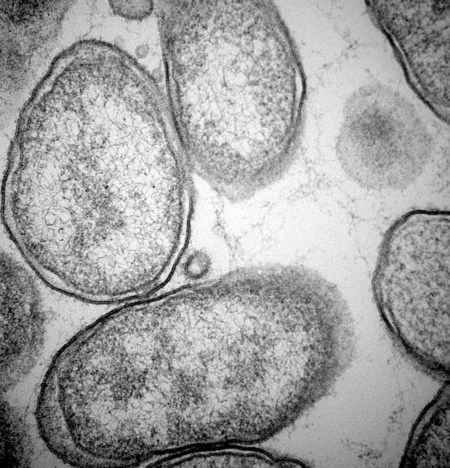
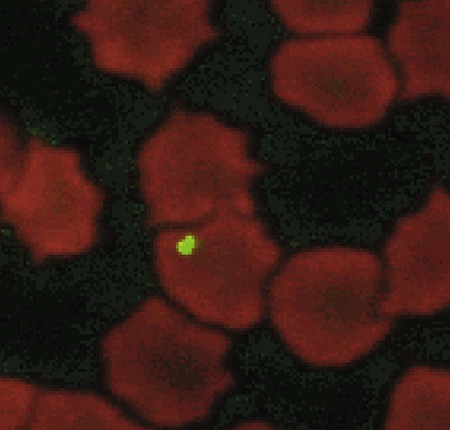
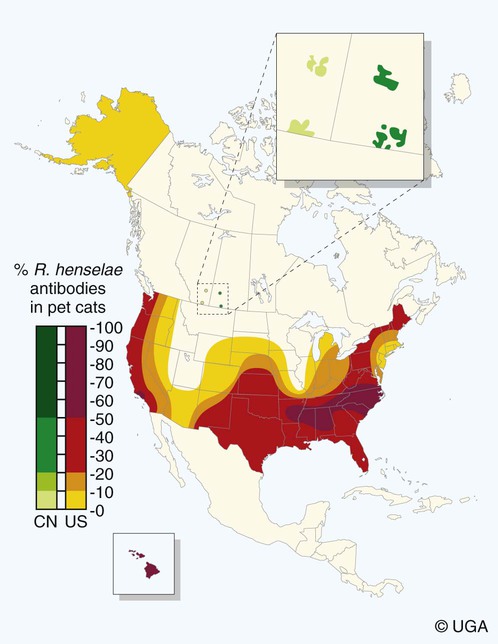
Bartonella is a fastidious genus comprising at least 22 species and subspecies of small, curved, gram-negative hemotropic α-proteobacteria. Each has become highly adapted to preferential mammalian reservoir hosts, in which they cause a long-lasting, intraerythrocytic bacteremia.41,89,90,202,229 Among Bartonella and their respective host examples, Bartonella henselae has coevolved with cats, Bartonella vinsonii ssp. berkhoffii has coevolved with wild canines, and Bartonella bovis has coevolved with cattle.41,90 Bartonella spp. are vector transmitted, and the vector preference for particular host species has resulted in the evolution of individual transmission cycles. Transmission can also occur by bites or scratches and sometimes multiple vectors. In the natural reservoir host, chronic bacteremia with a Bartonella sp. can frequently be detected by culture or polymerase chain reaction (PCR) testing in seemingly clinically healthy individuals. Mechanisms facilitating persistent bacteremia are still under investigation. It appears that intracellular, localization, and alteration of outer membrane components are important strategies for Bartonella persistence; inhibition of apoptosis of colonized vascular endothelial cells may also play a role.* Nonhemolytic intracellular colonization of erythrocytes would contribute to efficient vector transmission, protect Bartonella from the host immune response, and potentially contribute to decreased antimicrobial efficacy.338 Bartonella spp. include organisms that once comprised the genera of Bartonella, Rochalimaea, and Grahamella (Fig. 52-1 and Table 52-1).32,64,64 Bartonella bacilliformis, the type species, causes a focally occurring vasculoproliferative and hemolytic disease of humans in the Andes Mountains of Peru. Bartonella quintana, the cause of trench fever in World War I, also causes bacillary angiomatosis, endocarditis, and chronic lymphadenomegaly, predominantly in immunocompromised humans.107,132,224,228 B. quintana is transmitted by the body louse and has also been detected in cat fleas in France340; however, this potentially incidental finding may relate to recent feeding on an infected host. B. henselae, a feline-adapted Bartonella species, has usually been isolated from clinically healthy domestic cats, although some strains of the organism have been associated with mild illnesses and histopathologic lesions, and occasionally endocarditis.97 In fact, endocarditis has been one feature of illness that is occasionally reported in respective reservoir hosts for individual Bartonella species (see Etiology under Dogs, later). In contrast, humans with B. henselae infections have developed bacillary angiomatosis, visceral bacillary peliosis (extravasation of blood), relapsing fever with bacteremia, meningitis, encephalitis, neuroretinitis, endocarditis, and pyogranulomatous lymphadenitis (cat-scratch disease [CSD]), among other clinical conditions.225,187,329,335,362 B. henselae has also been associated with several clinical entities in dogs.115,153,291,362,398 Closely related to B. henselae, Bartonella clarridgeiae comprises approximately 10% to 30% of Bartonella isolates from clinically healthy cats* and was found in endocarditis lesions in one dog177 and in another dog with hepatopathy using PCR testing158 (see Canine Bartonellosis, later). B. clarridgeiae has been serologically associated with CSD-like illness in humans.45,234 Bartonella koehlerae133 was isolated from two healthy cats, a human, and a dog with endocarditis11,306; and B. bovis (formerly “Bartonella weissii”)27,330 was isolated from four cats; the pathogenic significance of these two species in cats has not been determined. Bartonella elizabethae was isolated from a human immunodeficiency virus (HIV)-infected human with endocarditis114 and by PCR testing in blood of a moribund dog with renal failure and anemia.291 B. vinsonii ssp. vinsonii has only been isolated from voles. B. vinsonii ssp. berkhoffii has been isolated from the blood of healthy and diseased dogs, from healthy coyotes (Canis latrans), and from one human with endocarditis.79,236,236 B. vinsonii ssp. arupensis was isolated from a cattle rancher with fever and bacteremia and has been associated with a case of endocarditis in a human.146,391 Bartonella washoensis, whose reservoir is the California ground squirrel, has been isolated from a dog with endocarditis and from a human patient with cardiac disease.101,240 Bartonella alsatica was isolated from one human with endocarditis and diagnosed by serologic testing in another human endocarditis patient.204,326 Bartonella grahamii was associated with neuroretinitis in one human.219 A novel Bartonella species, Bartonella rochalimae, has been associated with clinical illness in a human and is considered identical to a previously described isolate (“B. clarridgeiae–like”) causing endocarditis in dogs. Foxes, dogs, and other canids may serve as reservoir host for this newly characterized Bartonella.97,142,142 Additional species or subspecies, for which the pathogenic potential in cats, dogs, or humans have yet to be defined, include Bartonella birtlesii, Bartonella capreoli, Bartonella schoenbuchii, Bartonella tribocorum, Bartonella talpae, Bartonella peromysci, Bartonella taylorii, Bartonella doshiae, and Bartonella chomelii.* Other identified reservoir hosts for provisional members of the genus include grey squirrels (Sciurus spp.: Candidatus Bartonella durdenii”), flying squirrels (Glaucomys spp.: “Candidatus Bartonella volans”), and groundhogs (Marmota monax: “Candidatus Bartonella monaxi”). It is likely that new Bartonella pathogens and disease manifestations will be observed in the future. With respect to feline and canine infections, the following discussion of Bartonella in animals focuses on B. henselae, B. clarridgeiae, and B. vinsonii ssp. berkhoffii. These and other species of Bartonella infecting humans and their zoonotic implications are discussed later under Public Health Considerations for dogs and cats, respectively. TABLE 52-1 Comparison of Recognized Disease-Producing Members of the Genus Bartonellaa WWI, World War I; ?, unknown. aAlso includes other organisms of uncertain pathogenicity—B. birtlesii, B. capreoli, B. doshiae (rodent), B. koehlerae (cat), B. peromysci (rodent), B. schoenbuchensis, B. talpae (rodent), B. taylorii (rodent), B. tribocorum (rodent), B. vinsonii ssp. vinsonii (Canadian vole agent)—that infect small woodland mammals and herbivores, and other animals (fish and birds?). bNonhuman primates have been experimentally infected. dDenotes disease manifestations reported in dogs and humans. eSuspected, but not proven, vector. Since the first recognition of feline B. henselae infection in 1992,332 it has been established that natural infections of cats with Bartonella spp. are common. Blood culture and molecular methods have detected natural infections with B. henselae, B. clarridgeiae, B. koehlerae, B. bovis, and B. quintana in cats.† Serologic and blood culture data indicate that exposure to Bartonella spp., predominantly B. henselae, is most prevalent among cats in temperate regions of the world.295 Prevalence in domestic cats is lowest in Northern Europe and the Rocky Mountain regions of the United States and Canada, and greatest in warmer, more humid regions (Fig. 52-2). There are two main genotypes of B. henselae. The Houston 1 genotype is more prevalent in the Far East, and the genotype Marseille is predominant in western Europe, Australia, and the western United States. Both genotypes are equally prevalent in the eastern United States.95 A third genotype, designated Berlin, was identified in a cat from Germany.9 Seroepidemiologic studies in cats generally show a higher prevalence of seroreactivity with age, warmer temperature, and higher humidity, and in feral cats and those infested with fleas.‡ B. henselae bacteremia affects approximately 5% to 40% of cats in the United States, depending on geographic location, and is most common in temperate areas.53,86,177,273 Seroprevalence of over 90% is reported in some cat colonies.305 Prevalence rates of B. clarridgeiae infections were reported to be approximately 10% of cats with Bartonella bacteremia evaluated in the United States, 16% to 31% of cats with Bartonella bacteremia in France, and 31% of cats in the Philippines.91,186,186 B. koehlerae was isolated from 2 cats (from the same household in California), and B. bovis was isolated from 2 cats in Utah and 2 in Illinois.133,330 Domestic cats are considered the major reservoir and vector for human infections with B. henselae and for B. clarridgeiae. Cattle are the reservoir for B. bovis, and humans are the predominant reservoir for B. quintana. The reservoir for B. koehlerae is unknown although cats are suspected.58a In France, molecular methods detected B. quintana, B. koehlerae, B. henselae, and B. clarridgeiae in cat fleas, along with rickettsial pathogens.340 This finding suggests that fleas may be involved in the transmission of many of these Bartonella species for humans and animals; however, the fleas may have just fed on an infected host. Additional evidence that arthropods may serve as vectors of some Bartonella species comes from finding these organisms in questing ticks in California, and in Ixodes ricinus ticks in Italy, among other locations.76,78,122,298,351 Ticks have been proposed as the vectors for transmission of some Bartonella infections in humans, dogs, and other mammalian hosts.23,315,362,391 In addition, there is evidence of transstadial infection of I. ricinus ticks with B. henselae, and B. henselae was detected in the saliva of infected ticks.111 Biting flies have also been associated with Bartonella transmission.104 Interestingly, Bartonella spp. have been isolated from rodents in Alaska, where there are no tick vectors.281 Wild felids are also exposed to Bartonella spp.; 18% of panthers (Puma concolor coryi) in Florida and 28% of mountain lions (Puma concolor) in Texas had serum antibodies to B. henselae, and the prevalence of serum antibodies to B. henselae in free-ranging and captive wild felids in California was 30% to 53%.344,404 Prevalence of serum antibodies to B. henselae in mountain lions and bobcats (Lynx rufus) in Mexico, Central America, and South America ranged from 0% to 33%.98 Bartonella infections have also been documented in free-ranging wild and captive African lions (Panthera leo) and cheetahs (Acinonyx jubatus), using serology, PCR testing, and blood culture.95,295 B. henselae infection was identified by PCR in several species of neotropical felids in Brazil.168 B. henselae contains multiple genetically diverse strains. There are two recognized 16S ribosomal RNA (rRNA) types of B. henselae (Houston 1 [type 1] and Marseille [type II]) and at least two subgroups are within each type.276,406 Cats can be co-infected with B. henselae 16S rRNA types I and II and co-infected with B. henselae and B. clarridgeiae.178 In cats, co-infection also exists with hemotropic Mycoplasma spp. (see Chapter 31).250 Regional differences in prevalence of infection of cats exist with different genetic types of B. henselae.* Some evidence is suggestive of genomic variation in B. henselae during the course of infection in cats.22,207 Such variation may enhance the ability of B. henselae to persist in an infected cat for prolonged periods. Whether the 16S rRNA type is the most accurate means of describing genetic differences among B. henselae isolates has not yet been determined. Multiple other methods have been investigated, and the full extent of genetic diversity within B. henselae is still being defined.† Genetic variation makes vaccine development difficult (see Pathogenesis) but is useful in epidemiologic studies and may also be useful in furthering the understanding of the pathogenicity of various Bartonella isolates. B. henselae is naturally transmitted among cats by cat fleas (Ctenocephalides felis). B. henselae was transmitted among cats by transferring fleas fed on infected cats to specific-pathogen free cats, and by intradermal inoculation of flea excrement.93,150 Cats exposed to B. henselae–infected fleas that were confined to capsules that permitted fleas to feed, but prevented contamination of cats’ skin and haircoat with flea excrement, did not become infected with B. henselae.150 This finding suggests that transmission does not occur via flea saliva. As noted previously, ticks may also have a role in transmission. Cats were experimentally infected with B. henselae through intravenous or intramuscular inoculation with infected cat blood,233 and by intravenous, subcutaneous, intradermal, or oral routes of inoculation with plate-grown bacteria.1,166,167,308 B. henselae transmission has not occurred when infected cats cohabit with uninfected cats in a flea-free environment,1,167 indicating that transmission among cats does not occur through cat bites, scratches, grooming, or sharing of food dishes and litter boxes. Transmission did not occur when cats were inoculated intramuscularly with urine of bacteremic cats.230 Transmission also did not occur between bacteremic female cats and males during mating, or to the kittens of infected females either during gestation or in the neonatal period,1,174 again in flea-free environments. Bacteremia with B. henselae and B. clarridgeiae is commonly chronic and waxes and wanes; periods occur when bacteremia cannot be detected either by culture or by PCR testing. Although experimentally infected cats were not always followed for extended periods, some maintained B. henselae and/or B. clarridgeiae bacteremia for as long as 454 days.233 Relapsing bacteremia occurred at irregular intervals of between 1 and 4.5 months in some of these cats.175,233 Naturally infected cats maintained recurrent bacteremia for periods as long as 3 years; however, reinfection via fleas of cats living in private homes is a likely cause of prolonged recurrent bacteremia.10,237 Measured increases in interleukin-4 and serum antibody titers after the peak of bacteremia occurred concurrently with a decrease in the bacteremia to low or undetectable levels in one study.208 However, the effectiveness of antibodies in clearing bacteremia is not certain. In another study, kittens that did not produce measurable anti-Bartonella IgM or IgG antibodies had the same course of bacteremia as did kittens that produced high titers of anti-Bartonella antibodies.175 A study suggests that cell-mediated immunity is important in reducing the level of bacteremia in experimentally infected cats.209 There was no difference in antibody response or duration of bacteremia in splenectomized cats compared to nonsplenoctomized cats, though the average level of bacteremia in splenectomized cats was tenfold greater in the splenectomized cats.371 This suggests that phagocytosis is important in limiting bacteremia in infected cats. In a study of shelter cats, concurrent infection with B. henselae was most frequent in cats coinfected with feline leukemia virus than with feline immunodeficiency virus or feline panleukopenia virus.69a One conclusion is that latent FeLV infection may predispose cats to B. henselae infection or its persistence. Immune protection against Bartonella infections has been evaluated in several studies. Complete protection against reinfection is highly specific, even with strains of a given species of Bartonella, and likely in reactivity to the outer membranes of the organism.81 Cats previously infected with B. henselae 16S rRNA type II were protected from reinfection with B. henselae 16S rRNA type II but were susceptible to infection with B. henselae 16S rRNA type I.401 Cats infected with B. henselae type I or II were susceptible to challenge infection with B. clarridgeiae, and cats infected with B. koehlerae or B. clarridgeiae were susceptible to challenge infection with B. henselae type I or type II.403 In contrast, cats infected first with B. henselae type I were partially or completely protected against subsequent challenge infection with B. henselae type II.403 It is evident from these studies that the level of bacteremia and degree of susceptibility to reinfection after challenge inoculation vary with strains, as well as with species, of Bartonella.401 The scope of localization of Bartonella in cats has not been completely determined. Bartonella are intracellular bacteria, and B. henselae have been detected within erythrocytes of naturally infected cats341,355,355 and in vitro (Fig. 52-3).287 Bartonella are also endotheliotropic and may also be located intracellularly in vascular endothelial cells of infected cats, as has been suggested for rodents (Fig. 52-4).117 B. henselae are also found extracellularly in blood and tissues of infected cats.176
Bartonellosis
Etiology
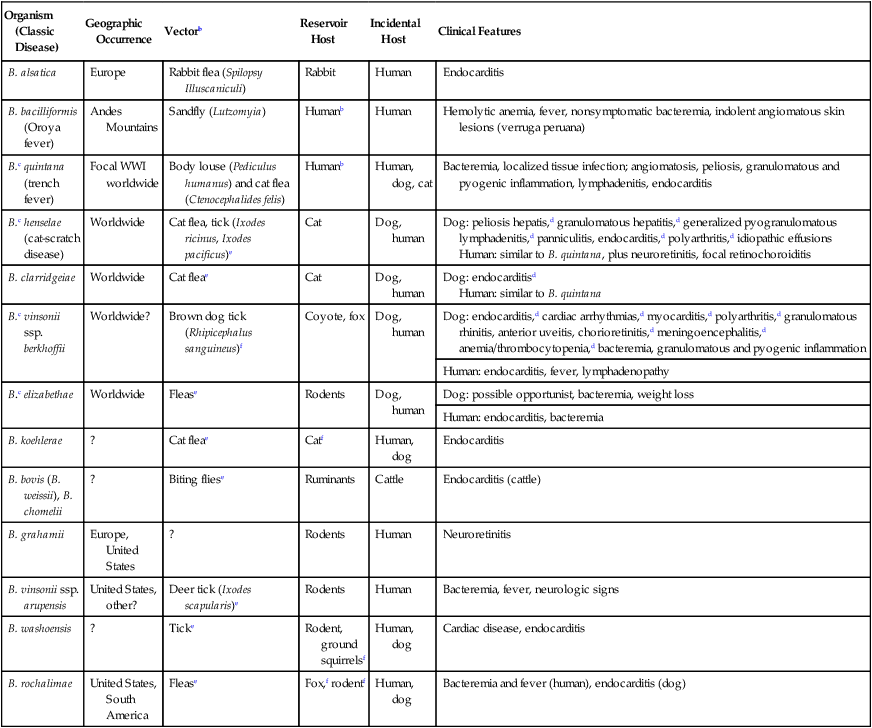
Organism (Classic Disease)
Geographic Occurrence
Vectorb
Reservoir Host
Incidental Host
Clinical Features
B. alsatica
Europe
Rabbit flea (Spilopsy Illuscaniculi)
Rabbit
Human
Endocarditis
B. bacilliformis (Oroya fever)
Andes Mountains
Sandfly (Lutzomyia)
Humanb
Human
Hemolytic anemia, fever, nonsymptomatic bacteremia, indolent angiomatous skin lesions (verruga peruana)
B.c quintana (trench fever)
Focal WWI worldwide
Body louse (Pediculus humanus) and cat flea (Ctenocephalides felis)
Humanb
Human, dog, cat
Bacteremia, localized tissue infection; angiomatosis, peliosis, granulomatous and pyogenic inflammation, lymphadenitis, endocarditis
B.c henselae (cat-scratch disease)
Worldwide
Cat flea, tick (Ixodes ricinus, Ixodes pacificus)e
Cat
Dog, human
Dog: peliosis hepatis,d granulomatous hepatitis,d generalized pyogranulomatous lymphadenitis,d panniculitis, endocarditis,d polyarthritis,d idiopathic effusions
Human: similar to B. quintana, plus neuroretinitis, focal retinochoroiditis
B. clarridgeiae
Worldwide
Cat fleae
Cat
Dog, human
Dog: endocarditisd
Human: similar to B. quintana
B.c vinsonii ssp. berkhoffii
Worldwide?
Brown dog tick (Rhipicephalus sanguineus)f
Coyote, fox
Dog, human
Dog: endocarditis,d cardiac arrhythmias,d myocarditis,d polyarthritis,d granulomatous rhinitis, anterior uveitis, chorioretinitis,d meningoencephalitis,d anemia/thrombocytopenia,d bacteremia, granulomatous and pyogenic inflammation
Human: endocarditis, fever, lymphadenopathy
B.c elizabethae
Worldwide
Flease
Rodents
Dog, human
Dog: possible opportunist, bacteremia, weight loss
Human: endocarditis, bacteremia
B. koehlerae
?
Cat fleae
Catf
Human, dog
Endocarditis
B. bovis (B. weissii), B. chomelii
?
Biting fliese
Ruminants
Cattle
Endocarditis (cattle)
B. grahamii
Europe, United States
?
Rodents
Human
Neuroretinitis
B. vinsonii ssp. arupensis
United States, other?
Deer tick (Ixodes scapularis)e
Rodents
Human
Bacteremia, fever, neurologic signs
B. washoensis
?
Ticke
Rodent, ground squirrelsf
Human, dog
Cardiac disease, endocarditis
B. rochalimae
United States, South America
Flease
Fox,f rodentf
Human, dog
Bacteremia and fever (human), endocarditis (dog)
Feline Bartonellosis
Epidemiology
Pathogenesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bartonellosis