transforming growth factor-β (TGF-β). Therefore osteoblasts and stromal cells have the ability to both up- and down-regulate osteoclastic bone resorption (Fig. 16-5). In conditions of inflammation and necrosis, inflammatory mediators, such as IL-1 and TNF-α, can stimulate osteoclasts directly, causing bone resorption independent of the presence of viable osteoblasts. Fig. 16-5 Schematic diagram showing interaction between stromal cells/osteoblasts and osteoclast/osteoclast precursors. The mineralized matrix of bone provides the organ’s strength. Bone organic matrix consists of type I collagen and “ground substance” (the noncollagenous extracellular matrix: water, proteoglycans, glycosaminoglycans, noncollagenous proteins, and lipids, which are discussed later). Type I collagen polymers are secreted by osteoblasts and assembled into fibrils that are embedded in the ground substance and then mineralized. The type I collagen molecule is composed of three intertwined amino acid chains, unique to which is the hydroxylated form of the amino acid proline (hydroxyproline). Type I collagen molecules have extensive cross-linkages among the amino acid chains within the molecule and between adjacent molecules. Collagen molecules are deposited in rows with a gap between each molecule and with the rows staggered so that the molecules overlap by one-quarter of their length. This specific packing of the collagen molecules and the cross-linkages contribute to the strength and insolubility of the fibrous component of the bone matrix. Other than in rapidly deposited bone (woven bone found in primary trabeculae, bone of early fetal development, and pathologic conditions such as fracture repair), collagen fibers are arranged in parallel lamellae (singular: lamella) and the tissue is called lamellar bone. In cortical (compact) bone, lamellae are arranged concentrically (Fig. 16-6). In trabecular bone, the lamellae usually are arranged parallel with the bone surface. The collagen content of bone and its lamellar arrangement give bone its strength and flexibility. The ground substance of bone, which also is synthesized by osteoblasts, consists of noncollagenous proteins, proteoglycans, and lipids. Many of the noncollagenous proteins are cytokines that are capable of influencing bone cell activity. These cytokines, such as TGF-β, may play pivotal roles in controlling the extent of bone formation and resorption in normal remodeling and in disease (Fig. 16-7). Also, among the noncollagenous proteins are enzymes that can function in the degradation of collagen (e.g., matrix metalloproteinases) and can destroy inhibitors of mineralization (e.g., pyrophosphatases). Other noncollagenous proteins in the matrix can function as adhesion molecules and help bind cells to cells, cells to matrix, and mineral to matrix. Examples of these are osteonectin and osteocalcin. The role of proteoglycans in bone matrix is uncertain; however, there is some evidence that they influence bone cell differentiation and proliferative activity. Lipids may assist in binding calcium to cell membranes and in promoting calcification. Fig. 16-6 Osteonal remodeling, cortical bone. Endocortical surface of bone has undergone extensive osteonal remodeling. Fig. 16-7 Schematic diagram of the relationship between osteoclasts, osteoblasts, and growth factors. Bone mineral is in the form of a crystal called hydroxyapatite. Fully mineralized bone composes approximately 65% of the bone by weight and consists in part of calcium, phosphorus, carbonate, magnesium, sodium, manganese, zinc, copper, and fluoride. The mineral content gives bone its hardness. The production of osteoid (unmineralized organic matrix) by osteoblasts is followed by a period of maturation, after which mineral is deposited in exchange for water. Mineralization in woven bone is initiated within cytoplasmic blebs (matrix vesicles) of osteoblasts in the osteoid (see Web Fig. 16-1). Initiation of mineralization involves concentrating calcium, phosphorus, and other elements in these matrix vesicles to a level that causes precipitation of the mineral in the form of amorphous (not yet crystalline) hydroxyapatite. Matrix vesicles contain phospholipids and enzymes such as alkaline phosphatase and adenosine triphosphatase in their membranes. It is speculated that the membrane phospholipids attract calcium and phosphorus to the surface of the vesicle and that the alkaline phosphatase and adenosine triphosphatase enzymes might function in the pumping of these ions into the cell against a concentration gradient. In the cortex and subjacent to articular cartilage (subchondral bone), bone is organized into osteons (also called Haversian systems), which are cylinders of concentric layers of lamellae that are oriented parallel to the longitudinal axis of the bone and contain centrally located vessels and nerves (Fig. 16-8; see also Fig. 16-6). The bone between the osteons is called interstitial lamellae. Layers of bone oriented parallel to the internal and external circumference of the bone (beneath the endosteal and periosteal surfaces) are called circumferential lamellae. The osteonal system