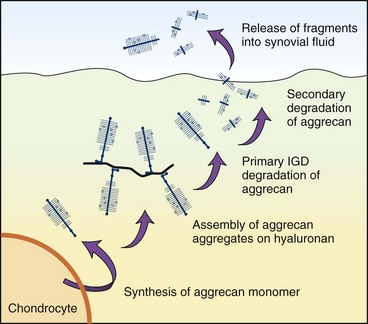
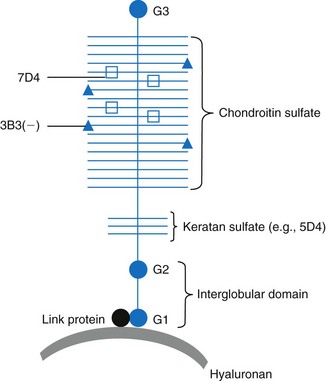
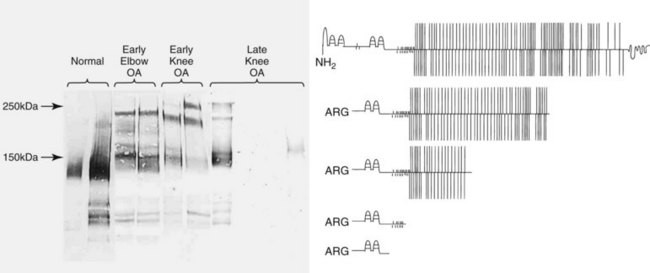
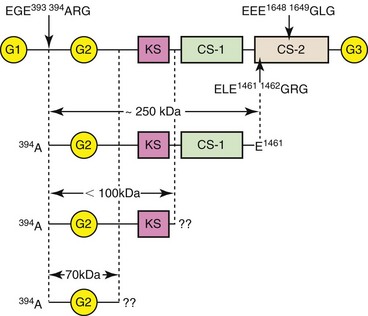
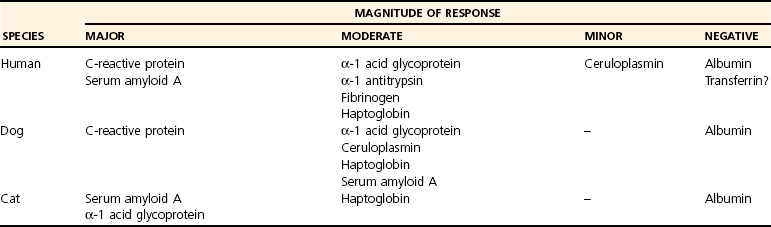
Chapter 3 Biologic markers (biomarkers) aim to provide information on the physiologic or pathologic status of a specific tissue or organ. The National Institutes for Health (U.S.) defines a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”9 Biomarkers may be used in several ways: • As a diagnostic tool for identification of those patients with a disease or abnormal condition • As a tool for staging of disease or classification of the extent of disease • As an indicator of disease prognosis • For prediction and monitoring of clinical response to an intervention. As such, biomarkers may be used as surrogate outcomes measures (or clinical endpoints) in phase I and II trials for efficacy and safety of new interventions, as well as in vitro studies or animal model studies. Typically, biomarkers are measured in serum, plasma, or urine, but they may also be measured in some other more local fluid such as saliva, synovial fluid,131 tears,129 aqueous humor,206 or cerebrospinal fluid,207 or in tissue biopsy samples. The perfect biomarker would be specific to the diseased tissue and pathology, sensitive to changes in disease progression or therapeutic intervention, and predictive of disease outcome. Clearly the subject matter of biomarkers is vast because it spans every body system and specialty. This chapter uses biomarkers from two areas of key relevance to small-animal surgeons, namely, osteoarthritis and inflammation, to exemplify the development, validation, and current uses of biomarkers. Osteoarthritis is a common, debilitating, degenerative joint disease that affects all mammals, including the horse, the dog, and man.176 Degradation of articular cartilage in the affected joint is a major feature62,214 of the disease, and osteoarthritis can be defined as a process of aberrant repair with gradual and progressive loss of articular cartilage through degradative mechanisms, along with remodeling of bone and synovial inflammation. Progressive deterioration of the articular cartilage leads to loss of function and, ultimately, failure of the joint. Articular cartilage is predominantly composed of water, collagen,63 proteoglycan, and glycoprotein.224 For further discussion of the basic structure of cartilage, please refer to Chapter 40, and for further background on the pathologic process of osteoarthritis, please refer to Chapter 68. Over the past three decades, one focus of osteoarthritis research has been the study of candidate molecular biomarkers for the early detection of osteoarthritis, monitoring of disease, or prediction of progression. The ability to detect cartilage loss in osteoarthritis is highly desirable, not only from a diagnostic, prognostic, and therapeutic perspective, but also as a surrogate outcomes measure in trials of candidate structure-modifying agents.67 The current imaging modalities of arthroscopy and magnetic resonance imaging (MRI) are costly, require general anesthesia, and yield results only when gross damage or fibrillation to the cartilage has already occurred. Cartilage destruction leads to an accumulation of breakdown products in the synovial fluid. These products are then released into the circulation and ultimately are filtered and excreted, or broken down in vivo (Figure 3-1). The potential exists therefore, for the analysis of fluids (such as plasma, serum, urine, or synovial fluid) that are easily obtained with minimal invasion, and that provide information regarding the integrity of the cartilage before the development of gross pathology, or any metabolic changes attributable to the treatment being studied. For a biomarker to be appropriate for use as a measure of the joint’s response to the test treatment, it “must reliably predict the overall effect on the clinical outcome”69—a standard that osteoarthritis biomarkers thus far have failed to meet. Osteoarthritis is a complex process, and numerous interdependent factors can affect clinical outcomes; the requirement for a biomarker to correlate with the clinical outcome while capturing the net effect of treatment has thus far has proven to be challenging.67 Nevertheless, continued progress in the field continues to suggest that perseverance may result in validated assays. Proteoglycan content in biologic fluids can be measured easily and cheaply with the use of the dimethylmethylene blue (DMMB) assay—a simple colorimetric assay that utilizes the formation of an insoluble precipitate between the DMMB and intact glycosaminoglycan (GAG) chains.46,66 The glycosaminoglycan content of synovial fluid, serum, or tissue culture medium increases as a result of inflammatory insult to the cartilage.8,73,78,217 The assay is nonspecific, and a large degree of error can be incorporated because of the necessity for serial dilution of highly concentrated samples. As a result, more specific markers of proteoglycan catabolism have been developed. The major proteoglycan of articular cartilage is the large aggregating proteoglycan, aggrecan. Chondroitin sulfate is a glycosaminoglycan that is covalently attached to specific proteins to form proteoglycans, which are abundantly found within the extracellular matrix of cartilage, particularly on aggrecan (Figure 3-2). The primary component of chondroitin sulfate is a repeating disaccharide sequence (D-glucuronate and N-acetyl-D-galactosamine) that can be sulfated (on the 4- or 6- position of the galactosamine) or can be nonsulfated.161 In the last two decades, monoclonal antibodies have been raised that detect carbohydrate structures within native (nondigested) chondroitin sulfate chains of glycosaminoglycan derived from articular cartilage.213 Keratan sulfate is also a glycosaminoglycan that forms side-chains in the aggrecan monomer, predominantly in the keratan sulfate-rich region close to the G2 domain (see Figure 3-2). 