CHAPTER 6 Body Cavity Fluids
Typically there is little fluid present in the peritoneal, pleural, and pericardial cavities, and thus they are considered potential spaces. Detailed physiologic descriptions of serous body cavity homeostasis are available (Bouvy et al., 1991; Forrester, 1988; Kirby, 2003). These serous body cavities are lined by specialized cells, termed mesothelial cells. Clinical signs of the presence of increased amounts of fluid include abdominal distension, abdominal pain, dyspnea, muffled heart sounds, and cardiac arrhythmia. Collection and evaluation of fluid from these sites may be therapeutic as well as diagnostic for the presence of inflammatory, hemorrhagic, neoplastic, lymphatic, or bilious conditions. Additionally, further diagnostic tests may be indicated by the cytologic characteristics. Removal and examination of fluid is highly recommended unless anesthetic risks or bleeding diathesis are present or further injury is likely.
COLLECTION TECHNIQUES
Abdominal Fluid
Place the patient in left lateral recumbency and restrain. Clip and surgically prepare an area (e.g., 4 to 6 inches square) with the umbilicus in the center. The urinary bladder should be emptied before performing paracentesis. Infiltrate a small area with local anesthetic, if desired. Use a 20-to 22-gauge needle or over-the-needle catheter to penetrate the abdomen. Attempt to obtain fluid in four quadrants, allowing the fluid to flow freely by gravity and capillary action. If needed, gentle suction with a 3- or 6-ml syringe can be employed. For a complete description of the technique, the reader is referred elsewhere (Ford and Mazzaferro, 2006). Allow the animal to rest quietly while fluid is being removed. Moving the animal or allowing the patient to move while the needle is in the abdomen can result in laceration of the organs. Some investigators prefer to have the patient standing for fluid removal; however, it is more likely that the omentum will occlude the needle if the patient is in a standing position.
Pleural Fluid
For removal of fluid from the thorax, the patient should be in a standing or in ventral/sternal recumbency. Clip the hair and surgically prepare the thoracic wall from the 5th to the 11th intercostal space. Infiltrate a small area at the 7th to 8th intercostal space at the level of the costochondral junction with local anesthetic. It is best to attach extension tubing to the hub of the needle or over-the-needle catheter and a three-way stopcock for removal of pleural fluid. Insert the needle or catheter into the chest wall at the surgically prepared site taking care to avoid the intercostal vessels located just caudal to each rib. For a complete description of this technique, the reader is referred elsewhere (Ford and Mazzaferro, 2006).
Pericardial Fluid
For removal of fluid from the pericardial sac, sedate the patient if necessary. Surgically prepare an area over the lower to mid 5th to 7th intercostal space bilaterally. Place the patient in lateral or sternal recumbency. Attach ECG leads to monitor for dysrhythmias during the procedure. Infiltrate an area at the costochondral junction, or approximately where the lower and mid thorax meet, with local anesthetic. Use an over-the-needle catheter or Intrafusor system with a three-way valve to which a 30-ml syringe is attached. Always maintain negative pressure on the syringe as the chest wall is punctured. Carefully advance the needle into the 4th intercostal space through a nick incision in the direction of the heart. Advance the needle until resistance is met (from the pericardium). A release will be felt as the needle enters the pericardial sac and a flash of blood is often seen. Thread the tubing or catheter so that it is securely within the pericardial sac. For a complete description of this technique, the reader is referred elsewhere (Ford and Mazzaferro, 2006).
SAMPLE HANDLING
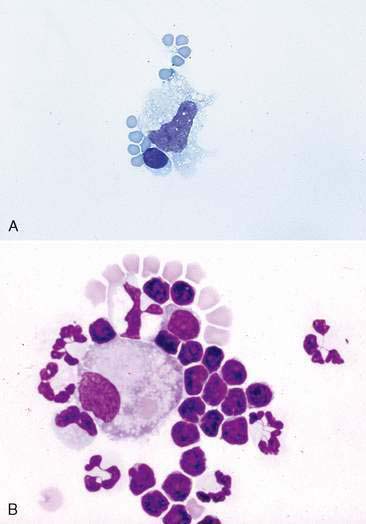
Note the color and character of the fluid initially upon removal (Fig. 6-1). If the fluid is clear initially then turns red, iatrogenic blood contamination is likely. The fluid should be collected into both a lavender-top tube (EDTA anticoagulant) for cytology and a red-top tube (or any sterile tube without additives) for potential bacterial culture. Also at collection, make both direct unconcentrated smears by a squash or blood smear technique and smears from spun samples. Romanowsky-type stains such as Wright stain or an aqueous-based Wright (quick) stain can be applied to a few slides for immediate in-house evaluation. The remaining unstained smears, as well as an EDTA and serum tube filled with fluid, should be submitted to the laboratory. This will allow the clinical pathologist evaluating the sample to compare the cellularity of the sample and the appearance of the cells at the time of collection with that which was submitted in the tube.
LABORATORY EVALUATION
Protein Quantitation
Protein quantitation is typically done via refractometry; however, some institutions will determine protein via spectrophotometry or automated analysis. Both methods offer accurate readings in a wide range of protein concentrations (George, 2001; George and O’Neill, 2001). It has been shown with canine effusions that refractometry underestimates the protein content when < 2.0 g/dl and that the spectrophotometry using the biuret method is more accurate when there is high protein content (Braun et al., 2001). Others have found that refractometry can be used accurately down to 1.0 g/dl (George and O’Neill, 2001). A similar finding of underestimating protein content in feline effusions with refractometry may occur (Papasouliotis et al., 2002). In that same study, a dry chemistry analyzer produced increased globulin concentration and therefore lower Albumin:Globulin (A:G) ratios when compared with a reference wet analyzer using the same biuret and bromocresol green methodologies (Papasouliotis et al., 2002). This finding is particularly important since decreased A:G ratios support a diagnosis of feline infectious peritonitis (Hartmann et al., 2003).
Red Blood Cell and Total Nucleated Cell Count
Although an initial impression of the cellularity and amount of blood can usually be made by visual inspection of the sample (see Fig. 6-1), knowledge of the actual cell counts for erythrocytes and nucleated cells is important for further classification of the type of fluid. With this information, one can begin to narrow down the list of possible causes for the abnormal fluid accumulation. For samples being submitted to a reference laboratory, placing some of the sample in a lavender-top tube (EDTA) and some in a red-top tube is recommended. The lavender-top tube contains anticoagulant, which prevents the sample from clotting if there is a high protein content. EDTA is bactericidal, however, and is contraindicated if a sample is to be cultured (Songer and Post, 2005). Thus, some of the fluid should also be put into a red-top tube or sterile tube without additives. The cell counts will be done either with a hemocytometer or an automated cell-counting instrument. If the amount of fibrinogen in the fluid sample is high, then the sample in the red-top tube is likely to clot, producing erroneous results.
NORMAL CYTOLOGY AND HYPERPLASIA
Normally, only a very small amount of fluid is found in the peritoneal, pleural, and pericardial spaces; thus cytologic evaluation is generally not typically performed unless an increased amount of fluid accumulates. Normal fluid is clear and colorless (see Fig. 6-1). Several types of cells may be found in body cavity effusions and their relative proportions vary depending on the cause of the fluid accumulation. Cells expected to be in normal fluid include mesothelial, mononuclear phagocytes, lymphocytes, and rare neutrophils. Mesothelium will easily become hyperplastic or reactive when increased body cavity fluid or inflammation is present.