provides channels for the vascular supply to the cortex and also acts as tightly bound cables, giving the cortical bone strength and limited flexibility. This osteonal system also might be important in limiting propagation of microcracks in bone by diverting cracks along cement lines, which are collagen-poor, proteoglycan-rich seams between adjacent remodeling or modeling units (see later discussion). In H&E-stained histologic sections of decalcified bone, cement lines appear as basophilic lines. Fig. 16-8 Schematic diagram of the structure of compact and cancellous bone. In contrast to the dense compact bone of the cortex and the subchondral bone plates, the bone in the medullary cavity is in the form of anastomosing plates or rods and is called cancellous, trabecular, or spongy bone (see Figs. 16-8 and 16-10). The orientation of the trabeculae usually reflects adaptation (modeling) to mechanical stresses applied to the bone. This is readily apparent when examining the trabeculae in the femoral neck, which are oriented in lines and arcs that are perpendicular to the stress applied and are thicker and more numerous on the ventral aspect (side of compression) compared with the dorsal aspect (side of tension). The lamellae within a trabecula usually are arranged parallel to the surface of the trabecula and are not arranged into tubes or osteons, as they are in cortical bone. The remodeling unit of cortical bone is called the osteon, whereas the remodeling unit of trabecular bone is called the basic structural unit. The shape of the osteon is cylindric; however, the basic structural unit has the contour of a shallow trench filled with parallel lamellae. The time required from initiation to completion of these remodeling units (from initiation of bone removal by osteoclasts to complete filling of the defect by osteoblasts), regardless of the type of bone, is estimated to be 3 to 4 months in humans. Basophilic cement lines that mark the limit of previous resorptive activity are usually somewhat scalloped, following the contours of the Howship’s lacunae created by osteoclasts, and are called reversal lines (where resorption stopped and the process was reversed by formation) (Fig. 16-9). Cement lines also can occur when osteoblast formation ceases and subsequently resumes, and these are called “resting lines” and are usually smooth, following the contour of the overlying surface. Fig. 16-9 Remodeling in compact subchondral bone adjacent to a joint with bacterial infection, bone, horse. Individual bones of the skeleton vary in their manner of formation, growth, structure, and function. Flat bones of the skull develop by the process of intramembranous ossification, in which mesenchymal cells differentiate into osteoblasts and produce bone directly, in the absence of a preexisting cartilage model. In contrast, most bones develop from cartilaginous models by the process of endochondral ossification, in which cartilage is invaded by vessels, undergoes mineralization, and forms primary (diaphyseal) and secondary (epiphyseal) centers of ossification. Bones forming by endochondral ossification, such as the long bones of the appendicular skeleton and the vertebral bodies, are divided anatomically into epiphyses, metaphyseal growth plates (physes), metaphyses, and diaphyses (Fig. 16-10). Arterial blood from the systemic circulation enters bones through nutrient, metaphyseal, periosteal, and epiphyseal arteries (Fig. 16-11). Nutrient arteries penetrate the diaphyseal cortex through a nutrient foramen covered by strong, protective fascial attachments; once within the medulla, these arteries divide into proximal and distal intramedullary branches. Other arteries penetrating the cortex are the proximal and distal metaphyseal arteries, which are smaller and more numerous than the nutrient arteries. They penetrate the cortex and anastomose with the terminal branches of the nutrient arteries in the medullary cavity, protecting against infarction in case of obstruction of a nutrient artery. Fig. 16-11 Correlation of long bone development and vascularization. Bone grows in length by interstitial growth within the metaphyseal growth plates (physes) (Fig. 16-12). The mineralized longitudinal septa of the growth plates serve as struts on which bone is deposited, a process called endochondral ossification. The metaphyseal growth plate is primarily responsible for lengthening the bone and is divided into a reserve or resting zone, a proliferative zone, and a hypertrophic zone (Fig. 16-13). The hypertrophic zone is sometimes further subdivided into zones of maturation, degeneration, and calcification. The resting or reserve zone serves as a source of cells for the proliferating zone in which cells multiply, accumulate glycogen, produce matrix, and become arranged in longitudinal columns. In the hypertrophic zone, chondrocytes secrete macromolecules that modify the matrix to allow capillary invasion and initiate matrix mineralization. The hypertrophic zone chondrocytes eventually die. The overall lengthening of the bone is due both to chondrocyte proliferation and hypertrophy. Calcification begins in the longitudinal septa of cartilaginous matrix between columns of chondrocytes. Matrix vesicles derived from chondrocytes (analogous to those described previously for mineralization of bone) form in the hypertrophic zone and initiate the mineralization process. The processes of mineralization and vascular invasion of the growth plate are codependent events. To supply salts for the mineralization, a nearby blood supply is necessary, and vascular invasion, a critical step in endochondral ossification, does not take place in mammalian growth plates unless there is mineralization of the longitudinal septum. Blood vessels from the metaphysis invade into the advancing growth plate, providing an entryway for osteoblasts that form bone on the cartilage spicules (see Fig. 16-13). The chondro-osseous junction in the metaphysis is a fragile lattice of bone-covered spicules of calcified cartilage (primary trabeculae). As the growth plate advances and elongates the metaphysis, the more mature trabeculae deeper in the metaphysis become fewer and thicker (secondary and tertiary trabeculae) and often contain residual fragments of cartilage. Fig. 16-12 Growth plate, long bone, dog. Fig. 16-13 Schematic diagram of the major blood supply to the physis. Growth of the epiphysis contributes both to the overall length of the bone and to the shape of the ends of the bone. This is accomplished by endochondral ossification at the articular-epiphyseal complex (AEC). The AEC is composed of permanent articular cartilage, as well as a subjacent temporary growth/epiphyseal cartilage that is vascularized and contains the same zones as the growth plate (Fig. 16-14). In the mature individual, endochondral ossification no longer occurs at the AEC. Although the articular cartilage remains throughout life, the epiphyseal cartilage is completely replaced by bone once growth has ceased (Fig. 16-15). Bone grows in width by intramembranous bone formation. Except for articular surfaces (including the ends of the vertebral bodies), the surfaces of bones are covered by periosteum. This covering is a thin membrane that is loosely attached to underlying bone except at heavy fascial attachments on bony prominences and at tendon insertions, where its attachments are strong and are associated with large vessels penetrating the underlying bone. Microscopically, the periosteum is composed of an outer fibrous layer that provides structural support and an inner osteogenic or cambium layer, capable of forming normal lamellar appositional bone on the cortex of growing bones and of forming abnormal woven bone formation in response to injury (Fig. 16-16). The periosteum is well supplied with lymph vessels and with fine myelinated and nonmyelinated nerve fibers that account for the intense pain that occurs with periosteal injury. Fig. 16-14 Articular-epiphyseal complex (AEC), bone, immature pig. Fig. 16-15 Articular cartilage, bone, adult dog. Fig. 16-16 Periosteum, bone, dog. Articular (hyaline) cartilage is normally a white to blue-white material with a smooth, moist surface. Cartilage thickness is greatest in the young and at sites of maximal weight bearing. Thinning and yellow discoloration occur in old age. At its margins, articular cartilage merges with a periosteal surface that is lined by fibrous tissue contiguous with the synovial membrane. Synovial fossae are normal depressions on non–weight-bearing articular cartilage surfaces (Fig. 16-17) that develop bilaterally in the larger appendicular joints of the horse, pig, and ruminant. These fossae are not present at birth but are fully formed by skeletal maturity. The function of synovial fossae is not known; however, they are significant because they are often mistaken for lesions. Adult articular cartilage contains no nerves or blood or lymph vessels, and its nutrients are obtained by diffusion from synovial fluid and to a lesser extent from subchondral vessels. In the immature skeleton, articular cartilage overlies the temporary growth cartilage of the epiphysis (epiphyseal cartilage). The epiphyseal cartilage is highly dependent on a network of blood vessels that originate from the perichondrium and the subchondral bone. Similar to the physis, it undergoes endochondral ossification and thereby contributes to the growth/development of the epiphysis. At skeletal maturity, the epiphyseal cartilage has been entirely replaced by bone, and the only joint cartilage remaining is the adult articular cartilage, which includes a thin subjacent zone of mineralized cartilage. In skeletally mature individuals, the junction between the unmineralized articular cartilage and the deeper mineralized cartilage forms a thin basophilic line in H&E stained sections called the tidemark (see Fig. 16-15). As animals age, multiple tidemarks can be formed, indicating an advance (thickening) of the mineralized layer of articular cartilage and thinning of the overlying nonmineralized articular cartilage. The mineralized cartilage serves to anchor articular cartilage to subchondral bone and limits the diffusion of substances between bone and cartilage. Fig. 16-17 Synovial fossa, joint, radius and ulna, bone, proximal