Paul S. Morley, J. Scott Weese, Consulting Editors Infection control, biosecurity, biocontainment, and biosafety are essential functions at all health care operations, including veterinary practices.* All veterinarians, at some level, recognize and act to prevent adverse outcomes in patients. However, as major outbreaks of health care–associated infections (HCAI) at veterinary hospitals have become increasingly publicized,1–12 it has become increasingly obvious that coordinated infection control practices are a critical component of delivering high-quality care at veterinary facilities, especially those with large caseloads and those that specialize in intensive care of patients. This threat is relevant to all veterinarians and all hospitals. A survey of veterinary referral hospitals at American Veterinary Medical Association (AVMA)–accredited institutions reported that 81% had recognized outbreaks of HCAI in patients within 5 years, and at least 45% had recognized more than one outbreak.13 The two agents most commonly associated with these outbreaks were Salmonella enterica and methicillin-resistant Staphylococcus aureus (MRSA), but 12 other agents were also implicated. Fifty-eight percent of the 38 participating hospitals had to restrict admissions to protect patients and allow mitigation, and 12 of these hospitals (all were equine hospitals) were temporarily closed. Even with very limited surveillance, 51% of these same hospitals recognized significant HCAI and illness within 3 years of interviews. The agent most commonly implicated in human illnesses was Cryptosporidium parvum. The standard of veterinary care is changing such that sporadic occurrences and outbreaks of HCAI may no longer be interpreted as unavoidable accidents if coordinated measures are not routinely used to minimize their likelihood. Our understanding about infection control issues in veterinary medicine has also advanced significantly during the past decade. As such, what may have passed as sufficient for infection control in veterinary practices 10 or 20 years ago may often not be sufficient today. It is also important to realize the important part that infection control and biosecurity must play in ambulatory practices and on our clients’ premises. Inherently, healthy animals with lower contagious disease risks represent a smaller proportion of hospital populations than they do among populations in their home environments. However, veterinarians are obviously called upon to contact those animals most likely to be shedding contagious pathogens regardless of whether this is in hospitals or in the field. In addition, there are several examples in which patients discharged from hospitals were the likely source of viral and bacterial infections for animals in their home environments.6,9 The magnitude of this potential hazard is perhaps best illustrated by the equine herpesvirus type 1 (EHV-1) outbreak, which occurred in association with the National Cutting Horse Association Western National Championship in Ogden, Utah, in May, 2011.14,15 It is likely that exposure to a single horse that was shedding EHV-1 at this event resulted in over 165 horses developing clinical disease that was known or suspected to be caused by EHV-1, and at least 13 of these horses were euthanized. The outbreak spanned at least 10 western U.S. states and two Canadian provinces. Clearly the need to apply sound biosecurity and infection control practices extends well beyond the walls of veterinary hospitals. However, it is also clear that these dramatic outbreaks are not the only circumstances in which HCAI affect patients. Lessons learned in human health care settings clearly show that endemic occurrence of HCAI typically creates a much greater overall burden in exacerbated morbidity and mortality than does the occurrence of epidemics. Unfortunately, there is insufficient information available from veterinary care settings to clearly say whether the same is true for animal patients, but there is no reason to believe that it would be different. Still, when studied systematically, the burden of disease associated with sporadic or endemic HCAI is substantial in veterinary patients. A recent publication of a multicenter study performed in five academic referral hospitals reported that nosocomial events monitored as seven different clinical syndromes were recognized during hospitalization in 19.7% (95% CI = 14.5% to 26.7%) of equine patients admitted for gastrointestinal disease at a rate of 3.9 events per 100 days of hospitalization (95% CI = 2.9 to 4.3 events per 100 hospitalization days).16 Other studies reported in equine patients have principally focused on HCAI related to surgical site infections.17,18 These rates are not dissimilar from rates reported for HCAI among humans in critical care settings. Thus, these notable outbreaks of HCAI and the emerging awareness of the importance of endemic occurrences should create an acute awareness that dramatically sharpens our focus on preventing all types of HCAI, including those that seem sporadic. Although the discussions in this chapter are mostly framed in the context of hospital settings, the concepts and issues apply much more broadly to ambulatory practices and animals’ home premises. Exposures to contagious disease threats, HCAI in patients, and zoonotic infections in care providers are undeniable risks in every veterinary practice. Because veterinarians have ethical and legal obligations to take reasonable protective actions to prevent their patients and employees from foreseeable harm associated with their actions (and inactions), it is clearly possible to not pay enough attention to infection control. The consensus opinion of veterinary infection control experts is that there is a recognizable standard of practice for infection control in veterinary medicine and appropriate effort must be given to the control and prevention of infectious disease transmission in all animal populations.19 The implication that should be recognized is that it is possible to commit malpractice by not paying enough attention to infection control while caring for patients. There are key areas that must be addressed in all comprehensive infection control programs, including patient contact, hygiene, surveillance, communication, and education.20 However, we cannot determine whether we are meeting an acceptable standard of care for infection control by listing the specific prevention strategies that are used, but rather we must evaluate the occurrence of HCAI in a veterinary practice along with how we respond to the identification of these HCAI. Few prevention practices for HCAI are universally considered applicable and efficacious for preventing HCAI in all settings. Rigorous attention to hand hygiene would meet these criteria, but many other common control methods such as the use of disinfectant footbaths or coveralls do not. Thus, it is not possible to judge whether a control program is sufficiently rigorous solely by evaluating procedures, protocols, and policies. Recognition of the risks for legal liability undoubtedly give reason for pause, but a better motivation for emphasizing infection control practices should be to provide the best care possible within the scope of a practice’s specialization. In order to create an environment where patient care can be optimized, it is incumbent upon veterinarians to actively manage the risk of HCAI in their patients. Achieving excellence in patient care and helping clients are undoubtedly among the highest priorities for all veterinary practices. However, the occurrence of HCAI in our patients is an ever-present hazard that interferes with our ability to deliver optimal patient care. Good infection control practices are not the only feature defining excellence in veterinary care, but it is impossible to achieve excellent patient care without appropriately controlling HCAI. The implications of suboptimal infection control may not always be readily apparent, but both sporadic/endemic infections and outbreaks can have a significant effect on patient morbidity, patient mortality, hospital economics, personnel health, personnel morale, and facility reputation. There