Mesothelial Cells
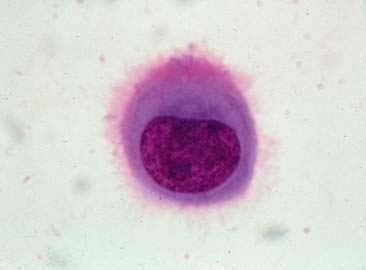
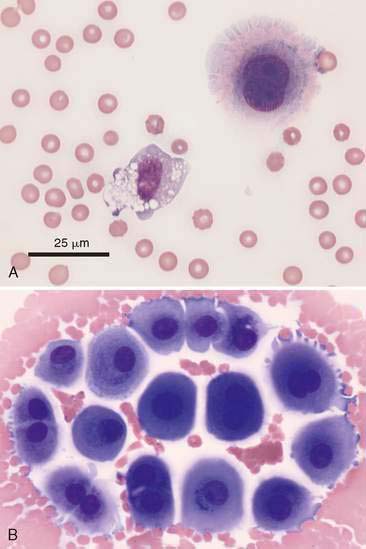
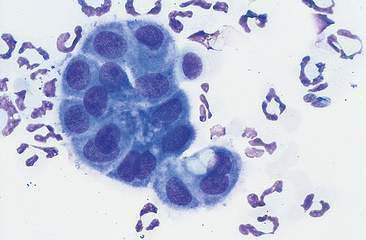
In most cases, the cytologist will find reactive mesothelial cells in body cavity fluids. These are considered as large mononuclear cells for the purpose of the three-part cell differential. Mesothelial cells may be seen as individualized cells or in variably sized clusters. They contain a moderate amount of medium-blue cytoplasm (Fig. 6-2). Hyperplastic mesothelial cells are large (12 to 30 μm) with deep-blue cytoplasm and may display a pink to red “fringed” cytoplasmic border (Fig. 6-3A&B). This feature helps identify these cells as mesothelial cells. These cells may contain one or more nuclei of equal size (see Fig. 6-3B). Nucleoli may be visible and occasional mitotic figures may be evident.
Macrophages
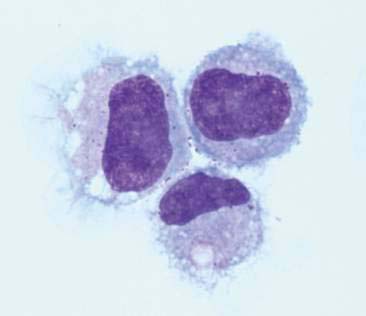
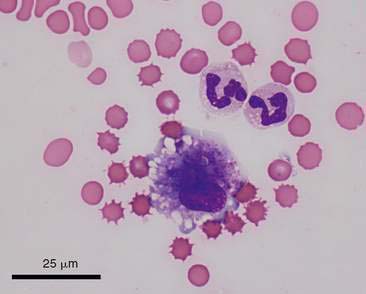
Macrophages are large mononuclear cells with abundant pale-gray to light-blue cytoplasm and a round to kidney bean–shaped nucleus (Figs. 6-3A and 6-4). The chromatin may be fine and nucleoli may be visible. Macrophages often contain vacuoles or previously phagocytosed cells and/or debris if there is inflammation or if the fluid has been present for a long time (Fig. 6-5). Macrophages are considered as large mononuclear cells for the purpose of the three-part cell differential.
Lymphoid Cells
Small and medium lymphocytes found in effusions appear similar to those found in peripheral blood. These nucleated cells often have a thin rim of lightly basophilic cytoplasm and a round nucleus. Lymphocytes are considered as small, mononuclear cells for the purpose of the three-part cell differential. In normal fluids they are present in higher proportions in cats and cattle than in dogs and horses. The nucleus nearly fills the cell, producing a uniformly high nuclear-to-cytoplasmic (N:C) ratio. The chromatin is finely stippled to evenly clumped; nucleoli are not visible (Fig. 6-6A&B).
Neutrophils
Neutrophils appear similar to those found in peripheral blood. They are medium-sized cells with pale to clear cytoplasm and a segmented nucleus. Neutrophils should be absent or present in very low numbers in normal fluid but they will be found in increased numbers with chronic fluid accumulation or with inflammation (see Figs. 6-5 and 6-6B).
GENERAL CLASSIFICATION OF EFFUSIONS
Effusions are usually classified as transudates, modified transudates, and exudates, related to the protein concentration, nucleated cell count, and cell types present (Table 6-1).
Transudate
Fluids are classified as transudates when they have a low protein content and a low cell count (protein < 2.5 g/dl and cells < 1000/μl). These fluids increase in volume in response to physiologic mechanisms, such as increased hydrostatic vascular pressure or decreased colloidal osmotic pressure, that cause the normal homeostatic mechanisms of fluid production and resorption to be overwhelmed. Some causes for transudate accumulation include severe hypoalbuminemia, portal hypertension, hepatic insufficiency, portosystemic shunt, and early myocardial insufficiency. The cells commonly found in transudates are similar to those in normal fluid, which are mostly mononuclear cells consisting of macrophages, small lymphocytes, and mesothelial cells (see Fig. 6-6A). Neutrophils may compose a small proportion of the population.
Modified Transudate
A fluid is classified as a modified transudate when a transudate changes its physical features. The accumulation of transudative fluid in a body cavity causes increased pressure, which is irritating to the mesothelial cells lining the space. They respond by proliferating and sloughing into the effusion. With time, the sloughed mesothelial cells die and in so doing release chemoattractants that draw small numbers of phagocytes into the effusion to remove cellular debris. The result is a mild increase in both total protein (≥2.5 g/dl) and nucleated cell count (less than 5,000/μl). Thus, modified transudates are generally transudates that have been present long enough to elicit a mild inflammatory reaction (see Fig. 6-6B). It is most often associated with cardiovascular disease or neoplastic conditions.
In cases of extended duration, modified transudates can have a cloudy to almost milky gross appearance. Such fluids strongly resemble chyle and, in fact, have in the past been called “pseudochylous effusions” (a term no longer used). The gross appearance of these fluids is the result of high lipid content (due to higher cholesterol content than serum) but is in no way related to a true chylous effusion, as there are no triglycerides or chylomicrons present (Hillerdal, 1997; Tyler and Cowell, 1989). The phagocytes attracted to remove cellular debris from transudates are rich in enzymes that digest protein but are virtually devoid of enzymes that will break down complex lipids. Consequently, while most of the constituents of dying cells are removed by phagocytosis, lipid content of the cells simply accumulates in the effusion. Effusions formed in this manner are easily distinguished cytologically from true chylous effusions.
The principle cellular constituent of the modified transudate is the reactive mesothelial cell (see Fig. 6-3A). Because of the ability of mesothelial cells to respond to irritation by proliferation, the presence of increased numbers of mesothelial cell clusters and rafts is a common finding in reactivity (see Fig. 6-3B). Mitoses are increased and occasional multinucleated reactive mesothelial cells are seen. Reactive mesothelial cells in clusters are capable of imbibing lipid from the effusion fluid and when they do, they take on the characteristics of secretory cells. In this form they must be differentiated from metastatic adenocarcinoma or mesothelioma. This may be done by critically evaluating the cell populations for criteria of malignancy.
As modified transudates mature, the proportion of inflammatory cells they contain will increase. In most cases the principal inflammatory cell is the nondegenerate neutrophil, but neutrophils rarely account for more than 30% of the total cell population. Over time, modified transudates gradually become cytologically indistinguishable from nonspecific exudates. One method used to distinguish modified transudates from exudates or transudates from exudates is measurement of C-reactive protein concentration in canine effusions (Parra et al., 2006).
Exudate
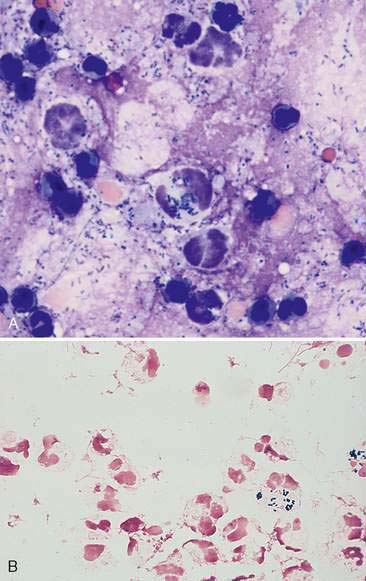
Exudates are the result of either increased vascular permeability secondary to inflammation or vessel injury/leakage (hemorrhagic effusion, chylous effusion). An exudative fluid usually contains both increased protein and an increased nucleated cell count. The total protein concentration is usually greater than 3.0 g/dl along with cell counts greater than 5,000/μl. Infectious causes for exudates include bacteria (Fig. 6-7A&B), fungi, viruses, protozoa such as Toxoplasma (Toomey et al., 1995), or helminthes such as Mesocestoides sp. (Caruso et al., 2003). Noninfectious causes involve organ inflammation such as pancreatitis, steatitis, inflammatory neoplasia and irritants such as bile or urine. Cytologic evaluation is useful to determine an underlying cause in cases of exudative effusions.
Most inflammatory effusions are cytologically nonspecific in terms of etiologic diagnosis. However, as with inflammatory responses elsewhere, cytomorphology provides significant clues as to the underlying cause. Neutrophilic inflammatory effusions indicate severe active irritation (Fig. 6-8). If neutrophils are degenerate, an effort should be made to identify bacterial organisms within phagocytes (primarily neutrophils). This is generally easiest at the feathered edge of the smear. If organisms are not seen, the fluid should still be cultured. Mixed and macrophagic inflammatory effusions reflect less severe irritation and are found with resolving acute effusions or in association with less irritating etiologic agents than bacteria (e.g., fungal organisms or foreign bodies). Chemical evaluation of effusion fluid is also useful in recognizing sepsis.
Septic effusions may be diagnosed by use of biochemical parameters in dogs and cats. It was shown that effusion fluid in dogs and cats with a pH <7.2, pCO2 >55 mm Hg, glucose concentration <50 mg/dl, and lactate concentration >5.5 mmol/L is highly likely to have bacterial infection (Swann et al., 1996). A study involving peritoneal fluid in dogs and cats evaluated blood and effusion fluid glucose concentrations. This study found that a difference of blood-effusion glucose >20 mg/dl provides a rapid and reliable means to differentiate septic and nonseptic effusion fluid (Bonczynski et al., 2003). In this study, a difference of blood-effusion lactate concentration in dogs <−2.0 mmol/L was 100% sensitive and 100% specific for the diagnosis of septic peritonitis.
SPECIFIC TYPES OF EFFUSIONS
Feline Infectious Peritonitis
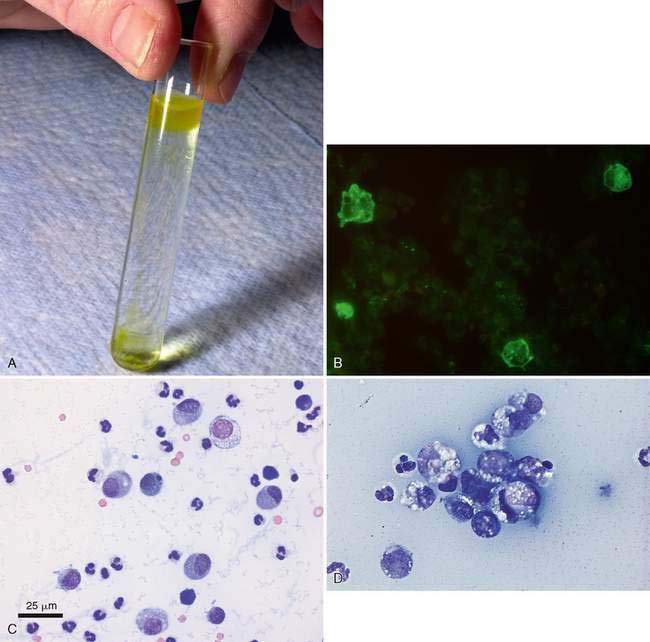
Feline infectious peritonitis (FIP) is unique among the causes of inflammatory effusion in that the fluid that accumulates is usually high in protein, yet has a low cellularity (McReynolds et al., 1997). Classification as an inflammatory effusion is based primarily on the presence of high total protein (often >4.5 g/dl) which is a reflection of a similar elevation in serum protein. Electrophoresis of either the effusion fluid or the serum reveals a polyclonal gammopathy. An albumin-to-globulin ratio of less than 0.8 on the fluid is very suggestive for FIP. It has been reported that if gamma globulin is greater than 32% of the protein in the effusion fluid, this is suggestive of FIP (Shelly et al., 1988). An additional test that can help rule in or rule out FIP is the Rivalta test. To perform this relatively simple test, place one drop of 98% acetic acid into 5 ml distilled water and mix well in a reagent tube. Then add slowly one drop of the effusion fluid to the surface of the acetic acid solution. A positive test requires that the drop of effusion retain its form on the surface or slowly sink to the bottom as a droplet or jellyfish-like shape (Fig. 6-9A). This test indicates a high protein content as well as fibrin and inflammatory mediators. This test has a positive predictive value of 86% and a negative predictive value of 97% (Hartmann et al., 2003). A more specific cytologic test for FIP involves immunofluorescence of intracellular feline coronavirus (FCo V) within effusion macrophages. When positive (Fig 6-9B), this test is diagnostic for FIP; however, the negative predictive value is 57%, so a negative test may miss cases of FIP (Hartmann et al., 2003).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree