Chapter 12 Biochemical markers of cardiovascular disease
INTRODUCTION
Biochemical markers of cardiovascular disease are important tools in assessing human heart disease. Assays based on natriuretic peptides and biochemical mediators of activation of the sympathetic nervous and renin–angiotensin–aldosterone system can be used for diagnosis, prognosis and treatment monitoring in a range of heart diseases. Measurement of cardiac troponins is the gold-standard for identification of myocardial disease in human beings. While it is extremely tempting to reach for an “off-the-shelf” assay kits developed for use in human beings and apply these to equine patients, this is often not appropriate for a number of reasons. Firstly, immunoassays developed in one species do not necessarily cross-react with the equivalent molecule in another species and it is important that any biochemical assay is either developed specifically for equine use, or if developed for another species, it should have been validated for horses before it is applied in clinical practice. Secondly, the prevalence of the target disorder within the species of interest must be considered. Diagnostic tests are often described in terms of their sensitivity (the proportion of true positives) and specificity (the proportion of true negatives). These can be regarded as inherent characteristics of the test; however, from a clinical perspective, it is much more relevant to know a test’s predictive value or how likely it is that the test will give a correct diagnosis of whether the tested animal is diseased or disease free.1 The predictive value is not only influenced by the accuracy of the test but also by the prevalence of disease within a given population and predictive values decrease as prevalence decreases. This issue is particularly relevant to biomarkers of cardiovascular disease in horses because disease states that are common in human beings such as heart failure and myocardial necrosis are extremely uncommon in horses. Consequently, biomarkers that are extremely important in human beings are not necessarily so useful in horses. A test may be extremely sensitive and specific in detecting a specific form of cardiovascular pathology, but this is not very helpful when the problem faced by the equine clinician is in finding the horse that actually has the pathology in question. Finally, in-depth knowledge of the neuroendocrine changes that accompany heart disease in horses is lacking compared to the wealth of data available in human beings. Nevertheless, research on equine cardiovascular biomarkers is underway and this is an interesting and exciting area of development in equine cardiology.
CATECHOLAMINES
Activation of the sympathetic (adrenergic) nervous system is a hallmark of heart failure2 (see Chapters 2 and 5). The β1– and β2-adrenoreceptors are important regulators of cardiac function and their characteristics change in human and canine cardiac disease. Atypical β-adrenoreceptors (β3 and β4) are also found in the cardiovascular system of some species. Myocardial tissues from failing human ventricles demonstrate a reduction in β-adrenoreceptor density and contractile response to β-adrenergic agonists. These changes are mediated by increased concentrations of noradrenaline (norepinephrine) in the vicinity of the receptors.2 There is wide variation in the distribution of cardiac β-adrenoreceptor types among species: in the equine heart the predominant adrenoreceptor is the β1 subtype and there is also evidence for atypical adrenoreceptors within the heart although these have not been fully characterized.3 In contrast to human beings, in horses, the density of β-adrenoreceptors did not change in heart failure but, in some cases, heart failure increases the expression of β2-adrenoreceptors.3
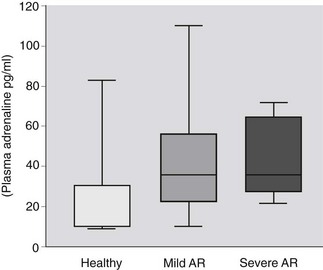
One method of documenting the increase of sympathetic outflow is by measurement of plasma catecholamine concentrations and these have been used as prognostic indicators in human congestive cardiac failure.4,5 Plasma concentrations of noradrenaline are increased in human heart failure and the clinical outcome is related to plasma noradrenaline concentrations.5 In contrast, plasma concentrations of noradrenaline did not differ among healthy horses and those with aortic insufficiency with and without clinical signs of cardiovascular compromise at rest whereas adrenaline (epinephrine) concentrations were significantly different among healthy horses and those with aortic regurgitation (Fig. 12.1).6 Although this finding is of interest as it provides evidence on the pathophysiology of equine heart disease, measurement of plasma catecholamines is limited in its usefulness in the clinical setting because there are circadian variations in plasma concentrations and samples must be kept on ice and processed very quickly. Furthermore, the sensitivity and specificity of plasma catecholamines in distinguishing horses with mild and severe and nonprogressive and progressive aortic regurgitation are low.6
RENIN–ANGIOTENSIN–ALDOSTERONE SYSTEM
The renin–angiotensin–aldosterone system (RAAS) is activated in low cardiac output states and serves to maintain arterial blood pressure by retaining sodium and water (see Chapters 2 and 5). Angiotensin II is a potent peripheral vasoconstrictor and together with increased adrenergic activity contributes to excessively increased systemic vascular resistance in heart failure. Angiotensin II also enhances release of noradrenaline. Sodium retention, mediated by aldosterone, contributes to oedema formation. Tissue angiotensin and aldosterone also have a key role in cardiovascular remodelling and fibrosis and this leads to reduced vascular compliance and ventricular diastolic dysfunction.2 Plasma renin activity and plasma aldosterone concentration increase and correlate with severity in canine dilated cardiomyopathy.7 There is also early activation of the RAAS in some dogs with mitral valvular insufficiency8 whereas other studies have shown that plasma aldosterone and angiotensin II decrease as cardiac decompensation ensues.9 Studies in horses with valvular insufficiency have shown that plasma aldosterone concentrations rise as the severity of valvular disease increases with significant differences detected between healthy horses and those with left ventricular and atrial dilation.10 In contrast, another equine study showed no activation of the RAAS in horses with aortic insufficiency (n = 25) compared to healthy horses (I. M. Bowen, C. M. Marr, J. Elliott, unpublished data).