are also liability implications for HCAI that occur in the absence of an appropriate infection control program. Although HCAI are an undeniable hazard associated with caring for patients, and although it is possible to reduce the risk of infections through a variety of prevention strategies, it is important to note that not all HCAI are preventable using practical and cost-effective control programs. In reality, even impractical and cost-prohibitive programs would not eliminate HCAI, since there is an undefined but undeniably significant “nonpreventable” fraction of infections that will occur despite any infection control practices. To be most successful, it is important that over time administrators and personnel responsible for infection control programs strive to better understand and target prevention efforts at this preventable fraction of all HCAI. In general, all comprehensive infection control programs center on four major goals: • Decreasing the likelihood of exposure to infectious agents among patients and personnel • Decreasing the intrinsic risks for HCAI • Maximizing participation of personnel in infection control activities • Optimizing the efficiency of infection control procedures and policies These goals are achieved through five general areas of action: • Decreasing direct and indirect patient contact • Recognizing high-risk patients and HCAI through efficient surveillance strategies • Maximizing communication and education among all personnel When initiating an infection control plan, it is important to take a global assessment of the contagious disease hazards in a practice, the level of risk aversion, and the resources that can be expended on infection control efforts. If a veterinary practice predominantly works in preventive health care under extensive field conditions, the contagious disease hazards may be less common and less severe than those encountered if a practice concentrates on intensive care of patients in a hospital. The specific disease hazards will also vary with the types of patients being managed (i.e., sick neonates vs. patients with acute GI disorders vs. reproduction cases, equine vs. bovine vs. camelid, etc.). Risk aversion is a concept that relates to how much a person or business is unwilling to accept or allow a negative event to occur. The inverse of risk aversion can be thought of as risk tolerance. The more risk averse a person is, the more it may be reasonable for him or her to initiate and maintain a rigorous infection control program. In contrast, a more risk-tolerant veterinarian may recognize the potential for contagious disease hazards in his or her practice but may not believe it is necessary to engage in preventive strategies that are seen as extreme. However, the risk tolerance of an individual or facility cannot be the sole deciding factor, since management decisions have impacts on patients and their owners, hospital personnel, and even animals and humans in the broader population. Thus, there is a baseline level of risk aversion that must be present. As our understanding of risks for HCAI methods for infection control advance, the bar is being raised, resulting in professional and legal risks should veterinarians fail to ensure that they are adequately pursuing infection control in their daily practices. The third component of this internal inventory is to assess the resources that will be available for infection control activities. The term resources in this context is intended to broadly encompass monetary resources, personnel time, and effort. Keeping in mind this assessment of mindset and resources, the next step in developing an infection control program is to elaborate what the specific goals will be. For example, specified goals for an infection control program might include protection of hospital personnel and clients from exposure to zoonotic disease agents, creating an environment where patient care can be optimized by minimizing the risk of HCAI, optimizing education of personnel and clients regarding important infectious disease hazards, and protecting the operational capabilities of the practice. Using a comprehensive, systematic process for evaluation of disease hazards and design of control systems will then allow design of a logical control system that triages efforts to optimize efficiency. One systematic approach that the authors have used successfully is Hazard Analysis and Critical Control Points (HACCP) methodology.21 After identifying which specific hazards (infections) are most likely to occur, as well as when and where in the systems these events might occur or be prevented, the next step is to define specific control measures. As mentioned previously, the majority of these prevention efforts are effective because they decrease the likelihood of exposing patients to infectious agents (i.e., by optimizing hygiene in the environment, personnel, or patients or by decreasing direct and indirect contact among patients). A few other prevention practices are effective because they reduce the inherent susceptibility to infection. To understand which control measures are of greatest importance and where efforts should be targeted, it is critical to consider the lifecycle and methods of transmission for the specific agents of concern. Among the questions that should be asked: Is the agent most likely transmitted through direct contact, or are respiratory aerosols or contaminated surfaces and fomites also important sources of exposure? Is there a subclinical carrier state associated with agent shedding or is shedding mostly restricted to clinically affected patients? Does the agent persist well in the environment and can common disinfection procedures readily inactivate organisms? Each infectious disease may be considered individually in this evaluation process, but it is useful to remember that control measures that are effective against one agent are usually effective against others, particularly if they share common routes of transmission or have common risk factors in patients. Design of infection control programs should focus on practical actions for known problems, but it is important not to ignore the potential for newly recognized and re-emerging diseases. Infectious diseases continue to emerge internationally and many are of relevance to large animal veterinary medicine. The general strategy used in infection control protocols should be sufficiently rigorous to protect against most emerging issues, at least at a basic level. However, infection control programs should also be adequately fluid so that they can be modified to address new issues. Another critical aspect in the practice of infection control is effective targeting of efforts. Taken to hypothetical extreme, the most rigorous infection control methods would prescribe that every patient be handled in complete isolation using barrier precautions verging on those used by “hazmat” personnel. Clearly this is not practical or needed in most situations, and yet, in a few rare circumstances this level of precaution can be warranted. In many more situations, some lesser level of precaution is warranted beyond that used in casual encounters with animals in their home environments. By their very nature, these extra control measures will inevitably inconvenience caregivers and clients in addition to increasing costs associated with care. These measures could also affect the quality of patient care and result in a corresponding increase in morbidity when taken to the extreme. The challenge is to target prevention efforts to just those patients that warrant increased concern and to use the most appropriate, albeit inconvenient, methods for controlling risks to personnel and other patients. Another side effect of the inconvenience created by infection control efforts is that people by their very nature gravitate to the most convenient methods for daily activities. As such, the more personnel are inconvenienced through infection control efforts, the less likely they are to follow prescribed policies unless they understand and believe that procedures are needed and have value. Thus, a critical component of any effective infection control program is maximizing