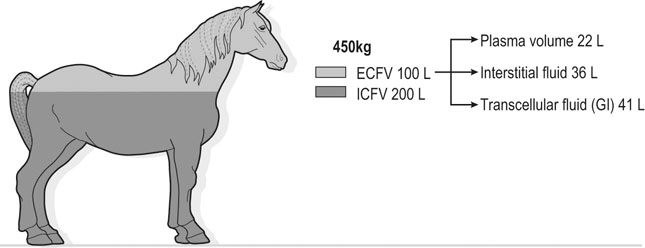
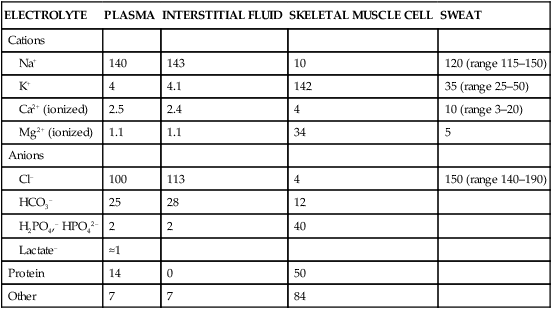
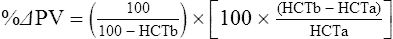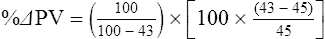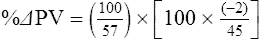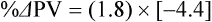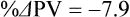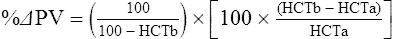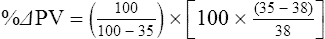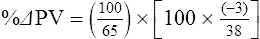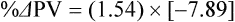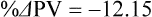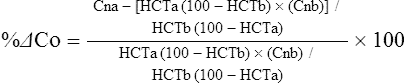The exercising horse produces a tremendous amount of metabolic heat. This byproduct of the transduction of potential energy into kinetic energy can raise core body temperature in the horse from 37°C at rest to temperatures exceeding 42°C in a matter of minutes.1–6 Normal cellular function, however, requires active and efficient ways to keep core body temperature within narrow limits. To do this a horse must move heat produced in the muscles to the periphery.1–6 Other mammalian species, like the rabbit and dog, have an enhanced countercurrent brain blood-flow mechanism that couples with panting to provide for cooling of the brain while permissively allowing heat storage in the rest of the body and a rise in body temperature.7,8 Similarly, horses have a singular anatomical feature involved in brain cooling; their internal carotid arteries are in close contact with the air-filled guttural pouches and it is suggested that blood reaching the brain has a lower temperature compared to mixed venous blood given this unique arrangement (see Chapter 41).9,10 Horses and humans are the only species that cool primarily through the evaporation of sweat.1,5,7,8 Sweating can produce tremendous fluid and electrolyte losses that, if uncompensated for, can lead to cardiovascular and thermoregulatory instability. These fluid deficits and their effects are even more pronounced during endurance exercise and exercise performed during periods of high environmental temperature and humidity.1–3,5–7 Work focused on preventing thermal injuries in horses has documented findings similar to those published in the human sports medicine literature: namely, that strategies aimed at maintenance of fluid and electrolyte balance prevent dehydration and provide thermoregulatory and cardiovascular stability.1–8,11–14 Data demonstrate that optimal fluid and electrolyte balance delays the onset of fatigue. The present chapter reviews the current literature on the effects of exercise and training adaptations on fluid balance and renal function in horses. Like all animals, the body of the horse is primarily composed of water and electrolytes. Those solutions are compartmentalized within and outside the cells. This combination of intracellular and extracellular water is referred to as the total body water (TBW). The TBW accounts for 50–70% of bodyweight, or 250–350 kg of the bodyweight of a typical 500 kg horse.1,2,11 TBW can be measured using various indicator dilution techniques, stable isotope techniques, and bioelectric impedance technologies.1,2,11 Each of these techniques has advantages and disadvantages, with the use of stable isotope infusion being one of the most accurate but technically demanding and bioelectric impedance technology the least reliable. The TBW is divided by cell membranes into two primary fluid compartments, the intracellular fluid compartment (ICF) and the extracellular fluid compartment (ECF).1,2,8,11 Approximately two-thirds of the TBW (~200 L) is contained within the cells of the body, leaving one-third of the water in the ECF space (~100 L) (Fig. 38.1). According to Carlson,2 the latter is further compartmentalized into fluid contained within the vascular space, the interstitial fluid space, the lymphatics, and transcellular fluids. This last category includes the fluid content of the gastrointestinal tract, which represents a large reservoir of fluid.2 The vascular space or total blood volume is filled with a mixture of fluid and cells, the latter primarily red blood cells, but also including white blood cells and platelets. Thus, the total blood volume (BV) is the combination of the plasma volume (PV) and the red cell volume (RCV) or stated as a formula:2 BV = PV + RCV. Blood volume varies from breed to breed, with age, body composition, hydration status, and training status.2,15 Across breeds, studies have reported total blood volumes ranging from 61 mL/kg in draft horses to 137 mL/kg in racehorses.1,2,11 In the average 450 kg