7D4, 3B3, CS846: Two examples of monoclonal antibodies recognizing chondroitin sulfate motifs (a sequence or structural pattern of biologic significance) are 7D4, which recognizes an epitope that is 6-sulfated and contains one nonsulfated disaccharide,7,116 and 3B3, which recognizes the neo-epitope, 3B3(−), found on native, non–enzymatically cleaved chondroitin sulfate chains that have a nonreducing termination of GlcAβ1,3GalNAc6S28,175 (see Figure 3-2). 7D4 and 3B3 are generally considered to be “anabolic” markers of cartilage turnover in osteoarthritis.29 The 7D4 antibody recognizes subtle combinations of sulfated and nonsulfated disaccharide isomers within the native chondroitin chain. The epitope recognized is expressed only weakly in normal adult cartilage but is found in increased concentrations in the developmental stages of cartilage growth, such as in the growth plates, in fetal cartilage, and during attempted repair early in osteoarthritis. It is also found in much higher concentrations in synovial fluid and cartilage from experimental models of osteoarthritis.25,30,116 The concentration of the 7D4 epitope, quantified by immunoassay of synovial fluid and stifle articular cartilage from sheep,132 dogs,184 and humans,209 was significantly increased in animals with traumatic or experimentally induced osteoarthritis compared with controls. Longitudinal analysis of 7D4 concentrations in canine synovial fluid using an enzyme-linked immunosorbent assay (ELISA), following resection and delayed repair of the cranial cruciate ligament, showed a marked increased in concentrations throughout the 5-month period after initial surgery.116 Similarly, another study showed that 7D4 concentrations in the synovial fluid of dogs with naturally acquired cranial cruciate ligament rupture were significantly higher than those from healthy control joints.117 Concentrations of 3B3 in the synovial fluid of human patients are raised following trauma to the cruciate ligament or meniscus89 and are significantly increased in the synovial fluid16 and serum36 from patients with chronic osteoarthritis. In the dog, a discrepancy in synovial fluid 3B3 levels between naturally acquired cranial cruciate ligament rupture and experimental transection was reported; only values in the naturally acquired cranial cruciate ligament rupture group were significantly greater than those in the healthy control group.117 A significant correlation between 3B3 and 7D4 levels was also reported.117 Synovial fluid 3B3 levels are significantly elevated in canine stifles following meniscectomy, with levels reaching a peak at 4 weeks, remaining significantly raised until 12 weeks post meniscectomy, and declining throughout subsequent measurements.24,131 Serum levels of 3B3 in dogs with cranial cruciate ligament rupture, osteochondritis dissecans, fragmented coronoid process, patella luxation, hip dysplasia, or infective arthritis were reduced compared with levels in normal dogs.91 Another anabolic marker for osteoarthritis is the antigen detected by the CS846 antibody assay. In fetal cartilage, or in cartilage undergoing attempted repair, large forms of aggrecan with at least one chondroitin sulfate epitope are synthesized. A commercial assay (Ibex, Montreal, Canada) detects molecules of fetal aggrecan released into the serum from cartilage following matrix metalloproteinase or ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) cleavage.83 In dogs with experimental cranial cruciate ligament transection, CS846 levels increased soon after injury and remained elevated for 3 months.142 Keratan Sulfate, 5D4: The monoclonal antibody 5D4 recognizes “oversulfated” forms of keratan sulphate.220 5D4 concentrations on average are 20 times higher in synovial fluid than in serum, suggesting local production of keratan sulfate within the joint.23,198 Synovial inflammation, which is often an accompanying feature of joint disease, may partially explain differences between measurements from blood and from synovial fluid in the same patient.147 The 5D4 epitope has been widely used in studies of experimental and naturally occurring canine osteoarthritis.8,19,107,110,139 Serum 5D4 concentrations were reduced in dogs with naturally acquired osteoarthritis.91 No correlation between synovial and serum 5D4 and 3B3(−) values was found. Experimental meniscectomy resulted in a rapid increase in synovial fluid 5D4 concentration, but by 12 weeks post meniscectomy, 5D4 concentrations were no longer elevated, in contrast to 3B3. It was suggested that the divergence of 5D4 may be due to the fact that it is derived predominantly from cartilage, whereas the 3B3 epitope may be derived in significant proportions from noncartilaginous tissues.131 Median concentrations of synovial fluid 5D4 were found to be upregulated after cranial cruciate ligament rupture and patella luxation and were inversely correlated with increasing duration of lameness; it is hypothesized that this may reflect changes in the metabolism and composition of proteoglycans in osteoarthritis joints. Previously published data identified an increase in the chondroitin sulfate : keratan sulfate ratio and that of chondroitin-4-sulfate to chondroitin-6-sulfate during the development of canine osteoarthritis, which may reflect the synthesis of proteoglycans more commonly associated with immature cartilage.146 Synovial fluid 5D4 was not significantly altered by tibial plateau leveling osteotomy surgery,82 and in dogs with naturally acquired stifle osteoarthritis (as a result of cranial cruciate ligament rupture), serum 5D4 concentration was not significantly associated with other disease features.112 In addition, synovial fluid 5D4 values from osteoarthritis joints were low, compared with values in contralateral normal joints. The authors concluded that serum 5D4 concentration is not a useful marker of stifle osteoarthritis in dogs; this conclusion is supported by other studies in which 5D4 concentrations confer no predictive value.111 BC-3, BC-14: The BC-3 antibody recognizes the new N-terminus of aggrecan generated by cleavage of the interglobular domain (IGD) by aggrecanases (Figures 3-3 and 3-4), while the BC-14 antibody recognizes that generated by the proteolytic action of matrix metalloproteinases.104,133 Detection of these epitopes is helping to identify the degradative enzymes responsible for the catabolism of aggrecan in osteoarthritis. In one study, the synovial fluid from some dogs with normal joints and from all dogs with early-stage osteoarthritis contained detectable quantities of BC-3 epitope, but a notable increase in the size of BC-3–positive aggrecan catabolites was seen in early osteoarthritis. However, few samples from late-stage osteoarthritis displayed BC-3–positive bands on Western blot, implying that BC-3 may be more useful as a marker of early-stage disease (see Figure 3-3).109 The new C-terminus of some fragments generated by secondary cleavage of canine aggrecan remains unknown at the current time (see Figure 3-4). Figure 3-4 Schematic to illustrate various BC-3–positive aggrecanase-generated aggrecan catabolites that may appear in canine synovial fluids. Aggrecanase enzymes (e.g., ADAMTS-4, ADAMTS-5) cleave aggrecan at the EGE393,394ARG site in the interglobular domain. However, C-terminal proteolysis by aggrecanases or matrix metalloproteinases can result in a variety of BC-3–positive catabolites.108 Detection of specific aggrecan fragments may provide useful information for future diagnosis and prognosis in osteoarthritis. CS, Chondroitin sulfate; KS, keratan sulfate. OA-1: Although detection and quantification of biomarkers in biologic fluids, such as those discussed earlier, provide evidence of change in cartilage matrix turnover, they do not necessarily represent the results of specific proteolytic pathways. In the past few years, an ELISA has been developed that used the monoclonal neo-epitope antibody OA-1, which specifically recognizes the N-terminal sequence “ARGSVIL,” present in the keratan-containing aggrecan fragment generated by aggrecanase-mediated cleavage at the Glu373-Ala374 bond of the IGD.180 The antibody appears to detect similar aggrecan fragments as the BC-3 antibody, for which no quantitative assay is currently available.104 In the near future, the OA-1 ELISA may serve as a biomarker assay for evaluation of both preclinical and clinical samples. Measurement of Type II Collagen Synthesis Type II collagen is synthesized as a pre-propeptide with N- and C-terminal globular domains. These pre-propeptides are translated on the ribosomes of the rough endoplasmic reticulum, then are directed across the membrane to the rough endoplasmic reticulum lumen; they are modified enzymatically122,238 according to collagen type and age.193 Formation of the triple helix from the procollagen chains is complex and multi-staged. The folded triple helix is then secreted into the extracellular matrix, but the final aggregation of procollagens and formation of fibrils require the removal of the C- and N-terminal domains to give tropocollagen. This cleavage (by the extracellular proteinases C- and N-proteinase) ensures intracellular helix formation cannot occur. The tropocollagens then spontaneously aggregate.191 At this stage, relatively minor amounts of types IX and XI collagen form a heterofibril with the type II chains. The N- and C-terminal propeptides are present in the highest proportion in fetal cartilage, are rapidly reduced at birth and are present only in very low levels in the cartilage of adults.157 PIICP: The predominance of collagen in articular cartilage and its synthesis therein can be exploited to estimate the status of cartilage collagen synthesis and therefore draw hypotheses regarding tissue status. Specific features, one on the N-propeptide and one on the C-propeptide, can be used to measure synthetic activity. The C-propeptide can be detected by the procollagen type II C-propeptide (PIICP) assay (also referred to as CPII). Both an immunoassay96 and a sandwich ELISA208 for PIICP have been developed. The ELISA detects the presence of the three 35 kDa C-propeptides, connected by disulfide links,163 which are released into the circulation following cleavage by C-propeptidease. Because the half-life of the cleaved propeptide is relatively short ( In a study of experimentally induced canine osteoarthritis (surgical transaction of the cranial cruciate ligament), serum PIICP concentrations were not significantly raised at 3 or 12 weeks post surgery,142 although other studies, using both direct biosynthetic64 and molecular biology143 methods, have found increased synthesis and gene expression of type II collagen in cartilage at similar time points in the surgical transaction of the cranial cruciate ligament model. Baseline concentrations of PIICP are reportedly higher in the serum of dogs and horses than humans; as a result, the greater systemic PIICP production may mask any upregulation that occurs from a single damaged joint.188 PIIANP/ PIINP: The N-terminus of the three α1 chains of type II collagen is produced in two isoforms, by alternative splicing of the Col2a1 gene transcript and by exclusion or inclusion of exon 2A. In chondroprogenitor cells200 in noncartilaginous embryonic tissue and in osteophytes and chondrocytes in fracture calluses,105 the N-propeptide includes a 69-amino-acid, cysteine-rich domain (this is termed type IIA collagen, or PIIANP). The other isoform (type IIB, or PIINP), excluding this globular domain, is produced by mature, adult chondrocytes.200 It has been demonstrated that chondrocytes in human osteoarthritis cartilage also produce the PIIANP isoform,249 suggesting hypertrophic change and a shift in cartilage type, which more closely resembles that of a developing joint.199 The cleaved PIIANP fragment can be detected by means of a competitive ELISA.192 PIINP in the plasma, urine, and synovial fluid lavage from humans, dogs, and rats has been measured following development of an ELISA.159 Plasma concentrations of PIINP in human patients with radiographically confirmed osteoarthritis and clinical symptoms of disease were almost five times greater than in control samples. Urine PIINP concentrations in all three species were between two and three times higher than those in plasma. Consecutive measurement of collagen types IIA and IIB may improve the accuracy of type II collagen synthesis estimates. It is thought that the structural design of mature collagen aids its longevity and provides an inherent degree of protection from proteolysis, because enzymatic cleavage sites in and between molecules are limited. Breakdown and turnover of cartilage collagen are largely mediated by a family of degradative enzymes called matrix metalloproteinases, named for the metal ion (generally zinc) present at the active site. Collagenolysis must, for reasons of functional continuation, be strictly controlled, and as a consequence, matrix metalloproteinase activity is tightly regulated at three levels: enzyme synthesis, activation, and inhibition. Of approximately 23 matrix metalloproteinases currently identified, four are known to play a primary role in cartilage collagen destruction: the collagenases MMP-1, -8, and -13, and the membrane-bound MMP-14.166 MMP-13 is considered the most important collagenase in cartilage; it shows a preference for type II collagen.123 In joint disease, increased expression of collagenolytic matrix metalloproteinases and decreased expression of their endogenous regulatory inhibitors, the tissue inhibitors of metalloproteinases (TIMPs), mean that the homeostatic balance between synthesis and destruction is lost, in favor of collagen breakdown. Collagen proteolysis happens soon after injury,136 and destruction is considered to be the “point of no return” in cartilage degeneration.243 Cleavage of the triple helix typically occurs at the Gly794-Leu795 bond in the collagen triple helix, resulting in the production of CTX-II: The type II collagen C-telopeptide fragment (CTX-II) ELISA utilizes a monoclonal antibody specific for a six-amino-acid sequence present exclusively in the C-terminal of type II collagen, 1161EKGPDP1166. The assay preferentially recognizes peptides with a free N-terminal glutamate and recognizes only those with a free C-terminal proline. The proteases responsible for the generation of this fragment are currently unidentified, but it is thought that they are located in the cartilage matrix or are produced by the chondrocytes themselves.39 In the surgical transaction of the cranial cruciate ligament model in dogs, CTX-II levels were increased in synovial fluid and serum after 3 weeks, with elevations maintained until 12 weeks, at which point urinary concentrations of the peptide (uCTX-II) levels were also raised.142 In humans, some circadian variation (<20%) occurs in levels of uCTX-II, as does a difference between premenopausal and postmenopausal women.39 In human osteoarthritis patients, concentrations of uCTX-II were found to be 1.53-fold higher than in healthy controls, and a trend toward an association between joint space narrowing and uCTX-II levels was noted.39 Increased amounts of urine and synovial fluid CTX-II have been detected soon after knee injury, and higher concentrations correlated with more rapid progression of destruction.39,77,136 The reactivity of this assay may be influenced by, or indeed dependent upon, the presence in this particular region of an inter-α-chain cross-link; the significance of this structural component remains unknown and unaddressed.177 Nonetheless, an increase in uCTX-II in combination with a decrease in PIIANP was shown to be predictive of osteoarthritis progression in the human knee.76 In contrast, a 5 year study reported that serum PIIANP levels increased progressively in all patients, while uCTX-II remained stable only in those patients in which joint disease did not worsen, rising in the early stages of disease that became more advanced.204 C2C/UC2C: Two assays exist for the detection of the neo-epitope generated at the C-terminal end of the A model of experimental osteoarthritis in sheep also found that levels of C2C increased following partial-thickness injury to the cartilage,138 and serum concentrations were elevated in osteoarthritis-susceptible compared with osteoarthritis-resistant guinea pigs.103 However, in a study of obese women with unilateral knee osteoarthritis, no association was found between C2C and radiographic joint space narrowing.144 One as yet unresolved issue concerns that of epitope stability, because further enzymatic cleavage of fragments by matrix metalloproteinases occurs. In addition, the rate of degradation of cartilage and progression of subsequent osteoarthritis is considerably faster in an experimental model than occurs in natural disease, leading to earlier production of peak levels, which also may be of higher concentration. Although faster disease progression is essential for laboratory study, it may explain discrepancies between associations arising from experimental studies and from studies concerning the same biomarkers in populations with natural disease. This area deserves further study. COL CEQ: Following modification of the C1,2C epitope antibody, an antibody was developed that identifies the C-terminus neo-epitope produced by collagenase digestion of equine type II collagen.13 An inhibition ELISA was developed, using the 234CEQ antibody and the peptide Cys-Gly-Gly-Asp-Gly-Pro-Hyp-Gly-Pro-Gln-Gly (C-G-G-D-G-P-POH-G-P-Q-G), representing the amino acid sequence for the collagenase-created C-terminus of the equine type II collagen. Concentrations of synovial fluid and serum Col CEQ have been shown to be significantly increased in osteoarthritis-affected joints and to rise as a result of exercise.73 HELIX-II: The helical region of type II collagen represents the major part of the collagen molecule. The HELIX-II ELISA37 was developed with the aim of detecting a sequence found only in the α1 chain of type II collagen. Unfortunately, because of a sequencing error in the genomic database used as reference, the erroneous sequence ERGETGPPGTS was used; the actual Helix-II epitope sequence in COL2A1 encodes ERGETGPPGPA.65 As such, reports in which conclusions based on the HELIX-II assay are drawn20,37,77 require reevaluation. TIINE: Recently, a biomarker for a type II collagen neo-epitope (TIINE) resulting from enzymatic cleavage of the type II collagen fibril has been identified.158 In vitro stimulation of cartilage explants for 22 days with the proinflammatory cytokines interleukin-1 and oncostatin M resulted in the generation of several peptides, which were identified using liquid chromatography and mass spectrometry. Several of these peptides contained the conserved C-terminal collagenase-generated COLL-2-1/COLL-2-1NO2: Located toward the N-telopeptide of the triple helix is the nine-amino-acid-long Coll-2-1 peptide (108HRGYPGLDG116), or the nitrogenated form, Coll-2-1NO2 (108HRGY[NO2]PGLDG116). Its detection implies a degree of helical “unwinding,” or further catabolism of the A small, but significant, portion of cartilage matrix proteins are noncollagenous, nonproteoglycan glycoproteins, such as link protein, chondronectin, fibronectin, cartilage oligomeric matrix protein, thrombospondin, and anchorin. Fibronectin may play a role in matrix organization and matrix-chondrocyte interaction, and link protein stabilizes the interaction between aggrecan and hyaluronic acid in the presence of temperature, mechanical force, or pH extremes.68,155 In addition, link protein may help to protect aggregates of proteoglycan from degradation. It has been demonstrated that although high concentrations of hyaluronidase and free radicals cleave proteoglycan aggregates, at lower concentrations, link protein demonstrates a protective role.189 Cartilage oligomeric matrix protein is a pentameric, noncollagenous glycoprotein and is a constituent of articular cartilage. It is thought its function might be to bind type I or II collagen fibers190 and to stabilize the network of the collagen fiber202 or to facilitate fibrillogenesis.85 Cartilage oligomeric matrix protein is needed for normal development, function, and matrix assembly, as illustrated by the discovery that cartilage oligomeric matrix protein mutations result in pseudochondroplasia and some forms of multiple epiphyseal dysplasia.165 The intact cartilage oligomeric matrix protein molecule has a high molecular weight (524 kDa) and structurally consists of five identical globular subunits attached to a central assembly domain by flexible strands. On the basis of these structural characteristics, cartilage oligomeric matrix protein is classified as a member of the thrombospondin family.169 Cartilage oligomeric matrix protein also exists as oligomeric reducible fragments of about 150 kDa, monomeric, nonreducible fragments (67 to 94 kDa) and occasionally as even smaller fragments (43 to 67 kDa)49—these latter fragments might be the result of cleavage by matrix metalloproteinases, particularly MMP-1 and MMP-13.75 Cartilage oligomeric matrix protein is found abundantly in cartilage, from which it was originally isolated,90 but it is also found in synovium (including synovial fibroblasts and synovial fluid),106,186 tendon,50 and meniscus.151,156 Cartilage oligomeric matrix protein concentrations in biologic fluids (most commonly, synovial fluid and serum, and less commonly, urine) can be assayed by means of ELISA.202,236 In early osteoarthritis, the metabolically active cartilage undergoes multiple attempts at repair, which are associated with increased levels of serum cartilage oligomeric matrix protein.35 In addition, synovial inflammation at this stage provides an additional source of cartilage oligomeric matrix protein. Reports focusing on early-stage osteoarthritis therefore have reported increased levels. Once osteoarthritis becomes established, however, the remaining cartilage is thought to be less metabolically active, explaining the apparently conflicting reports of lower levels of cartilage oligomeric matrix protein.35 Some species differences are apparent when the value of cartilage oligomeric matrix protein as a diagnostic or prognostic indicator of disease is considered.174,205 Cartilage oligomeric matrix protein has been used in a small number of canine studies. Several studies in experimental canine models of osteoarthritis indicate the usefulness of cartilage oligomeric matrix protein24,131,182 and its association with cartilage damage. However, a study using cartilage oligomeric matrix protein to investigate the potential structure-modifying properties of tibial plateau leveling osteotomy in dogs with naturally occurring cruciate rupture was unable to show any significant change in synovial fluid cartilage oligomeric matrix protein concentrations after tibial plateau leveling osteotomy compared with preoperative values.82 Acute phase proteins are a group of blood proteins that are secreted mainly by the liver in response to a variety of insults, including infection, trauma, and stress.56 As a consequence, circulating concentrations increase during early stages of the inflammatory response of the body.74 Acute phase proteins form part of the innate immune system and function mainly to reestablish normal homeostasis after the insult, and to inhibit microbial growth for a sufficient time to enable the acquired immune response to develop. Given that the magnitude of increase in acute phase proteins in the blood correlates with the severity of inflammation and tissue damage,56 they are ideal candidates to use as biomarkers. The systemic response to a variety of adverse stimuli (e.g., infection, trauma, tissue damage) is termed the acute phase response. It is nonspecific in nature and rapid in onset, in some cases developing before clinical signs are evident. The principal mechanism by which inflammatory insults trigger this response is through the release, from leukocytes (especially macrophages), of proinflammatory cytokines, including TNF-α, IL-1, and IL-6.12,51,93,240 These cytokines in turn stimulate various pathophysiologic responses, including pyrexia, leukocytosis, increased circulating cortisol, and decreased thyroid hormone concentrations, as well as decreased iron and zinc concentrations. The collective effect is to minimize tissue damage and stimulate repair mechanisms. In addition, TNF-α, IL-1, and IL-6 can alter synthesis of acute phase proteins, most of which are synthesized by the liver,2,11,153,242,247 as well as some extrahepatic sites.230,239 The synthesis of “positive” acute phase proteins increases as a result of the insult, leading to elevated circulating plasma concentrations; examples include haptoglobin, C-reactive protein, serum amyloid A, ceruloplasmin, fibrinogen, and alpha-1 acid glycoprotein (AGP).152 It is not surprising that the synthesis of “negative” acute phase proteins declines in response to challenge; the main examples are albumin and transferrin.152 Acute phase proteins have already been extensively studied in human medicine, and they provide valuable clinical information on the presence and extent of inflammatory lesions.128,219 Similar work is evident in a wide variety of veterinary species, including companion animals, horses, ruminants, and pigs.152 In addition to their classification as positive or negative acute phase proteins, these proteins are classified by their importance to the acute phase response in a particular species, such that major (>10-fold increase), moderate (2- to 10-fold increase) and minor acute phase protein (<2-fold increase) categories exist (Table 3-1).33,152 For instance, in humans, C-reactive protein and serum amyloid A are the major acute phase proteins; as an example, C-reactive protein concentrations can increase up to 200-fold in some types of inflammation.55 Furthermore, determination of C-reactive protein can provide information on prognosis and response to therapy.137,172 In contrast, haptoglobin, AGP, fibrinogen, and alpha-1 acid glycoprotein AAT are classified as moderate acute phase protein, because their concentrations increase far less markedly (typically ≈3-fold) during inflammation.55 Finally, ceruloplasmin is only a minor acute phase protein because a subtle change (e.g., increases of only 60% to 70% above baseline) in circulating concentrations is seen.55 Table • 3-1 Classification of Acute Phase Proteins in Different Species Modified from Murata H, Shimada N, Yoshioka M: Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J 168:28, 2004 and Ceron JJ, Eckersall PD, Martynez-Subiela S: Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol 34:85, 2005.
Biomarkers in Clinical Medicine
Biomarkers in Osteoarthritis
Noncollagenous Biomarkers of Osteoarthritis
Biomarkers of Collagen Turnover
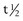 in cartilage = 16 hr,157
in cartilage = 16 hr,157 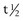 in serum = 18 hours),179 in theory PIICP is a good indicator of recent synthesis.
in serum = 18 hours),179 in theory PIICP is a good indicator of recent synthesis.
Measurement of Breakdown of Type II Collagen
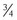 and
and 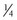 length fragments and the generation of two new termini, which can be measured to assess the rate of destruction. Only the
length fragments and the generation of two new termini, which can be measured to assess the rate of destruction. Only the 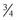 length fragment (the Col2-3/4 epitope) is detectable in the circulation; it is hypothesized that this is a result of the greater resistance of the longer fragment to proteolysis.47 In studies using immunohistochemistry, the N-terminal epitope of the
length fragment (the Col2-3/4 epitope) is detectable in the circulation; it is hypothesized that this is a result of the greater resistance of the longer fragment to proteolysis.47 In studies using immunohistochemistry, the N-terminal epitope of the 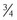 fragment was easily identified in human osteoarthritis cartilage, whereas very little C-terminal epitope was present, suggesting that the C-terminal epitope is rapidly cleared following collagenolysis, and that the helical region should not be considered uniformly susceptible to further enzymatic degradation. The type II collagen
fragment was easily identified in human osteoarthritis cartilage, whereas very little C-terminal epitope was present, suggesting that the C-terminal epitope is rapidly cleared following collagenolysis, and that the helical region should not be considered uniformly susceptible to further enzymatic degradation. The type II collagen 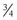 fragment is degraded by enzymes such as MMP-9,232 to release several smaller fragments. The enzymatic pathways of collagen catabolism are complex and as yet are not fully understood, and it is likely that the many enzymes involved are activated at different times and under different circumstances. The many pathways leading to osteoarthritis may therefore result in the formation of differing quantities of the various cleavage products, depending on the stage and type of arthritis.178
fragment is degraded by enzymes such as MMP-9,232 to release several smaller fragments. The enzymatic pathways of collagen catabolism are complex and as yet are not fully understood, and it is likely that the many enzymes involved are activated at different times and under different circumstances. The many pathways leading to osteoarthritis may therefore result in the formation of differing quantities of the various cleavage products, depending on the stage and type of arthritis.178
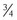 length fragment by the action of collagenases on the triple helix. The C1,2C ELISA assay was developed first and recognizes the sequence 900GPP(OH)GPQG906 common to the triple helix of both type I and type II collagen.14 Articular cartilage contains no significant levels of type I collagen, but this cross-reactivity was considered a disadvantage, and subsequently, an ELISA based on the C2C antibody was developed, specific to type II collagen, which recognizes the sequence 899EGPP(OH)GPQG906,177 This antibody recognizes the majority of collagenase-cleaved type II collagen and has also been used in studies of experimental arthritis (surgical transaction of the cranial cruciate ligament) in the dog,41,142 wherein levels of C2C in synovial fluid were found to be increased compared with those in normal dogs at 3 and 12 weeks. Additionally, urine (uC2C) and serum C2C levels were significantly raised 12 weeks after surgery compared with levels from normal dogs,142 and raised concentrations persist in long-term studies.41,84 However, opposing conclusions have also been drawn: A study of naturally occurring cranial cruciate ligament rupture found no significant differences in measured C2C in serum, urine, or synovial fluid,87 and the authors therefore concluded that this epitope may not be a specific marker for canine osteoarthritis, or that it may be unsuitable for the pathology associated with naturally occurring cranial cruciate ligament rupture.
length fragment by the action of collagenases on the triple helix. The C1,2C ELISA assay was developed first and recognizes the sequence 900GPP(OH)GPQG906 common to the triple helix of both type I and type II collagen.14 Articular cartilage contains no significant levels of type I collagen, but this cross-reactivity was considered a disadvantage, and subsequently, an ELISA based on the C2C antibody was developed, specific to type II collagen, which recognizes the sequence 899EGPP(OH)GPQG906,177 This antibody recognizes the majority of collagenase-cleaved type II collagen and has also been used in studies of experimental arthritis (surgical transaction of the cranial cruciate ligament) in the dog,41,142 wherein levels of C2C in synovial fluid were found to be increased compared with those in normal dogs at 3 and 12 weeks. Additionally, urine (uC2C) and serum C2C levels were significantly raised 12 weeks after surgery compared with levels from normal dogs,142 and raised concentrations persist in long-term studies.41,84 However, opposing conclusions have also been drawn: A study of naturally occurring cranial cruciate ligament rupture found no significant differences in measured C2C in serum, urine, or synovial fluid,87 and the authors therefore concluded that this epitope may not be a specific marker for canine osteoarthritis, or that it may be unsuitable for the pathology associated with naturally occurring cranial cruciate ligament rupture.
 fragment neo-epitope, GPXGPQG, the most abundant of which was 45 amino acids long and contained the sequence 862ARGDSGPPGRAGEPGLQGPAGPPGEKGEPGDDGPSGAEGPPGPQG.906
fragment neo-epitope, GPXGPQG, the most abundant of which was 45 amino acids long and contained the sequence 862ARGDSGPPGRAGEPGLQGPAGPPGEKGEPGDDGPSGAEGPPGPQG.906
 epitope. In young adulthood, serum concentrations of Coll-2-1NO2 are elevated, in contrast to those of Coll-2-1, which, assuming the continued health of the cartilage, are stable throughout life. Assays for the detection of both forms have been developed.48,94 Although Coll-2-1NO2 is the nitrated form of the peptide, its concentration is not necessarily indicative of the total quantity of Coll-2-1 epitopes present in the sample, but instead represents merely a portion, and direct extrapolation to the overall degree of catabolism cannot be performed.3 Nitration of Coll-2-1 requires oxidative stress, and as a result, measurement of the Coll-2-1NO2 epitope is perhaps more applicable to the study of rheumatoid arthritis, where higher levels are observed in disease. To date, few studies have utilized this assay in a veterinary species.
epitope. In young adulthood, serum concentrations of Coll-2-1NO2 are elevated, in contrast to those of Coll-2-1, which, assuming the continued health of the cartilage, are stable throughout life. Assays for the detection of both forms have been developed.48,94 Although Coll-2-1NO2 is the nitrated form of the peptide, its concentration is not necessarily indicative of the total quantity of Coll-2-1 epitopes present in the sample, but instead represents merely a portion, and direct extrapolation to the overall degree of catabolism cannot be performed.3 Nitration of Coll-2-1 requires oxidative stress, and as a result, measurement of the Coll-2-1NO2 epitope is perhaps more applicable to the study of rheumatoid arthritis, where higher levels are observed in disease. To date, few studies have utilized this assay in a veterinary species.
Noncollagenous, Nonproteoglycan Glycoproteins
Cartilage Oligomeric Matrix Protein
Biomarkers and Inflammation
Acute Phase Proteins and Inflammatory Biomarkers
The Acute Phase Response
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Biomarkers in Clinical Medicine
Only gold members can continue reading. Log In or Register to continue