awareness of personnel and educating them about potential hazards and the value of established control measures. Presenting nightmarish worst-case scenarios without other objective information may be initially effective in getting people’s attention, but continually using this approach as justification for infection control inevitably fails to truly motivate all or even most personnel. Establishing interactive communication regarding risks and concerns coupled with logical, objective, evidence-based presentations is clearly a better approach for convincing personnel of the need to fully participate in infection control efforts. However, this is also dependent upon engaging sufficiently in surveillance and investigation so that useful objective information will be available regarding the significance of specific disease risks in a practice. The principle of informed consent, as it applies to veterinarians caring for animals, implies that owners have the right to be provided with adequate information prior to treatment so that they can make appropriate decisions for their animals and themselves.22,23 As professionals with specialized training, veterinarians can be assumed to have knowledge about the health and care of animals that goes beyond that which someone without this training will have. As such, one of the components that experts generally agree is part of the informed consent process is the disclosure of potential risks that may be associated with a veterinarian’s management of his or her animals, particularly in situations in which there is greater than average risk for a particular adverse consequence.24 As such, it is reasonable and prudent for veterinarians to routinely disclose the potential for HCAI as part of the informed consent process for all patients. This is especially true for patients with an enhanced risk of acquiring HCAI (e.g., because of required invasive procedures or because patients are immunocompromised). However, this same principle can be applied in consideration of the general risk to all patients. For example, if it is known or suspected that there is an increased risk of nosocomial infections for patients at a facility, then there is likely an ethical and legal obligation to disclose how this risk pertains to new patients prior to their admission. It is extremely prudent to document the informed consent process in writing with clients. Often, there is reluctance to do so based on concerns that it may deter clients, particularly when other facilities do not address the issue. However, the informed consent process can be an opportunity to educate clientele about risks (and thereby increase their overall understanding) and highlight measures taken by the facility to mitigate those risks. As mentioned previously, procedures used to decrease risks of HCAI inherently increase labor and material costs related to patient care. If these infection control activities are an essential part of delivering veterinary care, it is reasonable that these costs should be passed on to clients. It should not be expected that these costs should reduce profits or that they be paid by veterinarians or hospitals. Remembering that only a portion of HCAI is preventable, if veterinarians have met the expected standard of care for infection control, then it is also reasonable to assume that extra costs incurred for treating complications associated with HCAI are paid by the client. In addition, if HCAI are an expected risk related to care of any patient, veterinarians and hospitals should develop plans for management of financial issues related to these risks. It is also important to differentiate consideration for how charges might be presented to the client from how costs for infection control activities are assessed. These costs might be passed on to clients by directly accounting for each item, which is most reasonable when there are specific charges that can be attributed to a specific patient (e.g., increased nursing or materials costs associated with care of a specific patient in isolation). However, this is less applicable when costs relate to care and protection of more than one patient (e.g., costs related to cleaning and disinfection of the hospital environment). In these cases it may make more sense to compensate for costs by aggregating expenses into a general fee category related to infection control (such as a daily surcharge) or to include these costs in overhead costs that are compensated by general admission or hospitalization fees. In special circumstances, such as when it is necessary to investigate or mitigate against suspected outbreaks of infections, it is useful to have a contingency fund that has been accrued and earmarked to pay for unforeseen expenses such as mass testing or additional labor related to cleaning and disinfection. Administrators should ensure that the potential for infection control emergencies is considered and that fee schedules will allow for development of this type of reserve fund. Much attention is paid to the possible role of the inanimate environment in HCAI of large animals. Contamination of the hospital environment by microorganisms is inevitable and entirely expected, and detection of microorganisms in a hospital environment does not, by itself, indicate anything of clinical relevance. A variety of factors are likely involved in the potential clinical relevance of contaminants, including the organism, numbers present, likelihood of contact with susceptible hosts, degree of susceptibility of potential hosts, environmental effects (temperature, ultraviolet exposure, humidity) and routine infection control protocols. Many microorganisms grow well on environmental surfaces, provided there is adequate moisture and organic debris, and may survive for very long periods. Even with relatively inhospitable conditions, many organisms persist for extended periods. If environmental pathogens are able to contact the appropriate body site of a susceptible individual in adequate numbers, disease could result. Environments where patients are housed or veterinary care is delivered can be expected to have greater numbers of environmental microorganisms than corresponding areas with lesser animal and human traffic.25 The relevance of environmental contamination is often difficult to determine. Even recovery of genetically indistinguishable organisms from the environment and from a patient does not confirm that exposure to an environmental reservoir was the source of infection since it can be difficult to distinguish cause versus effect. However, environmental contamination is of concern for both direct (e.g., oral inoculation from a reservoir of microorganisms in a stall) and indirect (e.g., transmission of an organism from an environmental reservoir to the hands of a care provider and then to an animal) transmission. Measures should be in used in all veterinary care environments to reduce the environmental pathogen load. This involves cleaning and disinfection of environmental sites. Cleaning is defined as the removal of all visible debris26 and is arguably the most important step in decontamination of animal environments. Even the best disinfectants will be minimally effective when used in the presence of moderate volumes of dirt and organic debris such as feces and bedding material. Dirt and debris hamper disinfection by inactivation of many chemicals, acting as a physical barrier between disinfectants and microorganisms and by providing a nutritional source for microorganisms. Not only does cleaning enhance efficacy of the disinfection process by providing optimal conditions for desired biochemical reactions, but cleaning can actually remove a majority of microorganisms so that fewer need to be killed by disinfectants. Therefore, removal of as much organic debris as possible is required for optimal disinfection. This involves manual labor, consisting of removal of all bedding and feces, and scrubbing of surfaces to remove adherent debris and biofilms. Detergents should be used to loosen organic debris, emulsify oils and fats, and remove biofilm. The disinfectant to be used must be considered when choosing a detergent because there can be interaction between chemicals in detergents and disinfectants. Because of the effort required to clean and scrub stalls and other large areas, less labor-intensive methods are often sought. For example, cleaning with high-pressure (>120 psi) power washers is frequently used in barns and hospitals. There are theoretical advantages that seemingly justify use of high-pressure washers, especially when they are designed to dispense high-temperature water and steam. Although power washing can be quite helpful in removing organic debris, this process can also exacerbate infection control problems by aerosolizing and widely dispersing infectious agents. Further, used indiscriminately, they can even damage surfaces, which then impairs subsequent cleaning and facilitates survival of pathogens. The true risk related to the use of high-pressure washers is unclear. However, the convenience of using high-pressure systems must obviously be balanced with more intensive manual cleaning methods in order to minimize untoward consequences. Using high-pressure systems on surfaces with large amounts of gross contamination should clearly be avoided.27 In areas likely to be contaminated with important contagious pathogens (e.g., isolation facilities), it is logical to use them minimally or at least only as a secondary cleaning process on surfaces that have been previously manually cleaned and disinfected. Disinfection is the process of substantially reducing the burden of microbial contaminants on inanimate objects in order to reduce the likelihood of transmission of infectious agents. This should be contrasted with sterilization, which is the inactivation of all microbial agents on an object, and is typically only performed on materials used in invasive procedures. Disinfectants are biocidal chemicals that must be used at appropriate concentrations, allowing for adequate contact time in order to achieve the intended effect. Even with proper cleaning and selection of an appropriate disinfectant, disinfection errors can occur. It is also important to remember that microbial responses to disinfectant exposures are not uniform. There is tremendous variation in the ability of microorganisms to tolerate cleaning and disinfection. Most enveloped viruses are easy to eliminate whereas protozoal oocysts, nonenveloped viruses, and bacterial spores may be difficult or impossible to kill with surface disinfectants.28 For disinfection to be effective, a few key factors must be considered: the presence of organic debris, disinfectant concentration, temperature, and contact time. Organic debris inactivates disinfectants to varying degrees, emphasizing the need for careful cleaning. Most disinfectants are available as concentrates and must be diluted prior to use. Excessively dilute disinfectant solutions may have little or no effect, whereas excessively concentrated solutions can be dangerous to use in addition to being wasteful of resources. Dilution of disinfectants is an important process and must be performed by measurement, not estimation. One method to easily ensure that disinfectants are appropriately diluted is to use metered dispensing units that can be either wall mounted or attached to the end of a hose. For some disinfectants, different concentrations may be recommended for different situations. Test strips that can commonly be purchased from a variety of vendors can also be used to verify appropriate dilution and activity disinfectant solutions. This is especially important when stock solutions are prepared for use over time. Cleaning personnel must be informed of the importance of disinfectant dilution and trained in proper methods. Contact time is critical, particularly for certain disinfectants and difficult-to-kill microorganisms. If disinfectants are applied and immediately rinsed away, there is little chance that they can be effective. Most disinfectants require 10 to 30 minutes of contact time to provide maximal microbial reduction. Chemical reactions that produce disinfection are slowed in cold temperatures, which should be considered when determining the amount of contact time that is required. Disinfectants should never be combined because of the potential for inactivation and production of noxious or toxic gases. It is critical to be aware that all disinfectants do not have the same effectiveness. As with antimicrobial drugs, disinfectants have a spectrum of activity that can be highly variable between disinfectant classes (Table 46-1). Choosing the most appropriate disinfectant can be complex, involving a variety of factors including spectrum of activity, relative efficacy in the presence of organic debris, toxicity to animals and humans, potential damaging effects on certain surfaces, cost, and potential environmental effects. There is no standard disinfectant to be used in all situations in large animal facilities, although oxidizing agents such as accelerated hydrogen peroxide and peroxymonosulfate are increasing in popularity because of their broad spectrum of activity, acceptable performance in moderate amounts of organic material, relatively rapid action at room temperature, relative safety for personnel, and environmental friendliness. Other options may be appropriate in certain situations. When disinfectants with a narrower spectrum of activity are used as the primary disinfectant, protocols should be in place to use alternative products should certain situations be encountered that require a higher level of activity. TABLE 46-1 Disinfectants Commonly Used in Veterinary Medicine* • There are differences among different types of quaternary ammonium compounds. • Commonly used primary environmental disinfectant, although the spectrum is not necessarily optimal. • Inactivated by anionic detergents. • Some residual activity after drying. • Less effective in cold temperatures and low pH. * It is critical to handle disinfectants and other chemicals using appropriate safety precautions. Refer to MSDS sheets for each product to obtain recommendations regarding appropriate handling and personal protective equipment that is recommended. The material to be cleaned and disinfected can also have a tremendous impact on efficacy. Many surfaces found in large animal clinics and farms are not amenable to thorough cleaning and disinfection. These include unsealed wood surfaces, unsealed block, dirt flooring, and areas that are difficult to clean. For stalls, solid walls with a sealed surface are optimal for disinfection. This may be difficult to achieve; however, certain procedures can be performed to facilitate disinfection.27 Cement block walls can be reasonably well sealed with at least two coats of good-quality enamel. Wood walls can be sealed using two or more coats of marine epoxy. Regular maintenance of stalls is required to seal defects that occur from kicks or chewing. The optimal floor surface, in terms of cleaning and disinfection, is a smooth, solid, completely sealed surface. However, surfaces meeting these criteria are not all amenable to animal housing, so a compromise may be required. Finding the right balance of floor cushion, traction, durability, ease of cleaning, and cost is difficult. Regardless, certain key factors should be considered. The floor should be completely sealed so that water (and pathogens) cannot seep underneath, since this creates an obvious environmental reservoir and prevents adequate contact with disinfectants. Damaged stall matting has been cited as the likely environmental reservoir associated with serious outbreaks of HCAI.1 If obtaining a seamless, water-impervious floor surface is not possible, floor coverings should be completely removable (i.e., rubber mats over sealed concrete floor) and must be removed regularly for disinfection. Dirt, sand, and other organic materials cannot be disinfected and are therefore unsuitable for permanent flooring in hospital stalls or other veterinary care facilities. If sand is required for care of orthopedic patients, plans should be made to completely remove and discard this material between every patient. For items that are used in patient care, disinfection processes are sometimes divided into three categories: high-, intermediate-, and low-level. High-level disinfection involves elimination of all viruses and vegetative bacteria but is not expected to inactivate bacterial spores, fungal spores, or protozoal oocysts. High-level disinfection is, in reality, difficult to attain and is uncommonly used. Intermediate disinfection involves eliminating all vegetative bacteria but not necessarily all viruses (especially nonenveloped viruses), spores, or oocysts. Low-level disinfection results in elimination of most but not all potentially pathogenic bacteria and enveloped viruses. Items requiring disinfection should be classified as to the level of disinfection required; Table 46-2 shows examples of these. TABLE 46-2 Examples of Items Requiring Different Levels of Disinfection After use, all items in a patient’s stall should be considered to be potentially contaminated with pathogens that the animal might be shedding. Thus, for patients suspected or known to have contagious diseases, every item in the stall should be treated as a source of infectious material and must be appropriately decontaminated or discarded. Many stall items may be difficult to disinfect, or it may not be possible to be confident in the ability to fully decontaminate them. If it is cost effective, disposal of these items is ideal. Otherwise, standard principles of cleaning and disinfection apply. It must be recognized that rough, damaged, and permeable surfaces may be very difficult to adequately disinfect. Particular attention should be paid to disinfection of surfaces that patients contact orally such as feeders and water bowls as well as surfaces likely to be contacted by hands of personnel. Appropriate decontamination of water bowls can be problematic. Ideally, the water supply should be turned off, and the bowl should be drained, cleaned, and applied with disinfectant for an appropriate time before the bowl is rinsed and the water is turned on again. Nets used to hold hay have a high likelihood of contamination and are difficult to disinfect without use of gas or plasma sterilization techniques. As such, their use should be avoided if possible. Certain items may be at particular risk for contamination. For example, thermometers used on animals with salmonellosis are almost assuredly contaminated, and complete disinfection of digital thermometers is very difficult. It is reasonable to dedicate thermometers to individual patients and discard them when they are no longer required. An alternative is the use of disposable temperature strips, which are sometimes used in human medicine. These have not been specifically validated but are routinely used in some large animal hospitals.29 Twitches and muzzles have a high potential for contamination with pathogens such as Streptococcus equi and MRSA. Rope used for twitches is extremely difficult to disinfect, apart from removing the rope for autoclaving. Chain twitches are easier to disinfect, but many find them less desirable to use. The ideal twitch material is easy to disinfect, is atraumatic, and provides good grip on the nose. It is debatable whether the ideal material is available at this time. Twitches should be routinely disinfected, to the best of the ability considering the material. Consideration should be given to regularly changing or autoclaving rope used for twitches, and routinely decontaminating twitches with the understanding that complete disinfection may not be possible. Twitches used on animals that are known to harbor or are likely harboring nasal or upper respiratory tract pathogens should be considered contaminated and the material discarded or disinfected. Few areas show a greater difference in approach to infection control between human and veterinary medicine than the handling of hypodermic needles after use. In human medicine, recapping of needles is strictly forbidden because of the potential for needlestick injuries and subsequent exposure to life-threatening bloodborne pathogens. In veterinary medicine, recapping of needles is very common and needlestick injuries are often not perceived to be a significant health threat to veterinary personnel. Currently, there are minimal risks to veterinary personnel in almost all situations regarding bloodborne transmission of infectious agents from large animals. However, infectious disease hazards clearly are continuing to emerge, and it is prudent to develop safe practices that minimize the potential for exposure to bloodborne pathogens that might be transmitted from domestic large animals. In addition, physical trauma from the needle and accidental inoculation of some vaccines (e.g., RB51 modified-live Brucella abortus vaccine), medications (e.g., hormones, cancer chemotherapeutic drugs), or bacteria can lead to serious outcomes. Rigorous use of hand-hygiene procedures is one of the oldest recognized infection control measures, and it is perhaps the single most important infection control practice. Unfortunately, extremely poor compliance with best practices in human and veterinary hospitals as well as in the community negates much of the potential benefit. CDC guidelines suggest that health care workers in human hospitals should wash hands or use hand-sanitizing products before and after every contact with patients, as well as prior to eating, and after using the restroom. It is hard to argue that less stringent procedures are warranted in veterinary hospitals. There are numerous indications for hand-hygiene procedures in health care settings that have been identified as part of guidelines for human medicine. As evidenced by Box 46-1, hand hygiene is needed frequently in a clinical situation, something that may be problematic in typical settings where veterinary care is provided to large animals. Maintaining optimal hand hygiene can be especially important for ambulatory clinicians and yet can also be particularly difficult to achieve because of logistical difficulties. A variety of options exist for decontaminating hands. The most common practices are hand-washing and use of waterless hand-sanitizing solutions. Both are acceptable methods in most situations, and each has individual benefits and drawbacks. Washing with antibacterial soap has been standard method of decontaminating hands of health care workers for over a century. However, the potential benefits of this practice generally are never realized because of failure to use appropriate methods or failure to wash hands at all.30,31 Common errors in hand-washing include inadequate duration of hand-washing, failure to use soap, use of contaminated items to dry hands, and contamination of hands immediately following hand-washing (i.e., through contact with contaminated faucets, door handles, etc.). An appropriate technique for hand-washing is outlined in Box 46-2. The importance of the type of soap used in hand-washing is also often overlooked. Plain soaps, those without antibacterial agents, have minimal direct antibacterial activity. The main benefit of these is assistance with removal of dirt and organic debris, although efficacy data are conflicting. The use of plain soap can slightly decrease bacterial numbers on hands, but studies in human medicine have failed to demonstrate effective removal of significant pathogens from the hands of hospital personnel.32 Also, the routine use of plain soap can at times be associated with increases in numbers of bacteria carried on hands if use of these products results in skin damage and irritation through drying.33 Plain soaps are therefore not an optimal choice for routine use in medical settings, but use is certainly preferable to not washing hands in the absence of suitable alternatives (such as in field settings). Most soaps used in hospital settings contain biocides such as triclosan or chlorhexidine. Both of these can produce greater decreases in skin contamination compared with plain soap, and both compounds have antibacterial effects that may persist after application, although the duration of activity is shorter against gram-negative organisms (especially Pseudomonas spp.).31 Iodine and iodophors have been used as skin disinfectants and have a broader antibacterial spectrum. However, both (particularly iodine) can be irritating to skin and are less commonly used for routine hand-washing and skin disinfection in medical personnel. In addition, some people become sensitized to skin contact with these products. Compliance with hand-washing protocols is often poor for a variety of reasons. The time required is a major factor, particularly in situations in which contact with a large number of patients is likely. Although hand-washing requires less than a minute to complete, if it is indicated 100 times in a day, this represents a significant amount of time cumulatively committed to this activity. Lack of convenient access to proper hand-washing facilities is also frequently a problem. If access to sinks with running water, soap, and disposable towels is not convenient (i.e., not adjacent to where animal contacts occur), it is less likely that hand-washing protocols will be rigorously followed. Regardless of the type of soap used, frequent hand-washing leads to skin irritation and damage, which compounds compliance problems by making people reluctant to wash their hands, and skin damage associated with hand-washing increases potential for colonization or transient carriage of potential pathogens.33–36 Nail care and jewelry can also be significant impediments to achieving optimal levels of hand hygiene.37 Colonization with higher bacterial numbers, colonization with bacterial pathogens, and outbreaks of infectious disease have been reported in human hospitals in association with effects related to long fingernails and artificial nails.38 Studies have shown that these hamper effective hand disinfection, and many health care facilities, particularly intensive care units, prohibit their staff from having long ( For many years, waterless hand sanitizers have been described as an alternative to hand-washing, but in the past decade, expert opinion and evidence supporting use has evolved to where use of these products is often the primary method for hand-sanitizing in health care settings. These products are effective, inexpensive, easy to use, and easier to make widely available throughout health care settings than are hand-washing stations. Most waterless hand sanitizers use varying concentrations of alcohol (isopropanol, ethanol, n-propanol, or a combination), although a few products contain alcohol plus biocides, or biocides (e.g., triclosan and benzalkonium chloride) alone. Alcohol products are most commonly available, with concentrations ranging from 60% to 95%. Most products are available as gels or liquids, but newer products are dispensed as foams, which may increase acceptability of products. Products with alcohol concentrations greater than 95% are less effective because the presence of water is important for the bactericidal activity. Alcohols have excellent effect against gram-positive and gram-negative bacteria, but not bacterial spores or protozoal oocysts.30,31 Antiviral effects are variable, with less reliable effects against nonenveloped viruses. Alcohol-based products have been shown to have good effect on reduction of hand contamination of health care workers and have been shown to be more effective than standard hand-washing.39 Frequent contact with alcohol can lead to significant drying of skin, so most commercial products contain emollients, humectants, or similar skin-conditioning agents. Alcohol-impregnated towelettes are also commercially available but contain a small volume of alcohol and are no more effective for hand decontamination than washing with plain soap.40 One concern that is sometimes expressed about waterless hand sanitizers is their activity in the presence of organic debris. This is a particular concern in large animal practice, where the likelihood of gross contamination is high and access to hand-washing facilities is sometimes limited. However, a study of the efficacy of hand sanitizers on hand disinfection after performing physical examinations in horses reported that both alcohol and alcohol/chlorhexidine hand sanitizers were more effective for decreasing bacterial contamination than washing hands for 15 seconds with antibacterial soap.41 This level of disinfection was noted even though the culture fluid collected off the hands was visibly dirty, indicating that there was a reasonable amount of gross contamination. Thus, waterless hand sanitizers are likely to be effective under normal large animal practice situations. When there is readily apparent contamination of the skin, however, debulking of the hands is probably required for optimal effect. It is important to remember that alcohol-containing products are flammable. Although very rare, fires have been associated with exposure to open flame. Common sense and ensuring that hands are rubbed together until all the alcohol has evaporated should greatly minimize any risks. While most available products contain only alcohol as a sanitizing agent (in addition to humectants or emollients), some commercial products contain alcohol plus other biocides such as chlorhexidine. Some of these products have sufficient efficacy such that they are widely used for presurgical hand disinfection. The main advantage of using this combination of products is the residual antibacterial effect that the chlorhexidine confers. Some users complain of a sticky feel to the skin, which is one of the reasons that this combination is less commonly used for routine situations. One of the main advantages of waterless hand sanitizers is their portability. Although it is difficult to make the water sources needed for hand-washing portable or always accessible, these products can be easily placed in ambulatory vehicles, can be carried by individual staff, and can easily be placed throughout hospitals. As such, there is a greater likelihood of obtaining compliance by facilitating access. Unfortunately, regardless of the types of products used, compliance with hand hygiene is often a major problem.42,43 Infection control programs need to address hand-hygiene compliance through a variety of means to increase use of this critical infection control tool. Scrubbing of the hands and forearms with disinfectant solutions has been a standard practice prior to surgery, but in the past decade use of scrubless or brushless protocols for hand antisepsis has become increasingly common. Recommended techniques for presurgical scrubbing vary somewhat but typically involve a systematic 2- or 3-minute scrub of all hand and forearm surfaces.44 Other studies have demonstrated that a two-step approach, using a 1- to 2-minute surgical scrub followed by application of an alcohol-based hand sanitizer is as effective as a 5-minute surgical scrub.45 One concern with repeated surgical scrubbing is the potential for skin irritation and subsequent increases in bacterial contamination. This has led to increasing use of brushless techniques, especially with the use of waterless hand sanitizer containing 1% chlorhexidine gluconate and 61% ethanol, which has been shown to be equal or superior to brush application of 4% chlorhexidine soap.46 There may be some concern about the sole use of brushless techniques in situations in which there may be moderate debris contamination as could be encountered in veterinary applications. A variety of hybrid techniques have been recommended, including performing a thorough hand wash with soap or full surgical scrub at the beginning of the day followed by application of a waterless hand sanitizer product as the sole hand antisepsis technique for subsequent procedures. The main advantage of these may be elimination of alcohol-resistant bacterial spores.47 One factor to consider, however, is that alcohol products may work better on dry hands, so hands should be allowed to thoroughly dry if hands are washed prior to application of an alcohol hand sanitizer.47 Barrier nursing techniques are an important infection control tool. The basic premise for their use is that placing some type of barrier between caregivers and patients prevents skin or clothing from being contaminated, whereas the contaminated outer barrier item can be discarded or left in the contaminated environment. Prevention of contamination of personnel’s skin, regular clothing, personal items, and medical instruments can substantially reduce the risk of transmission of pathogens between animals, contamination of the general environment, and zoonotic transmission. Basic barrier techniques should be used in all veterinary hospitals. Standardized, clean protective outwear should be worn over hospital-dedicated attire for any patient contact, regardless of the anticipated nature of contact or the assumed infectious disease status of the patient. The need for other barrier items varies with circumstances and is often dictated by the type of disease syndrome being managed (e.g., diarrhea or gastrointestinal disease, respiratory disease, wound infection, fever of unknown origin) as opposed to documentation of specific infections or diseases. Other factors that might indicate a need for additional barriers include farm of origin (e.g., farms with endemic S. equi or rotavirus infections) or a patient that is considered to have an increased risk of infection (e.g., compromised neonates). Gloves, gowns, and overboots are the most commonly used items for additional barrier protection, but masks, caps, and eye protection may also be required at times. In some facilities, overboots are not used in all areas, but personnel are required to wear footwear that can be readily disinfected. Disinfection of this footwear is required after exiting potentially contaminated areas. The greatest limiting factors associated with use of barrier precautions in infection control are poor compliance and improper use. Written protocols should be developed that document practical use protocols for barrier precautions. These should specifically state when additional barriers are required; it is not possible to achieve consistent results if protocols are ambiguous or suggest too much discretionary interpretation. In addition, it is critical to educate personnel regarding the need for barrier nursing precautions and on proper application. Many human hospitals require physicians and nursing staff to undergo practical training in proper use of barrier precautions to avoid inadvertent spread of contaminants, and this type of active training may help to ensure uniform understanding and application of protocols. It is also important to regularly monitor compliance with protocols. Standard protective outerwear for large animal veterinary personnel should include clean coveralls, lab coats, scrubs, or other dedicated clothing (hospital uniforms). Protective outerwear should be worn for every animal contact and should be changed regularly. This includes any time outerwear becomes visibly soiled or otherwise contaminated with body fluids perceived or known to be contaminated with potential pathogens (e.g., feces, blood, nasal exudates, urine, or uterine fluid). In addition, outerwear should be changed frequently (at least daily) because gross contamination does not need to be present for pathogen contamination to have occurred. Hospital personnel should change their hospital outerwear before leaving the building to decrease the risk of transfer of infectious agents from the hospital to the community. In order to facilitate this control measure, it is optimal from an infection control perspective for veterinary practices to provide laundry services or laundry facilities. Clothing that is potentially contaminated with biohazardous material should be handled appropriately so that personnel handling laundry are aware of the hazards and how to reduce risks for exposure. Gloves are an important component of barrier precautions if used properly. The Centers for Disease Control and Prevention (CDC) recommends glove use by health care workers to reduce the risk of transmission of infections from patients to personnel, to prevent health care workers’ skin flora from being transmitted to patients, and to reduce transient contamination of the skin on hands of personnel by microorganisms that can be transmitted from one patient to another.48 The same concepts apply to veterinary medicine. In certain situations, glove use has been shown to be an effective means of reducing pathogen transmission in human medicine.49 However, incorrect use can negate these effects or even be harmful. Common errors with glove use include failing to wear gloves when needed, not changing gloves after contact with infectious items, touching items (e.g., pagers, cell phones, pens, medical supplies) while wearing contaminated gloves, contamination of hands or clothing while removing gloves, and failing to wash hands after glove removal.50 Although gloves are used to prevent contamination of hands, the potential for inadvertent contamination through micro-breaks in the glove surface or contamination during glove removal necessitates use of hand-washing or application of hand-sanitizing solutions in conjunction with their use.48 There are no widely accepted standards in veterinary medicine for when gloves must be used, apart from the use of sterile gloves during surgical procedures. Examination gloves that are clean but not sterile are often used when handling wounds or infected body sites, and for contact with animals known or suspected to be shedding contagious pathogens. Despite relatively widespread use of examination gloves in a variety of circumstances in veterinary medicine, it is quite unusual for practices to have formal protocols regarding how and when they should be used. Although protective gowns have traditionally been used during surgery, their use as a barrier garment when contacting high-risk patients is increasing in both human and veterinary medicine. The CDC has produced guidelines for human medicine stating that “gowns should be worn by personnel during the care of patients infected with epidemiologically important microorganisms to reduce the opportunity for transmission of pathogens from patients or items in their environment to other patients or environments.”48 In general, gowns should be worn whenever direct or indirect contact with patients or their environment may result in contamination of caregivers’ clothing or skin that facilitates transmission of pathogens. The ideal barrier gown would cover all areas of the body that might become contaminated, prevent penetration of liquids, would be of adequate strength to resist tearing and puncture under normal activities, would be comfortable to wear for long periods, would be available in appropriate sizes for all personnel, would be easy to put on and remove without contamination of regular attire, would be nonabrasive to skin, would be unlikely to startle patients, and would be of acceptable cost. Unfortunately, a product with all of these characteristics does not currently exist, and facilities must prioritize the relative importance of different gown properties. This is often difficult because neither the overall effectiveness of gowning nor the effectiveness of different gowns in veterinary situations has been adequately evaluated. The problem most likely to be encountered with barrier gowns in veterinary practice is poor resistance to liquids, especially under direct contact or pressure. In large animal practice, there is a greater likelihood of contact with relatively large volumes of fluids (e.g., with diarrheic animals) or direct contact with patient surfaces that would have moist secretions or excretions (e.g., animals with nasal discharge or large infected wounds). The types of anticipated activities may also have an impact on the required size of gown. Gowns that do not completely cover the legs and feet may be useful in some circumstances but ineffective in situations with prolonged direct contact such as with assisted neonatal nursing. In human medicine, there are conflicting data regarding the efficacy of gowning for prevention of hospital-associated infections.49,50 Gowns may be more effective for reducing infection of personnel. It is possible that the most significant advantage in some situations is not the protective effect of gowns or other protective outerwear, but rather the raised awareness of the potentially infectious nature of the patient, which may in turn encourage concurrent application of other important infection control practices.
Biosecurity and Infection Control for Large Animal Practices

How Much Is Enough? How Much Is Too Little?
Principles of Infection Control
Informed Consent and Implications for Infection Control
Paying for Infection Control Activities
Environmental Hygiene
Cleaning
Disinfection
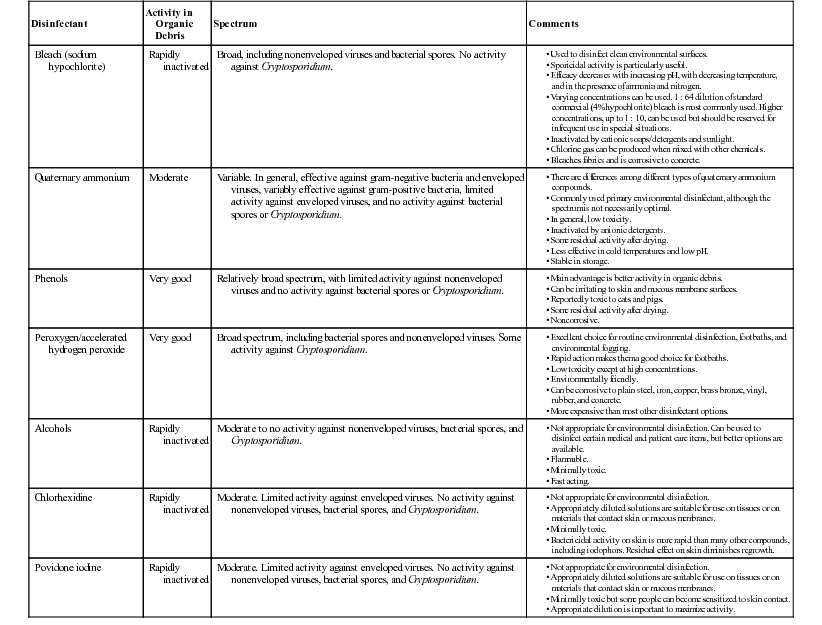
Disinfectants
Disinfectant
Activity in Organic Debris
Spectrum
Comments
Bleach (sodium hypochlorite)
Rapidly inactivated
Broad, including nonenveloped viruses and bacterial spores. No activity against Cryptosporidium.
Quaternary ammonium
Moderate
Variable. In general, effective against gram-negative bacteria and enveloped viruses, variably effective against gram-positive bacteria, limited activity against enveloped viruses, and no activity against bacterial spores or Cryptosporidium.
Phenols
Very good
Relatively broad spectrum, with limited activity against nonenveloped viruses and no activity against bacterial spores or Cryptosporidium.
Peroxygen/accelerated hydrogen peroxide
Very good
Broad spectrum, including bacterial spores and nonenveloped viruses. Some activity against Cryptosporidium.
Alcohols
Rapidly inactivated
Moderate to no activity against nonenveloped viruses, bacterial spores, and Cryptosporidium.
Chlorhexidine
Rapidly inactivated
Moderate. Limited activity against enveloped viruses. No activity against nonenveloped viruses, bacterial spores, and Cryptosporidium.
Povidone iodine
Rapidly inactivated
Moderate. Limited activity against enveloped viruses. No activity against nonenveloped viruses, bacterial spores, and Cryptosporidium.
Effect of Surface Material on Cleaning and Disinfection
Animal Contact Items
Level of Disinfection
Example Items
High level
Endotracheal tubes
Tonometer tips
Cystoscope
Intermediate level
Dental equipment
Endoscopes
Thermometers used on multiple patients
Vaginal specula
Low level
Thermometers used on individual patients
Feeding instruments
Muzzles
Nasogastric tubes
Oral specula
Stethoscopes
Hand Hygiene
Hand-Washing
 inch or longer) or artificial nails. The role of these factors in risks to patients in veterinary settings is unclear, but some veterinary facilities have developed similar protocols and restrictions.
inch or longer) or artificial nails. The role of these factors in risks to patients in veterinary settings is unclear, but some veterinary facilities have developed similar protocols and restrictions.
Waterless Hand Sanitizers
Surgical Hand Antisepsis
Barrier Protocols and Protective Attire
Use of Barrier Precautions
Limitations to Barrier Precautions
Protective Outerwear
Gloves
Gowns
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Biosecurity and Infection Control for Large Animal Practices
Chapter 46
