Fig. 1.
Circadian controlled cocaine intake is dysregulated for more than a month after episodic social defeat stress. Upper panel portrays hourly cocaine intake as mg/kg/h for previously stressed and control rats. Circadian-like cocaine self-administration behavior was maintained in control rats (open circles), whereas stressed rats (filled circles) intensely self-administered cocaine for 24 h, effectively abolishing the circadian pattern of intake (p < 0.01). Motor activity for each hour of the binge is revealed by the distance (cm) traveled (center panel ) and the duration (s) of focused stereotypes (lower panel ), occurring over 5 min sampling intervals (Reprinted from (73). With permission).
3.1 Controllability: Inhibiting the Persistence of Cocaine Bingeing
Behaviorally sensitized animals will self-administer significantly more cocaine during conditions of extended access (75). In particular, 24-h binges reveal that prior injections of cocaine, morphine or exposures to episodes of social defeat stress increase the persistence of responding for cocaine, corresponding to increases in the cumulative intake of cocaine as compared to nonsensitized controls (see Fig. 2). The persistence of cocaine responding can be compared across various treatment groups by performing survival analyses of the percentages of animals responding under each condition over consecutive hours of the binge (see Fig. 3). Vulnerability to cocaine addiction varies significantly across different populations of drug users. These data support the hypothesis that stressors, similar to a history of drug administrations, can potentially facilitate binge behavior (76). Since the introduction of “stress” to the field of physiology and medicine, this concept has been linked to the pathogenesis of several CNS disorders (77, 78). Empirical evidence supports the hypothesis that certain types of environmental stressors enhance the incidence of psychiatric illness (for reviews see 76, 79, 80). However, stress-induced increases in cocaine bingeing are sensitive to parameters of stress exposure including the intensity, duration, intermittency, and controllability of the stressor (64). Brief intermittent social stress episodes appear to be critical for the emergence of social stress-induced binge cocaine taking in rodents (75, 81). Brief episodes of social defeat stress sensitize behavioral, physiological and neural responses to subsequent stressors and stimulant challenges. Conversely, repeated aggressive acts that are also inherently stressful do not induce behavioral sensitization or promote an increase in cocaine bingeing (see Fig. 4) (82).
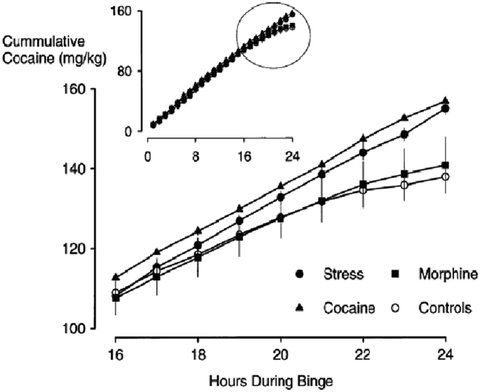
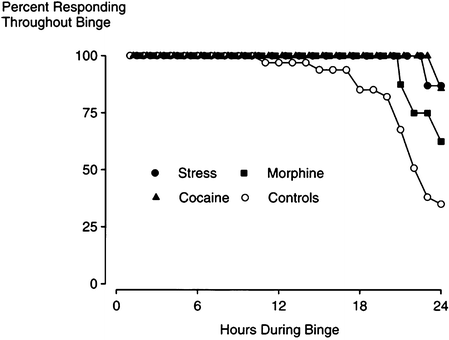
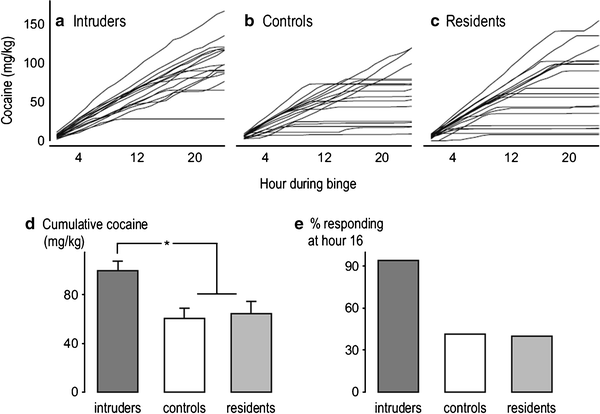

Fig. 2.
Behavioral sensitization increases the persistence of responding for cocaine during conditions of continuous access. The pattern of cocaine self-administration during a 24-h binge in stress-sensitized (filled circles, n = 8), cocaine-sensitized (filled triangles, n = 8), morphine-sensitized (filled squares, n = 8), and control rats (open circles, n = 24) is shown in the top insert. The last 8 h of the 24-h binge in groups of sensitized and control rats are highlighted in the lower graph. Total cocaine intake at hour 24 is significantly different between stress or cocaine-sensitized and control rats (Reprinted from (75). With permission).

Fig. 3.
Intermittent social stress, cocaine or morphine subsequently increases the persistence of cocaine taking during a 24-h binge. Portrayed here is the percentage of rats responding at each hour of a 24-h binge in previously stress-sensitized (filled circles, n = 8), cocaine-sensitized (filled triangles, n = 8), morphine-sensitized (filled squares, n = 8), and control (open circles, n = 24) rats. The percentage of stress or cocaine sensitized rats were significantly different from controls as indicated by survival analyses (Reprinted from (75). With permission).

Fig. 4.
Intermittent social defeat stress, but not offensive aggression, increases the persistence of cocaine self-administration behavior during a 24-h cocaine binge (0.3 mg/kg/infusion). Individual cumulative records of cocaine intake are portrayed for (a) previously defeated intruder rats, (b) control rats, and (c) resident rats with a history of offensive aggression. (d) portrays the total amounts of cocaine intake (mg/kg) during a 24-h binge by intruder, resident, and control rats. Intruders accumulated significantly more cocaine than controls and residents. (e) portrays the percentage of intruder, resident, and control rats that continued responding for cocaine after hour 16 of a 24-h binge.
Episodic social defeat stress, like intermittent psychomotor stimulant administration, depends on NMDA receptor activation in the VTA during the development of augmented behavioral responses (83, 84). Brief exposures to stress appear to stimulate neural responses that undergo adaptations in dopamine cell bodies and that intensify drug taking behavior over the later hours of a 24-h binge. Blockade of NMDA receptors in the VTA during episodic social defeat protects against the behavioral tendency to prolong cocaine self-administration during later hours of continuous (24 h) cocaine access (see Fig. 5). The impact of brief episodes of social stress on persistent cocaine bingeing is also long lasting, as it persists for at least 2 months after the last social defeat event, a further indication that enduring neuronal plasticity in brain reward processes is induced by some stressors (73).
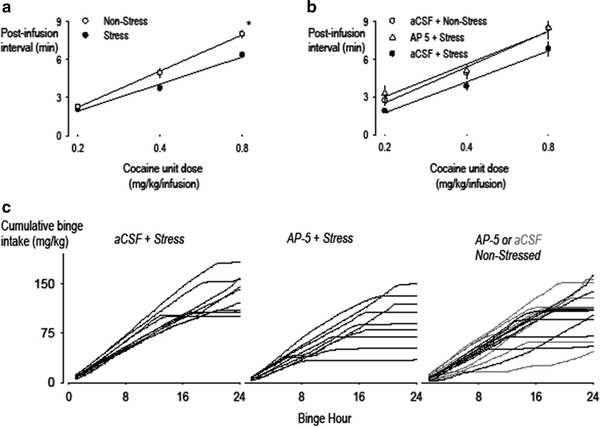

Fig. 5.
NMDA receptor activation is required during brief episodes of social stress to facilitate persistence in responding for cocaine during the later hours of a cocaine binge. Panels (a) and (b) reveal the average post-infusion interval following variable doses of cocaine delivered over the 24-h binge for groups of previously non-stressed or defeated rats, control rats injected with aCSF (open circles) and defeated rats injected with AP-5 (open triangles) or aCSF (filled circles). Panel (c) shows individual cumulative records for cocaine intake by defeated rats receiving intra VTA aCSF vehicle (left figure) or the NMDA receptor antagonist AP-5 (middle figure), immediately prior to each stress episode, and for non-stressed rats (right figure) receiving either AP-5 (black lines) or aCSF (gray lines) infusions (Reprinted from (83). With permission).
3.2 Controllability: Stability of the Rate of Cocaine Intake During a Binge
In addition to the process of behavioral sensitization, a history of extended access to self-administered cocaine (i.e., 6-h sessions/day, which leads to a significant escalation of intake over consecutive sessions (56)) also increases cocaine intake during a 24-h variable-dose binge (see Fig. 6, top) (85). The process of escalation entails an increase cocaine intake during a 24-h binge, particularly during the first half of the binge (see Fig. 6, top). In addition, the persistence of cocaine intake over the later hours of a 24-h binge is intensified, primarily, by the earlier experience of intermittent social defeat stress (see Fig. 6, bottom), or intermittent administration of psychomotor stimulants (75).
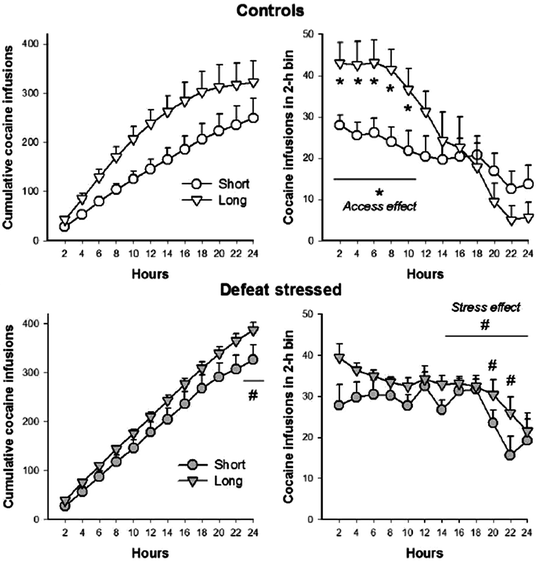

Fig. 6.
Repeated extended daily access to cocaine self-administration increases the rate of cocaine intake, and episodes of social defeat increase the persistence of cocaine intake, during a 24-h variable-dose binge. The upper panels show control conditions and the bottom panels show social defeat stressed rats, with or without a history of extended access to self-administered cocaine. Panels on the left portray cumulative records for cocaine intake, and the right panels show the average number of infusions obtained every 2 h. During the earlier hours of the binge, animals with a history of extended access (6 h/day) displayed an increase in cocaine self-administration, particularly in control groups. During the latter half of a binge, stressed rats accumulated significantly more cocaine than controls, which was characterized by more persistent responding, that also resulted in an overall increase in cocaine self-administration during a 24-h binge (Reprinted from (85). With permission).
Experimental binge assays that use multiple doses of cocaine within a 24-h interval of unlimited access have begun to elucidate features of dysregulation in the rates of cocaine taking. Initially, experiments were designed to examine the effects of social defeat stress on an animal’s ability to adjust to varying doses of cocaine reinforcement, while levels of cocaine intake increase during unlimited access to the drug (60). This goal was accomplished by a systematic analysis of the post-infusion interval across varying unit doses of self-administered cocaine (0.2, 0.4 or 0.8 mg/kg/infusion, in irregular sequence) conducted over a 24-h continuous access binge. Previous observations suggest that rats begin to lose control over the rate of intake only after extended hours (>10 h) of cocaine self-administration (60, 75). Therefore, the effect of behavioral sensitization could arguably either strengthen or weaken the response contingency for cumulative cocaine infusions at the time during which persistence of cocaine taking is expected to occur. An analysis of post-infusion intervals reveals that social defeat stress decreases the length of the intervals that follows doses of cocaine infused over the entire binge (see Fig. 5a and b). Second, it is clear that sensitized rats continue to adjust their rate of responding to varying unit doses of cocaine, as indicated by a lack of variation in the post-infusion interval for each dose delivered over the course of a 24-h binge (see Fig. 5a). In support of the hypothesis that prolonged cocaine bingeing develops as animals gain control over their rate of cocaine self-administration, rats have been observed to significantly reduce their overall variation in responding over repeated 16-h access binges (see Fig. 7). Specifically, variations in responding that are prominent after 10 or more hours of cocaine self-administration are largest during an initial binge, as compared to subsequent binges. Thus, continuous binge intake appears well controlled, as defined by the ability to adjust to varying doses.
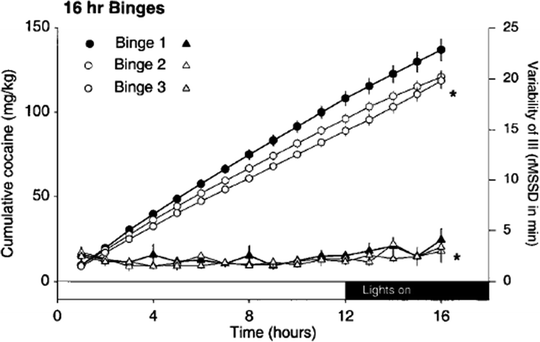

Fig. 7.
Cumulative cocaine intake does not increase over three repeated 16-h binges separated by 10 days, and variability in the post-infusion interval (III) decreases over each consecutive binge. Black, white, and gray circles indicate the average number of infusions accumulated during a first, second and third 16-h binge, respectively. Black, white, and gray triangles indicate the pattern of self-administration [average root mean square of successive differences (rMSSD)] over each hour for the first, second, and third binge. The shaded block indicates the inactive phase of the light/dark cycle (Reprinted from (66). With permission).
An animal’s ability to continue responding in a reliable and stable manner for cocaine over long periods of time occurs in the presence of intense stereotyped behavioral routines (see Figs. 1 and 8) (86). Tolerance to the rate disruptive effects of cocaine-induced stereotyped behavior may contribute to an ability to self-regulate higher amounts of cocaine taking, although this has not been experimentally verified.
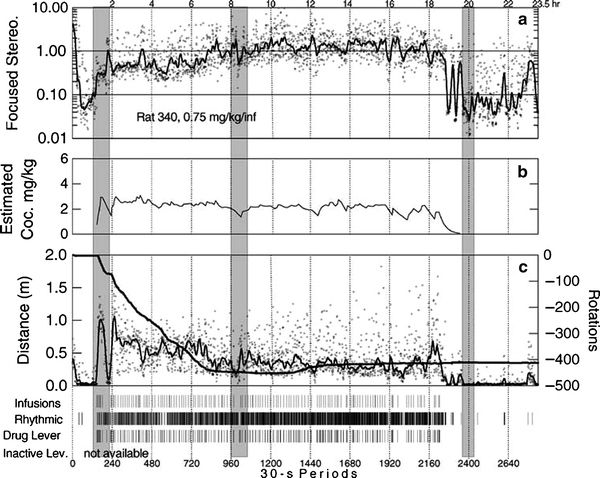

Fig. 8.
Cocaine self-administration throughout a binge occurs with the expression of intense stereotyped behavioral routines. Estimated cocaine intake and behavioral recordings within a 23.5-h binge period for an individual rat: Panel (a) portrays the focused stereotypy score over 30 s intervals. Panel (b) reveals the amount of cocaine self-administered, as calculated from an exponential decay function assuming a cocaine elimination half-life of 13 min. Panel (c) reveals the distance traveled over 30-s intervals across the binge. The dark line represents the cumulative net rotations throughout the cocaine binge. Event records at the bottom of the figure depict the time of occurrence for each cocaine infusion and response on the active lever. “Rhythmic” identifies the 30 s intervals when the peak in the power spectrum of the vertical force recordings fell within the 7.5–12.0 Hz frequency band, which is characteristic of stereotyped head movements (Reprinted from (86). With permission).
Individual variations in cocaine intake can predict vulnerability to cocaine taking behavior during prolonged binges (87). Adaptations in dopaminergic signaling in response to extended access to cocaine (14, 15, 64) strengthen subsequent responding for cocaine by increasing response rates, stabilizing the ability to adjust to changes in unit doses of the drug and increase the persistence of responding. Intermittent social stress episodes that facilitate prolonged bingeing correspond with decreased functional activity of cells in the infralimbic and prelimbic prefrontal cortex and increased functional activity of the amygdala (73); changes that are consistently observed in cocaine addicts (88). The prefrontal cortex and amygdala are critical for the attentional, goal-directed, repetitive, and hedonic components of cocaine self-administration behavior (15). Future experiments that systematically dissect the roles of particular forms of neuronal plasticity on binge-like forms of cocaine taking are bound to provide much needed translational information for the initiation, termination, persistence, and reoccurrence of cocaine abuse (89, 90).
4 Opiate Binge
Estimates report that over 500,000 Americans try heroin each year, and in 2007, approximately half of that number were dependent on, or abusing, the drug (39). Heroin abuse is sustainable for years and is characterized by stable and regular patterns of intake (91). This style of opiate use deviates from the cyclical patterns of behavior typically associated with other drugs of abuse, as in the case of cocaine bingeing (92). The use of opiates, such as heroin, results in severe physical dependence within a rather short period of time. Therefore, maintenance administrations of opiate agonists are often necessary to relieve withdrawal symptoms during periods of abstinence (93). Transitioning from initial use to heroin abuse is characterized by significant increases in daily intake, yet these increases usually occur at seemingly predictable intervals in order to reduce the opportunity for withdrawal symptoms to occur. As a result of intense withdrawal, opiate bingeing is likely to occur more frequently than other forms of bingeing (e.g., food, sex, cocaine), leading to notably high rates of morbidity and mortality (91).
In 1962, James R. Weeks first implemented the use of a swivel to allow for minimally restrictive intravenous access to morphine in rats in order to examine the effects of opiate self-administration over extended periods. By incorporating the use of an operant behavior (e.g., lever pressing) with intra-jugular catheter infusions, Weeks demonstrated how morphine can be reliably self-administered over long periods of time. Predictive, external and face validity of continuous access to opiates was thus established, as morphine was found to maintain stable levels of operant responding during 25–30 h of unlimited access across an extended range of doses. Small increases in the FR requirement increased behavioral responding during continuous access, but did not alter the rate of drug intake. Rates of intake are, however, controlled by the unit dose of morphine infused (i.e., higher doses promoted fewer infusions). The pattern of intake clearly reveals the rapid reinforcing and sedative effect of opiates since each infusion was immediately followed by a long pause in responding. In fact, Weeks found that patterns of responding were well maintained with a 10 mg/kg morphine dose that results in only two infusions/hour. The opioid receptor antagonist nalorphine systematically increased rates of responding confirming a critical role for opioid receptors in morphine’s reinforcing effects. Classical withdrawal signs (e.g., tremors, diarrhea, increased respiration, circling, and rearing (94, 95)) appear within 24 h following the discontinuation of a morphine binge documenting that physical dependence can be maintained by conditions of extended access (it is important to reemphasize here that physical dependence was initially established in this pioneering study via experimenter-administered morphine prior to self-administration).
It is difficult to model uncontrollable opiate use during a single binge since most animals will only self-administer during their active phase, due to a circadian cessation of drug taking at the onset of the inactive phase. Continuous access to opiates over numerous days reveals a gradual increase in daily intake; an effect that holds true for humans, nonhuman primates, and rodents (53, 55, 96, 97). Experimental approaches that model the transition to heroin dependence have revealed both escalated intake and persistent patterns of responding over repeated opiate binges; however, this effect takes an extended period of time to arise as compared to behavioral changes observed after only a few cocaine binges (55). Koob and colleagues (98) provide a clear example of how prolonged heroin access (23 h/day) influences patterns of self-administration over repeated days. During the first week of access, intake is relatively stable, with responses occurring primarily during the dark (i.e., active) phase. An escalation of heroin intake is observed in the first 4 days of access as indicated by increased numbers of self-infusions within the first hours of each binge. Within 3 weeks, a diurnal pattern of responding clearly emerges. Specifically, the nocturnal peak and the diurnal nadir during each photo cycle increases over consecutive days of access. Within 3 weeks, average daily intake curves are significantly elevated. Thus, it appears that during unlimited access to heroin, like cocaine bingeing, a shift in an individual’s control over intake occurs in two separate ways: (1) by inducing an “escalation” in the rate of intake at the beginning of access and (2) by progressively increasing the “persistence” of responding over long periods of time.
In response to opiate administration, both tolerance and sensitization occur concomitantly. Tolerance to the depressant and analgesic effects of opiates after repeated administration depends on excitatory amino acid receptors (99, 100). At the same time, excitatory amino-acid-dependent behavioral sensitization becomes evident (100). Excitatory amino acids appear critical for the induction of tolerance and sensitization to opiates and psychomotor stimulants, however, they do not appear to play a role in their long-term expression (101, 102). This implies that immediate responses to opiates promote lasting changes in down-stream neural mechanisms. Both tolerance and sensitization processes may contribute to opiate dependence, opiate bingeing and a propensity for relapse to drug taking after long periods of abstinence (103). Significant decreases in the expression of dopamine transporters and opiate receptors, in the ventral tegmental area, and hypothalamic nuclei, respectively, are likely to contribute to the dysregulation of normal behavioral routines and progressive increases in opiate intake (104, 105). The emergence of increased wakefulness over successive days of opiate intake, and corresponding changes in the pattern of food intake, imply that the types of neural mechanisms that control circadian processes (i.e., behaviors following the sleep/wake cycle) become disrupted after repeated opiate administration, particularly after high levels of intake that occur during opiate binges (96, 106).
5 Alcohol Binge
Half of the population of the USA occasionally drinks alcohol, and about 20% binge drink (i.e., more than 5 drinks on one occasion) each month (39). Binge drinking most often occurs at weekly intervals, and only 7% of the total population consists of binge drinkers that do so more than five times in a given month. Increasingly more adolescents take part in alcohol bingeing, further emphasizing the need to understand the neurobiological factors that contribute to alcoholism and interventions for treatment (39, 107, 108).
Humans, nonhuman primates, and rodents present an extensive range of individual preferences for alcohol within and across their respective populations, at least when self-administering orally (109–111). Individual preferences for alcoholic solutions have helped identify trait characteristics that may relate to genetic, environmental or developmental predispositions for bingeing behavior (112–114). Irregular patterns of alcohol intake are commonly reported when using animal models that provide continuous access to alcohol, and it is therefore difficult to achieve the high blood concentrations observed clinically after bingeing (115). Only a small portion of monkeys (Macaca fascicularis) allowed continuous access to alcohol are inherently susceptible to developing patterns of intake that lead to high blood concentrations, an effect that occurs predominately in males (116). These monkeys steadily increase their daily alcohol intake within the first 2 weeks of access, until approximately 4 g/kg are consumed during each session. As with humans, binge drinking behavior in monkeys is limited almost exclusively to the active phase of the light/dark cycle, with minor changes in the pattern of daily intake for up to 6 months (116).
Despite taste preferences, voluntary alcohol drinking can be intensified in rodents and monkeys by manipulating the initial exposure to alcohol using alcohol fading schedules, or forced exposures to an alcohol-rich vapor (117–119). Alcohol fading procedures typically begin by allowing access to a sweetened drinking solution, to which an alcohol concentration is gradually added, and alcohol content is progressively increased over repeated trials. Eventually, the sweet is faded out of the solution in a gradual manner over consecutive days. This method can achieve dependence on alcohol, and levels of intake increase significantly over time, with blood concentrations reaching ca. 150 mg/100 mL during daily binges (120). Binge-like drinking in rodents persisted for weeks after inducing dependence, indicative of lasting changes in the brain that sustained binge patterns of alcohol intake. Fading procedures can identify environmental factors that promote high levels of intake, as rhesus monkeys (Macaca mulatta) with a history of consuming sweetened alcohol solutions in a social environment at later time points drank high levels of unsweetened alcohol when isolated from their social group (121). However, the impact of environmental and social stressors on binge drinking is not obvious, as mostly decreases in alcohol intake have been reported in response to acute and chronic stressors (122–124). In light of these and epidemiological data, there remains substantial evidence to support an interaction between the effects of stress and alcohol intake (63, 125).
Exposure to ethanol vapors also readily induces alcohol dependence, and facilitates escalated or binge-like patterns of intake in rodents (126, 127). Intermittent exposure to alcohol vapor facilitates an escalation of voluntary intake more effectively than continuous vapor exposure (128). Intermittent exposures to alcohol vapor also progressively sensitized withdrawal behaviors in mice, indicating that a sensitization process, in addition to tolerance, contributes to increased binge drinking (129). In fact, administration of alcohol, like opiates, clearly induces both tolerance and sensitization and both of these processes depend on NMDA receptor activation (130–133). Unfortunately, it is difficult to identify with precision whether or not the consumption of alcohol during binges after experimental manipulations results from increased rates of intake within a given time or if intake is prolonged over an access period, although both patterns of responding are likely to occur (116). It is clear, however, that prolonged intervals of alcohol deprivation after daily limited access can substantially increase responding for alcohol when access is renewed, a behavioral phenomenon shared with other types of experimental bingeing that are typically dependent on an oral route of administration (134).
6 Binge Eating
Binge eating disorder is the most common eating disorder affecting approximately 5% of the general population (i.e., this disorder is more highly represented in the population than both anorexia nervosa and bulimia nervosa combined) (30). Patterns of behavior that characterize binge eating are typical of other abusive behaviors that involve bingeing. For example, binge eaters display self-imposed restrictions over food intake, or over intake of a specific type of food, for sustained intervals of time. These periods of caloric deprivation are subsequently followed by prolonged episodes of high calorie binge intake (135). During these binges, significantly higher amounts of food are consumed in comparison to what constitutes a normal meal, and the binge is most often followed by feelings of remorse and guilt (2).
Experimental attempts to model binge eating behavior in animal models were initiated in the mid-twentieth century. Immediately following the advent of operant conditioning procedures, and with the development of experimental conditions that promote increased or decreased rates of responding for fluids and palatable foods, it became possible to explore protocols that could be used for inducing a substantial increase in food intake (136–138). Earlier work on compulsive overeating identified how social stressors (e.g., social isolation, maternal separation, etc.) and environmental stressors (e.g., food deprivation, footshock, tail-pinch, etc.) can immediately and persistently escalate eating behavior in rats (139–141) and monkeys (142). Given the strong correlation between stress and overeating in clinical populations, results from these types of experiments have provided much insight into the neural mechanisms mediating stress-induced consumatory behaviors. For instance, acute stressors that induce a strong and rapid increase in food intake depend upon dopaminergic and opiate receptor activation (139, 143). Chronic mild stressors also lead to elevations in feeding, and an interaction between stress and diet is correlated with increased brain expression of brain-derived neurotrophic factor (BDNF), arginine vasopressin (AVP) and the cocaine-amphetamine regulator of transcription (CART) in male, as well as leptin in female, rats (144).
Experimental conditions that restrict food availability and limit access to food at specific times can greatly increase food consumption during intervals of access, particularly in rodents (32, 145). Limited access conditions have proven to be useful in examining numerous consequences of binge eating due to the face validity of this procedure, since the human condition is also characterized by cycles of food restriction and overeating (135). Binge eating by rats during limited access can be accomplished without food restriction, but simply by permitting access to fatty and sweet foods within predetermined intervals (e.g., for 1–2 h several times a week). Intermittent access to a high calorie diet escalates over the first few access sessions, and this form of bingeing behavior remains stable for extended periods of time. Escalated food consumption during binges can be inhibited by the administration of GABA-B agonists and opioid antagonists in lower dose ranges that do not affect normal feeding behavior (146, 147).
Intermittent access to a sugar solution has been employed to study how high sugar intake induces behavioral and neural adaptations that are similar to those induced by drugs of abuse. Intermittent exposure to sugar can induce behavioral cross-sensitization to psychomotor stimulants and produce a withdrawal syndrome during intervals when sugar access is discontinued (148, 149). Typically, food intake is associated with an initial rise in the release of dopamine into the nucleus accumbens, possibly indicative to its hedonic effects; this effect dissipates with renewed access (150). With repeated sugar binges, however, accumbal dopamine release continues to be reliably elevated (151). Likewise, withdrawal from a sugar binge corresponds to a decline in dopamine concentrations with a concomitant increase in acetylcholine levels in the nucleus accumbens. This result is similar to the effects of withdrawal from opiates and psychomotor stimulants (149). Increases in dopamine D1 receptor expression, decreases in dopamine D2 receptor expression and decreases in the expression of enkephalin in the nucleus accumbens have been reported after intermittent access to sweetened solutions, which has also been reported after psychomotor stimulant and opiate administration (149, 152, 153). Allostatic shifts in the modulatory effect of opioids, dopamine and acetylcholine within the nucleus accumbens in response to eating behavior is potentially related to a negative affective state that facilitates later bingeing (154). Thus, models of addiction to sweet substances have led to the proposal of neural “triggers” that may underlie the emergence of binge-eating patterns of behavior (154).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