horse, total BV would be about 36 L, PV ~20 L and RCV around 16 L. 1,2,11 The eloquent work of Persson15 and many others 2,16–18 has shown that there is a strong relationship between red blood cell volume and aerobic performance in the horse. Oxygen uptake and delivery is dependent on both optimal volume to insure cardiac filling pressure and an optimal number of red blood cells to carry oxygen. Much focus has been placed on the need to have red blood cells to carry oxygen to the working muscles. However, while RCV and PV are usually looked at independently, they are interdependent in the optimization of blood flow during exercise. Blood flow can be affected by changes in viscosity; thus, too many red blood cells and not enough plasma can cause a substantial change in viscosity, as well as other factors related to resistance to flow. The values for BV, PV, and RCV in the literature also vary with the differing methodologies used in different laboratories to measure and/or calculate total blood volume.2,15–18 For the most part, studies of equine blood volume have not used direct measurement of BV. Instead, they have generally used dye or indicator dilution techniques to measure PV and then calculated BV using the measured PV and hematocrit (HCT):2,15–18 The measurement of PV using dye or indicator dilution techniques requires the use of an indicator that stays within the vascular compartment for a long enough time to reach full steady-state distribution without substantial removal through the metabolism of the dye by the tissues.2,17 Ideally, this requires an indicator that binds to a large molecule that does not readily leave the vascular compartment. Two substances commonly used to measure PV in the horse are indocyanine green (IC-green or cardiac green) dye and Evans Blue dye.2 While IC-green dye has been used to measure plasma volume in the horse, one must caution that because it is rapidly cleared from the vascular compartment, it is better suited for the repeated injections required for the measurement of cardiac output. The rather short half-life of IC dye means it can be cleared before reaching full distribution, affecting the accuracy and repeatability of PV measurements in the horse. Evans Blue dye binds to albumin and, thus, has a relatively long half-life and stays for the most part in the vascular compartment.2,15,17 However, one must caution that albumin can shift out of the vascular compartment if there is an increase in hydrostatic pressure induced by manipulations such as exercise or epinephrine infusion. Thus, the measurement of PV using the Evans Blue dye dilution technique requires that a horse be standing relatively quietly and unperturbed by exercise or pharmacological manipulations for the 15- to 20-min mixing period between collection of a blank plasma sample and injection of the dye and the collection of a postinjection blood sample.17 Anything that disturbs the steady state of the cardiovascular system affects distribution of the dye; therefore, one should view with extreme caution published studies reporting PV, BV, and RCV values calculated using a postinjection sample obtained after exercise as the readings can be skewed in two ways:15 first, decreases in PV due to water shifts out of the vascular compartment caused by increases in hydrostatic pressure that would give artificially high concentration of the dye and, second, errors caused by the loss of dye from the vascular compartment due to extrusion of albumin out of the bloodstream by the same increases in hydrostatic pressure.3,19–22 Plasma volume can decrease 15–20% after only three 1-min steps of an incremental exercise test;19 therefore, errors in the measurement of plasma volume due to non-steady-state sampling would also affect the calculation of BV and RCV using the above formula. Another factor that must be considered in the calculation of total BV in the horse is the splenic reserve volume. The horse is somewhat unique compared to most other mammalian species in that the spleen is a very capacious and capricious organ, storing between 6 and 12 L of red-cell-rich blood at rest.2,15,20,22 Splenic blood typically has a hematocrit of ~65–75%.2,15,20,22 Thus, studies of total BV become somewhat problematic because measurement of the total circulating BV requires an accounting for the splenic reserve volume, a measurement that requires mobilization of the splenic red cell reserve. Most studies to date have utilized exercise or infusion of epinephrine or an α-adrenergic agonist drug to cause splenic contraction, with a blood sample obtained for the measurement of hematocrit after the accommodation and the mixing of the extra volume of blood. Complete mixing takes only 1–2 min; however, in many studies, the hematocrit used to calculate BV and RCV was taken at the end of or after an incremental exercise test.15,18 While this is an accepted way to cause splenic contraction and a viable way to estimate the contribution of the splenic reserve to the total circulating blood volume, the resulting hematocrit values are skewed upward by the dynamic fluid shifts caused by the changes in flow and hydrostatic pressure induced by the exercise or pharmaceutical manipulation. Therefore, the hematocrit used to calculate total BV would reflect both the contribution of splenic reserve mobilization and reductions in plasma volume and would be an overestimation of total blood volume. This is essentially an offset error and, because acute reductions in PV caused by exercise-induced fluid shifts are linked to exercise intensity,3,19–21 the fluid shifts that lead to this overestimation only become a problem if a study’s experimental design uses different exercise intensities to measure the hematocrits used in comparisons between treatment groups or comparisons made before and after training. For example, if one calculates BV using a hematocrit obtained at the 10 m/s step of a treadmill test it will yield a different result than the value calculated using the hematocrit collected at the 11 or 12 m/s step of an incremental treadmill test. As mentioned above, the absolute value for resting plasma volume can be determined using Evans Blue dye. However, measurement of PV during exercise and any resulting decreases are problematic because of mixing time, the requirement for steady-state conditions, and the potential for overwhelming the vascular space with dye through repeated injections. Percent changes in PV can be measured using changes in protein concentration.19 However, because some protein leaves the vascular compartment, this method tends to underestimate the reduction in PV.19 To get around these methodological problems, studies of human athletes23–25 have utilized changes in hematocrit to calculate percent changes in PV (Box 38.1,). Absolute volume changes in liters are then calculated using the previously measured absolute resting PV determined using Evans Blue dye.23–25 The calculation of percentage change in PV using hematocrit is feasible because red blood cells do not leave the vascular compartment like protein molecules and any change in their concentration must be due to changes in plasma volume.23–25 In humans, the calculation is simple and involves the use of a pre-exercise hematocrit (HCTb) and hematocrits measured during or after exercise (HCTa).23–25 For example, if the hematocrit measured before exercise (HCTb) was 43 and the hematocrit measured in a blood sample obtained after 10 min of exercise was 45, then the change in plasma volume is – 7.9%. Thus, one can see that a relatively small change in hematocrit represents a much larger change in plasma volume. However, it is important to note that the use of this formula requires that there is no addition of red blood cells to the central circulation or change in the size of the cells.23–25 The latter has been shown to not be a problem if exercise duration is less than 120 min.25 The former makes the use of this formula problematic for those doing horse research because the spleen adds RBCs to the central circulation. Fortunately, McKeever et al. have developed a correction factor obtained after comparing sequential blood samples taken from splenectomized and intact horses.19 These studies demonstrated that the spleen contracts very rapidly with both the extruded volume and cells accommodated and mixed with the central circulation within the first 1 to 1.5 min of exercise.19 Changes in hematocrit from the point of full mixing onward paralleled each other in both groups of horses. Thus, the changes in hematocrit from that point on were due to decreases in plasma volume caused by fluid shifts and loss of water from the vascular compartment.19 More importantly, the difference between the pre-exercise and the 2-min values for hematocrit in the intact horses represented an offset due to splenic reserve mobilization that could be used as a correction factor.19 The example in Box 38.2 demonstrates how to use hematocrit in the horse to calculate percent changes in PV. For example, if a horse had a resting hematocrit (HCTb) of 35 and the hematocrit measured at 2 min of exercise (HCTraw) was 55 then the difference between the two would be the calculated correction factor (HCTcorrected) to be used to correct all the hematocrits measured after the 2-min point of exercise onward. Thus, in the example in Box 38.2, if the uncorrected hematocrit obtained at 15 min of exercise was 58 (HCT), then the value for HCTa to be used in the formula would be obtained by subtracting the correction factor from the uncorrected hematocrit. More recently, multifrequency-bioelectrical impedance analysis (MF-BIA) has been applied to estimate fluid volumes and their fluctuations in adult horses. This technique offers the advantage of providing immediate results and is non-invasive. Indicator dilution methods have been used to estimate total body water (TBW), extracellular fluid volume (ECFV), intracellular fluid volume (ICFV) by difference of these two, and plasma volume (PV). Specifically, deuterium oxide has been used to estimate TBW in adult horses,26–28 bromide and sodium thiocyanate have been used to determine ECFV,27,29,30 and plasma and blood volume have been estimated by use of Evans blue dye.17,27 However, indicator dilution methods are not clinically applicable in situations of rapidly changing fluid balance because they require time to reach steady state, prolonged equilibration periods, and specialized laboratory equipment, and as such they are not practical for everyday clinical use.31 In studies recently performed in adult horses at rest, after prolonged exercise and after experimentally induced fluid shifts, MF-BIA appears to be a useful technique to estimate changes in TBW and ECFV, but not as reliable to estimate ICFV.31,32 Normal cellular function is vitally linked to maintenance of fluid, electrolyte and acid–base balance within a narrow range.2,3,25,33–35 Thus, the composition of both the plasma within the vascular compartment and the fluid within the intracellular fluid space is tightly controlled.2,3,25,33–35 Key to maintenance of the internal environment is a regulation of overall plasma osmotic concentration or osmolality as well as the concentration of key electrolytes such as sodium, potassium, chloride.2,3,25,33–35 Sodium is the major cation contributing to osmolality and is the major cation in the extracellular fluid space.2,3,25,33–35 Potassium on the other hand is the primary cation found within the cells.2,3,25,33–35 Other important cations include calcium and magnesium, both primarily intracellular ions.2,3,25,33–35 When considering exercise, it is the calcium found within the muscle in the tubular sarcoplasmic reticulum that is important.2,3,25,33–35 This calcium plays a vital role in the process of excitation contraction coupling. Magnesium found within the cells is an important cofactor in many of the reactions involved in various metabolic pathways.2,3,25,33–35 Major anions include chloride, bicarbonate, and the phosphates.2,3,25,33–35 Normal values for resting concentrations of the major electrolytes found in plasma, interstitial fluid, intracellular fluid and sweat can be found in Table 38.1. All of the electrolytes contribute to the osmotic concentration of the body fluids, a variable that is tightly regulated to prevent cell dehydration or cell swelling. Table 38.1 Electrolyte composition (mEq/L) of plasma, interstitial fluid, intracellular fluid (muscle), and sweat The osmotic concentration or osmolality of the plasma is essentially the same as that of the rest of the interstitial fluids.36,37 Normal plasma osmolality in the horse and most other mammals averages 290 mOsm/L.2 Plasma osmolality is the total number of dissolved particles in solution, independent of the elemental species making up that solution.36 Plasma osmolality reflects the osmolality of both the extracellular fluid space and the intracellular fluid space36 and is important for two reasons. First, large molecules in solution exert osmotic force across semipermeable membranes such as capillaries and cell membranes. Thus, plasma osmolality is a measure of the total ‘osmotic pull’ or osmotic force that is exerted by the sum of freely moving particles in solution exerting an effect on water in surrounding tissues.36,37 Because water tends to move down a concentration gradient from an area of low concentration to an area of high concentration, an increase or decrease in plasma osmolality has the capacity to dramatically alter normal cellular function by causing fluid shifts into and out of the cells, shifts that can decrease cell function.36,37 Second, a change in osmolality reflects expansion or contraction of the extracellular fluid compartment.36,37 Optimal cardiovascular function is highly dependent on fluid volume status and mechanisms associated with the maintenance of plasma osmolality and extracellular fluid volume serve as one of the first lines of defense in the regulation of cardiac filling volume and pressure and ultimately mean arterial pressure and the ability to perfuse the tissues.20,36,37 During exercise the horse can lose tremendous volumes of isotonic to slightly hypertonic sweat, presenting a serious challenge for maintaining the volume and the composition of the body fluids.1,2,20 Dramatic changes can compromise cellular function.37 It can also compromise cardiovascular stability through a reduction in venous return and cardiac output. Therefore, it is vitally important that the body regulates plasma osmolality within very narrow limits.37 Defense of osmolality is vitally intertwined with the defense of extracellular fluid volume, plasma volume, and cardiac filling pressure.20,37 Thus, defending plasma osmolality involves an integrative response of multiple systems, including the cardiovascular, neural, endocrine, and renal systems.6,12,20,36–39 Changes in plasma osmolality are sensed by specialized cells within the supraoptic and paraventricular nuclei of the hypothalamus.20,36–38 These osmoreceptors are very sensitive and changes in plasma osmolality as small as 2 mOsm/L can evoke a change in the synthesis and secretion of the hormone arginine vasopressin (antidiuretic hormone) by the posterior pituitary.38 Changes in circulating vasopressin concentration occur rapidly and can cause dramatic alterations in renal handling of water within minutes, thus correcting volume deficits and swings in the concentration of osmotically active substances through losses of plasma water or electrolytes in the sweat.38,39 Vasopressin also stimulates thirst and drinking, which ultimately affects water balance and osmolality.38 When considering the effects of acute exercise on changes in key electrolytes one must distinguish between changes in the concentration versus changes in the total content of those electrolytes.25,40 By definition, the concentration of a substance is the amount of solute in a given volume of solvent. Content, on the other hand, is the total amount of that solute in the fluid compartment or body depending on the focus of analysis. For example, normal plasma concentration sodium is 140 mEq/L or, put another way, there are 140 mEq of sodium per liter of plasma. Plasma content of sodium would be obtained by multiplying the concentration of sodium by the plasma volume:25,40 Calculation of changes in the content of key electrolytes and other substances allows one to determine if changes in the concentration of a substance is the result of addition or loss of the substance or just due to changes in plasma water.25,40 When viewed on a whole-body level, changes in content allow one to make calculations giving insight into how concentrations of tightly regulated plasma constituents are affected through routes of intake or loss that affect whole-body balance of said constituent.25,40 Acutely, calculation of relative or percent changes in the content of plasma volume and the plasma constituents gives one insight into the dynamic changes that occur in response to the challenge of exertion.25,40 To that end, human exercise physiologists for years have used key formulae to calculate percentage changes in plasma volume and percentage changes in the content of various plasma constituents during exercise (Box 38.3).23–25 If one knows the resting plasma volume, then once one calculates the percentage change in the content of a plasma constituent, one can calculate the total amount of that substance lost during exercise from the vascular compartment.25,40 On a practical level, calculation of changes in the content of various plasma constituents can give insight into their disposition. An example of this would be an examination of sodium and chloride losses during short-term versus endurance exercise. Plasma sodium and chloride concentrations are held within very narrow limits. During short-term exercise, plasma sodium and chloride concentrations undergo minimal changes.40 However, the plasma content of sodium and presumably chloride decreases, suggesting the fluid shifts that occur during short-term exercise involve an isotonic shift of fluid.40 During longer-term exertion there can be minimal changes in plasma sodium concentration but content can change dramatically.25,40 With chloride there is a dramatic disproportional decrease both in the plasma concentration and content due to large amounts lost in the sweat.25,40 Measuring total content lost gives a more complete picture of how much supplementation must occur to replenish exercise-related losses. When one looks at changes in plasma potassium concentration and content one sees a different picture. Both the concentration and content of potassium go up during high-intensity exercise. When one looks at the change in content one can see that the change in concentration is due to both the loss of plasma water and the addition of potassium to the plasma when it leaks out of the contracting muscle cells. Hypothetically, fluid and electrolytes can shift from the intracellular space to the extracellular space as well as between each of the compartments through active, passive, and facilitative mechanisms.6–8,13,20,21,25,41,42 This dynamic exchange of fluid and electrolytes between compartments moves nutrients and waste products, provides fluid and electrolytes for the production of sweat and allows the horse to defend the internal environment of the cells.6–8,13,20,21,25,41,42 To maintain cellular homeostasis the horse must regulate blood volume, blood pressure, and the osmotic composition of the intracellular and extracellular fluid compartments. Acute fluid and electrolyte shifts have differing functional significance related to the timing of the response during exercise. Early shifts appear more related to a system-wide redistribution of blood and fluid from capacitance vessels and the interstitial space so as to increase venous return and augment cardiac output.6–8 Later responses provide fluid and electrolytes for the production of sweat and thermoregulation6,13,19,25 Finally, decreases and depletion of fluid stores lead to dehydration, thermoregulatory, and cardiovascular instability and fatigue. 6,13,19,25 This latter challenge stimulates an expansion of plasma volume and the contents of the various electrolytes, a beneficial adaptive response known as a training-induced hypervolemia. 6,13,19,25 Senay43 demonstrated, in humans, that in the first seconds at the onset of exercise, there is a rapid net movement of protein and fluid from the interstitial space and lymphatics into the vascular compartment.43 This inward flux of protein and water causes a transient, short-lived increase in plasma volume, an intercompartmental shift of body fluids that couples with a redistribution of blood from the venous capacitance side of the vascular system to the arterial side to provide adequate venous return to maintain cardiac filling pressure.6–8,43 This important redistribution of blood and fluid from the capacitance side of the vascular system is important because of the need for extra venous return at a time when there is rapid vasodilation in the working muscles.6–8,43–45 Similar phenomena have been demonstrated in dogs and probably also occur in horses.44,45 Studies have demonstrated a shift in the albumin-to-globulin ratio in the horse consistent with an inward flux of fluid from the interstitial space.46 At rest albumin is the primary protein found in the vascular space and globulin represents the most prevalent protein found in the lymphatics.46 This dramatic change in albumin-to-globulin ratio suggests that the horse experiences a similar influx in fluid at the onset of exercise as described in humans.43,46
Body fluids and electrolytes
Responses to exercise and training
Introduction
Body fluid compartments
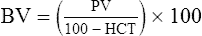
Plasma osmolality and the concentration of key electrolytes
ELECTROLYTE
PLASMA
INTERSTITIAL FLUID
SKELETAL MUSCLE CELL
SWEAT
Cations
Na+
140
143
10
120 (range 115–150)
K+
4
4.1
142
35 (range 25–50)
Ca2+ (ionized)
2.5
2.4
4
10 (range 3–20)
Mg2+ (ionized)
1.1
1.1
34
5
Anions
Cl–
100
113
4
150 (range 140–190)
HCO3–
25
28
12
H2PO4,– HPO42–
2
2
40
Lactate–
≈1
Protein
14
0
50
Other
7
7
84
Plasma concentration versus plasma content

Effects of acute exercise on fluid and electrolyte balance
Intercompartmental fluid shifts at the onset of exercise
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Body fluids and electrolytes: Responses to exercise and training