end, adult horse. The articular capsule consists of outer fibrous and inner synovial tissue layers. The outer layer is a heavy sheath that contributes to joint stability and at its insertion, attaches to bone at the margins of the joint, thereby enclosing a segment of bone of variable length within the joint cavity. It is well supplied with blood vessels and nerve endings. The inner synovial tissue layer is called the synovial membrane and covers all the inner surfaces of the joints except for the surface of the articular cartilage. The synovial membrane is normally very thin, lacks a basement membrane, and is barely visible grossly. In fact, collection of normal synovial membrane for histologic evaluation is best done immediately after opening the joint because otherwise, it will retract and become difficult to locate. The inner surface of the synovial membrane may be flat or may contain tiny projections (villi). Synovial intimal or lining cells, one to four cells thick, form a discontinuous surface layer and include two cell types: (1) A cells, which are macrophages that are responsible for the removal of microbes and the debris resulting from normal wear and tear in the joint (Fig. 16-18) and (2) B cells, which are fibroblast-like cells that produce synovial fluid. Normal synovial fluid contains hyaluronic acid, lubricin (a water-soluble glycoprotein), proteinases, and collagenase and is clear, colorless to pale yellow, and viscous. In addition to lubricating the joint surfaces, it supplies oxygen and nutrients to and removes carbon dioxide and metabolic wastes from the chondrocytes in articular cartilage. The synovial subintima can be classified according to the type of tissue that predominates (areolar, adipose, or fibrous), and contains blood and lymph vessels that supply and drain the intraarticular structures. Adipose tissue sometimes accumulates in the deep layers of the synovium, forming fat pads that serve as soft cushions in joint cavities (e.g., the infrapatellar fat body of the stifle joint). Fig. 16-18 Synovial membrane, joint, dog. The subchondral bone acts to support the overlying cartilage and dissipate concussive forces to the peripheral cortical bone (Fig. 16-19). The thickness of the subchondral bone plate varies in proportion to the degree of weight bearing. Increased subchondral bone thickness is a common pathological finding in osteoarthritis. In larger animals (nonrodent species), subchondral bone often is composed of compact osteonal bone rather than trabecular bone. A tendon is a unit of musculoskeletal tissue that transmits force from muscle to bone. Morphologically, tendons are similar to ligaments and fascia; however, ligaments join bone to bone and fascia connects muscle to muscle. The musculotendinous unit is composed predominately of parallel arrays of closely packed collagen fibers and rod- or spindle-shaped fibroblast-like cells (tenocytes) within a well-ordered extracellular matrix (Fig. 16-20). Collagen fibrils are bundled into large fibers that are evident throughout the tendon and are visible under light microscopy as a crimped or a sinusoidal pattern that facilitates a 1% to 3% elongation of the tendon. This elongation of the individual fibers serves to buffer the tendon from sudden mechanical loading. Water represents approximately 55% of the weight of tendon, is present mainly in the extracellular matrix, and is believed to reduce friction, facilitating the gliding of fibrils in response to mechanical loading. The major fibrillar component of tendon is type I collagen, which constitutes approximately 80% of the dry weight, whereas type III collagen is present in the endotenon and epitenon. The remainder of the tendon components include elastin, proteoglycans, and inorganic components. Collagen is synthesized by the tenocytes and constitutes the basic structural unit of tendon. The collagen polypeptides form a triple helix, which self-assembles into collagen fibrils with intermolecular cross-links that form between adjacent helixes. The collagen polypeptides and the ensuing triple helix are synthesized inside the cell, secreted into the extracellular matrix, and assembled into the microfibrillar units that constitute the collagen fibers. This step is promoted by a specialized enzyme called lysyl oxidase, which promotes cross-link formation—a process involving placement of stable cross-links within and between the molecules. Crosslink formation is the critical step that gives collagen fibers their strength. Tendon is stronger per unit area than muscle, and its tensile strength equals that of bone, although it is flexible and slightly extensible. The parallel arrangement of tendon collagen fibers resists tension so that contractile energy is not lost during transmission from muscle to bone. The mechanical properties of tendons depend on the collagen fiber diameter and orientation; however, the proteoglycan components of tendons also are important to the mechanical properties. While the collagen fibrils allow tendons to resist tensile stress, the proteoglycans allow them to resist compressive stress. Fig. 16-20 Tendon, joint, mouse.
Bones, Joints, Tendons, and Ligaments
Structure and Function of Bone
Prominent cuboidal osteoblasts (arrows) line the endosteal surface. Hematopoietic marrow is present (above the endosteal surface). More mature (older) osteocytes are present deeper in the cortex (below the endosteal surface) and are recognizable as small elliptical cells (arrowheads) residing in lacunae. The more recently embedded (younger) osteocytes are in larger lacunae closer to the endosteal surface. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
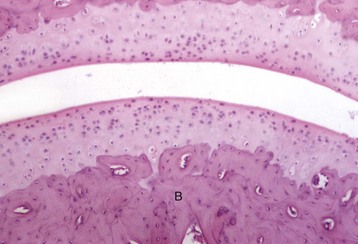
Quiescent osteoblasts (arrows) on the endosteal surface of adult bone are fusiform with little cytoplasm and form an inconspicuous layer between the cortical bone (below the endosteal surface) and active hematopoietic marrow (above the endosteal surface) of the adult axial skeleton. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
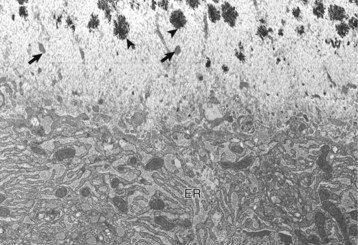
Osteoblast (below) with abundant endoplasmic reticulum (ER) on an actively mineralizing surface. Only a portion of the cell, not including the nucleus, is shown. Cell processes (arrows) of the osteoblasts extend out into the osteoid (above). Mineralization (black spicules) is initiated within matrix vesicles (arrowheads), then extends onto the adjacent collagen. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
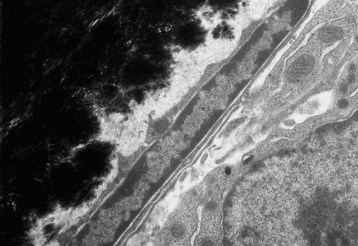
Quiescent osteoblast has reduced cytoplasmic volume due to the less-developed or less-active organelles, which are necessary for collagen synthesis. The narrow rim of unmineralized (light gray) bone matrix between the osteoblast and the mineralized bone (black) is termed the lamina limitans. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
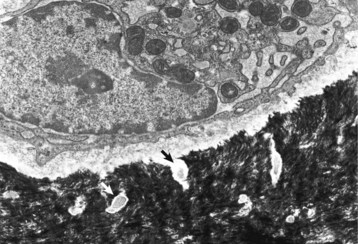
A recently embedded osteocyte still has residual rough endoplasmic reticulum and Golgi apparatus that were used during its osteoblastic period. Cell processes extend into the mineralized matrix (black) through tunnels called canaliculi (arrows). TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
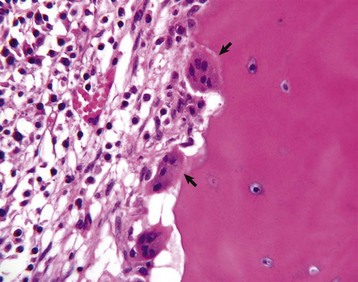
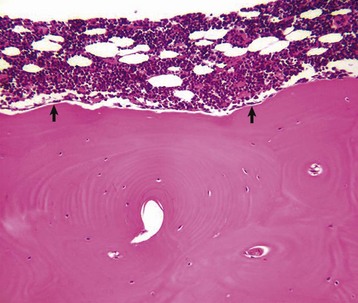
Several multinucleated cells (osteoclasts) (arrows) are resorbing endocortical bone and creating a scalloped endosteal surface. These scalloped cavities of resorbed bone are called Howship’s (or erosion) lacunae. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
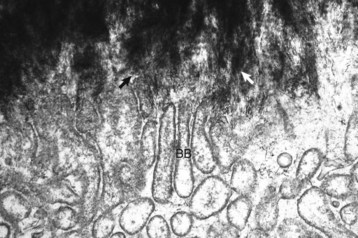
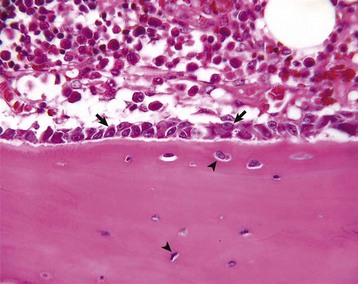
Spicules of hydroxyapatite (arrows) are apparent between the projections of the osteoclast brush border (BB). The crystals have been separated from the matrix and are in the process of being dissolved by acid and enzyme secretions across the brush border. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
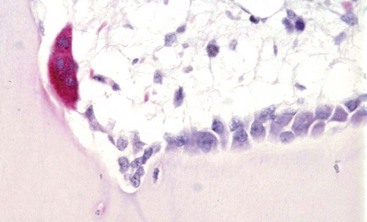
Tartrate-resistant acid phosphatase-positive osteoclast (multinucleated cell with red cytoplasm) on the surface of mineralized bone in an area of active remodeling. Plump, cuboidal osteoblasts line the remainder of the bone surface. Marrow space is above, bone is below. TRAP stain. (Courtesy Dr. C.S. Carlson, College of Veterinary Medicine, University of Minnesota.)
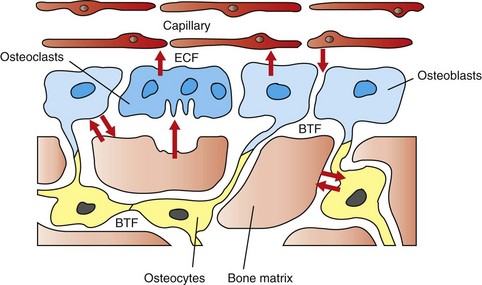
The hypothesized intracellular and extracellular movement of calcium (arrows) in bone and the relationships of osteoblasts (blue, single nuclei), osteocytes (yellow), and osteoclasts (blue, multiple nuclei) to blood vessels, extracellular fluid (ECF), and bone tissue fluid (BTF) compartment is shown. (Redrawn from Matthews JL, Vander Wiel C, Talmage RV: Adv Exp Med Biol 103:456, 1978.)
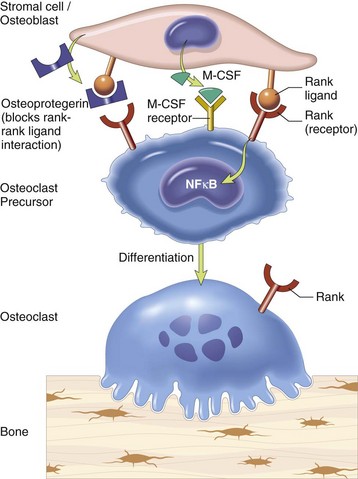
Stromal cells and osteoblasts produce “rank ligand,” which binds to the “rank” receptor on the osteoclast and its precursors; this binding stimulates osteoclasis. Conversely, osteoblasts and stromal cells can inhibit the activation of osteoclasts by secreting osteoprotegerin, which can bind to “rank ligand” and block its binding to “rank receptor.” M-CSF, Macrophage colony-stimulating factor; NFκB, nuclear factor κ B. (From Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
Bone at the Organic Matrix and Mineral Level

Collagen fibers are birefringent when viewed in appropriate plane with polarized light; alternate lamellae polarize when viewed in the same plane. This alternating pattern of birefringence demonstrates the parallel arrangement of collagen layers in lamellar bone. Much of the cortex has been remodeled into osteonal bone (concentric layers with central channel for vessels and nerves), but there are a few small areas of cortex that remain unosteonized (unremodeled). Unstained and fully mineralized. Polarized light micrograph. (Courtesy Dr. L. P. Krook, College of Veterinary Medicine, Cornell University.)
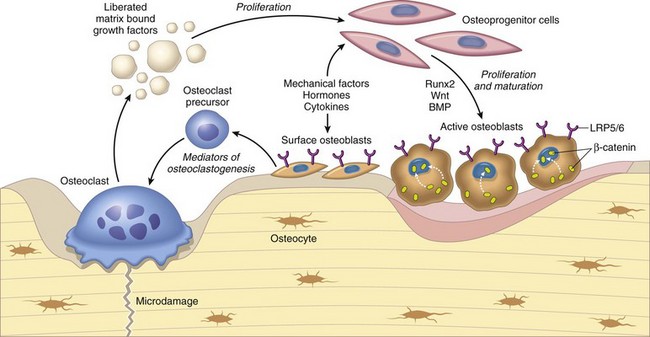
Osteoclasts are able to liberate and activate growth factors from the matrix that are stimulatory to osteoblast progenitor cells and “couple” the process of osteoclastic bone lysis with subsequent bone formation. (From Kumar V, Abbas AK, Fausto N et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
Bone as a Tissue
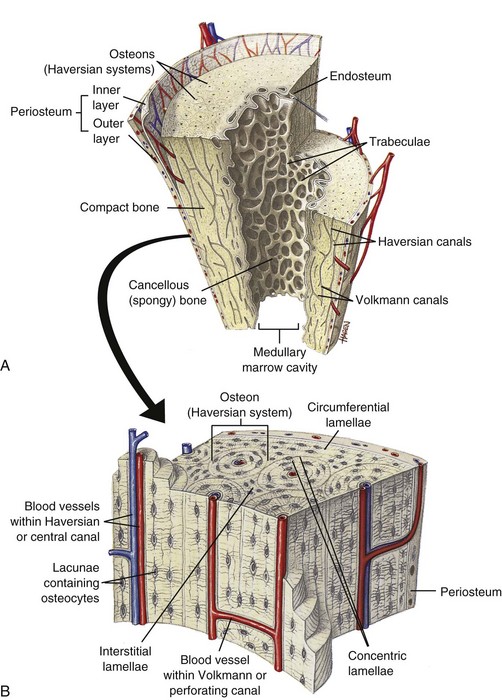
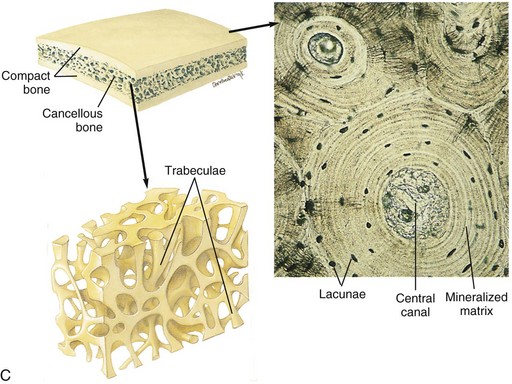
A, Longitudinal section of a long bone showing both cancellous and compact bone. B, Magnified view of compact bone. C, Section of a flat bone. Outer layers of compact bone surround centrally located cancellous bone. Fine structure of compact and cancellous bone is shown at a higher magnification. (From Thibodeau GA, Patton KT: Anatomy and physiology, ed 6, St Louis, 2007, Mosby.)
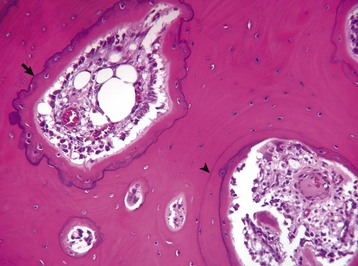
Resting cement lines appear as basophilic smooth lines indicating where formation has temporarily stopped (arrowhead). Reversal lines are scalloped basophilic lines (arrow) indicating where bone resorption stopped and was followed by formation. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
Bone as an Organ
Blood Supply to Bone
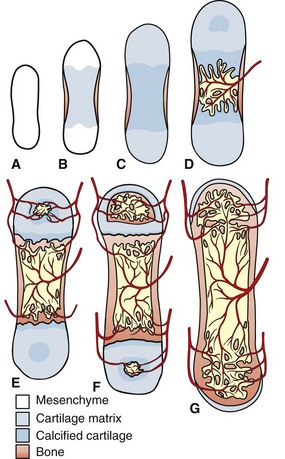
The primitive mesenchyme that makes up the skeletal primordia contains no blood vessels (A). This mesenchyme condenses and undergoes central mineralization; a bony collar forms in the periosteum of the diaphysis (B and C). The nutrient artery enters the mineralized cartilaginous tissue in the diaphysis, bringing osteogenic and osteoclast precursors and enabling endochondral ossification to occur (primary center of ossification) (D). Similarly, the epiphyseal arteries bring these cells to the secondary centers of ossification in the epiphyses (E). Extensive anastomoses develop as the bone continues to develop and as the growth plates close (F and G). (Redrawn from Banks WJ: Applied veterinary histology, ed 3, St Louis, 1993, Mosby.)
Bone Growth
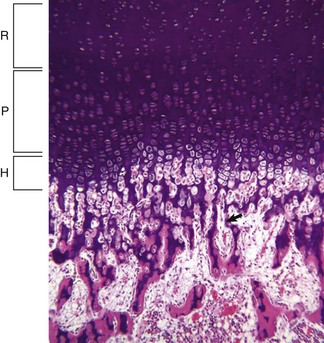
Resting (R), proliferating (P), and hypertrophic (H) zones of the growth plate are visible. Apoptotic chondrocytes are released from their lacunae by invading vessels and chondroclasts leaving only the longitudinal septa (arrow) as a template on which bone will be deposited to form a primary trabeculae. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
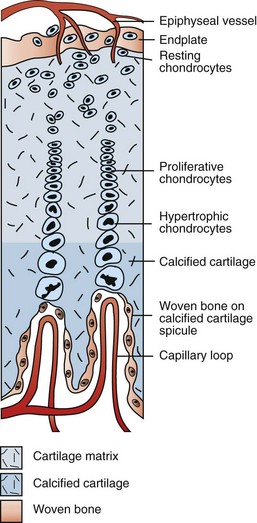
Branches of the epiphyseal artery supply the resting zones of the growth plate. Branches of the metaphyseal artery form capillary loops at the metaphyseal side of the physis where endochondral ossification is occurring. (Redrawn from Banks WJ: Applied veterinary histology, ed 3, St Louis, 1993, Mosby.)
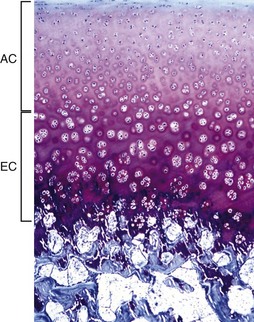
The AEC is composed of a layer of articular cartilage (AC) and a subjacent layer of growth (epiphyseal) cartilage (EC). The growth cartilage is present only in immature individuals, and its structure and function is similar to that of the physis. Compare with Fig. 16-15. Toluidine blue stain. (Courtesy Dr. C.S. Carlson, College of Veterinary Medicine, University of Minnesota).
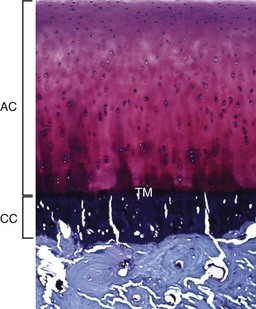
The articular cartilage is present throughout life; however, the epiphyseal cartilage is absent in adults. In adults, endochondral ossification has ceased and the tidemark (TM) delimits the boundary between the uncalcified articular cartilage (AC) and the calcified cartilage (CC). Compare with Fig. 16-14. Toluidine blue stain. (Courtesy Dr. C.S. Carlson, College of Veterinary Medicine, University of Minnesota.)
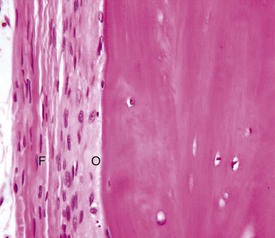
The outer fibrous layer (F) and inner osteogenic layer (O) line the periosteal surface. The osteogenic layer is able to rapidly deposit woven bone as a nonspecific response to injury. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
Structure and Function of Joints
Articular Cartilage
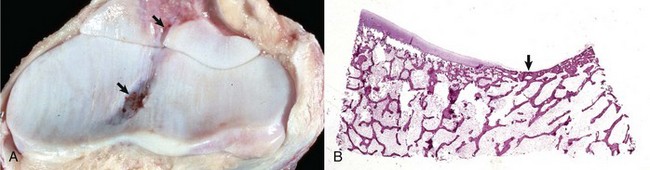
A, Synovial fossae are depressions in the cartilage on the non–weight-bearing surfaces of the sagittal ridge of the radius and in the semilunar notch of the ulna (arrows). The parallel linear grooves apparent on the weight-bearing surface (articular cartilage) of the radius are the result of degenerative joint disease. B, Histologically, the surface of the synovial fossae is covered by a thin fibrous membrane (arrow) rather than articular cartilage. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
Articular Capsule/Synovium/Synovial Fluid
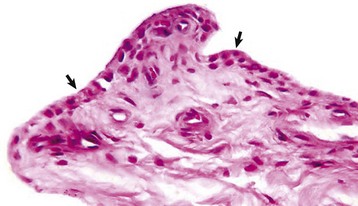
The normal synovial membrane (arrows) consists of an incomplete layer of histiocytes (phagocytic cells) and fibrocytes with subjacent loose fibrous and/or fibrofatty tissue. The joint lumen is at the top of the figure. H&E stain. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
Subchondral Bone
Structure and Function of Tendons and Ligaments
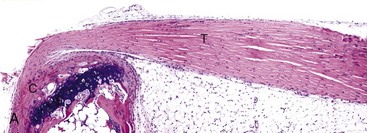
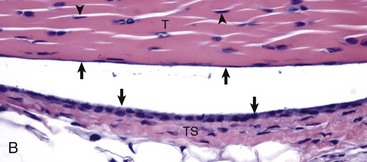
A, Gastrocnemius tendon (T). The tendon is composed of parallel arrays of closely packed collagen fibers and low numbers of fibroblast-like cells called tenocytes (see higher magnification in B; arrowheads). The tendon is covered by flattened synoviocytes (see higher magnification in B), so it may glide smoothly over the synoviocytes of the tendon sheath. C, Calcaneus insertion. H&E stain. B, Higher magnification of tendon sheath. The enveloping structure enclosing the tendon is the tendon sheath (TS), and it is lined by synoviocytes (arrows). T, Gastrocnemius tendon. H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine