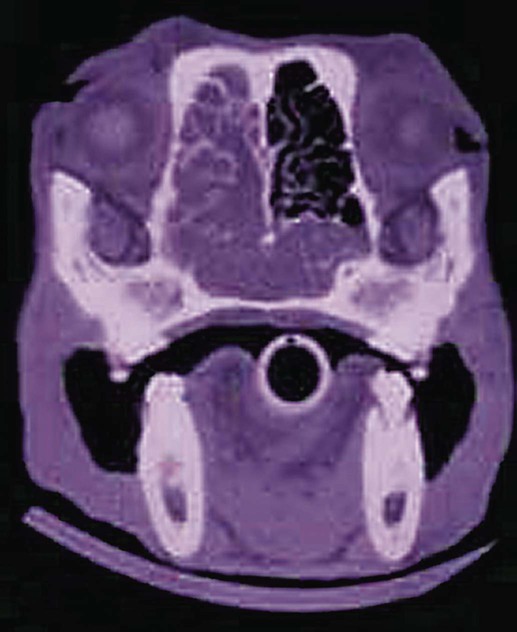
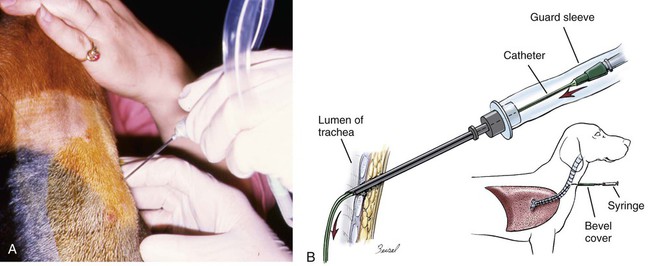
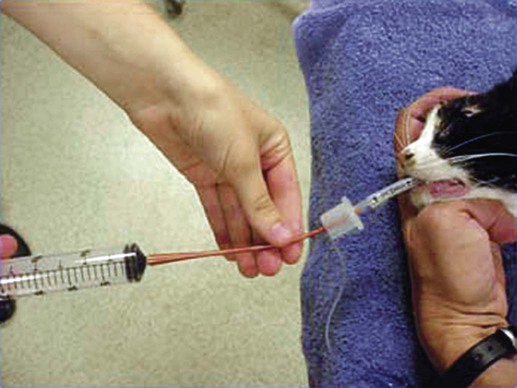
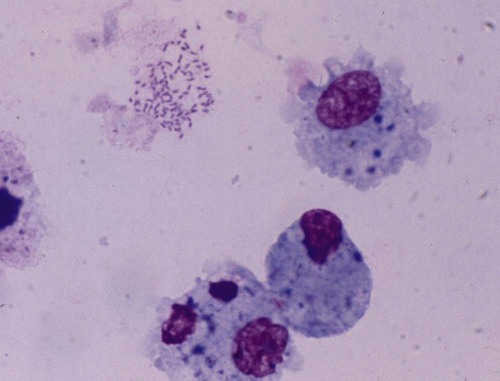
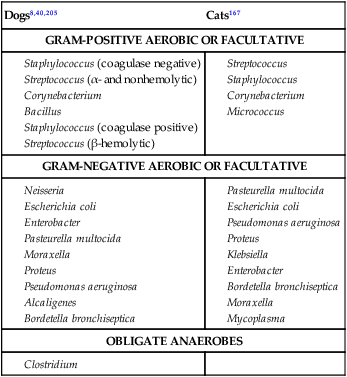
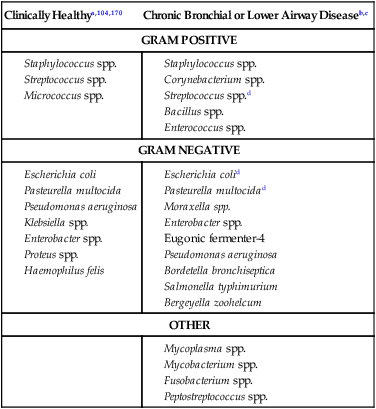
Surveys of the bacterial microflora of the nasal cavities, tonsils, and pharynx of clinically healthy dogs and cats have found many types of aerobic and facultative anaerobic bacteria (Table 87-1 and Box 87-1). Greater numbers of organisms are routinely cultured from the rostral than from the caudal nasal cavity. Because of marked individual variations, expecting to find the same organisms as flora of the nasal cavity and pharynx in each animal is not possible, but the presence of a certain range of flora can be predicted. TABLE 87-1 Bacterial Isolates from Nasal Swabs of Clinically Healthy Animalsa aBacteria are listed in approximate order of frequency of isolation. Bacteria are prevented from entering the lower respiratory tract by filtration of inspired air in thenasal turbinates, sneezing or coughing of inhaled particulate matter, and mucociliary clearance mechanisms. Despite these barriers, the healthy tracheobronchial tree and lung are not continuously sterile. Airways down to the first bronchial division are contaminated with low numbers of organisms in clinically healthy animals. Studies using guarded culture swabs or tissue samples of the lower trachea of clinically healthy dogs have found some bacteria in 40% to 50% of samples (Box 87-2). Small numbers of bacteria (fewer than 2 × 103 colony-forming units [CFU]/mL) are also frequently cultured from the lower airways of clinically healthy cats (Table 87-2). Oropharyngeal bacteria are aspirated and may be present for an unknown interval in the healthy tracheobronchial tree and lung. Aerobic bacteria have been isolated from 37% of lung tissue samples, whereas only 10% of dogs examined had no growth from cultures of multiple samples of their lung.129 Most of the bacteria cultured from the trachea and lungs are identical to those found in the pharynx of these same dogs. Finding large concentrations (105 CFU/mL or more) of bacteria or cytologic evidence of inflammatory cells in tracheobronchial washings makes their presence of more concern to the clinician. TABLE 87-2 Bacterial Isolation from Lower Respiratory Tract of Cats aCollected by bronchoscopic alveolar lavage. Organisms isolated from the upper respiratory tract are found in lesser concentrations in the lower airways down to first bronchial division of healthy cats. Colony counts under 2 × 103 CFU/mL. No anaerobic bacteria or Mycoplasma isolated in healthy cats. bCollected by endotracheal tube bronchial lavage, transtracheal lavage. cReferences 14, 38, 62, 76, 146, 166, 198. dAnimals with these isolates have been septicemic, either as a cause or effect. Bart M, Guscetti F, Zurbriggen A, et al. 2000. Feline infectious pneumonia: short literature review and retrospective immunohistochemical study on the involvement of Chlamydia spp. and distemper virus. Vet J 159:220–230. Although not specific to the respiratory system, the minimum database, including a complete blood cell count, serum biochemical profile, and urine analysis, can provide useful information regarding the systemic health of the patient. Especially in the case of lower respiratory and pleural infections, systemic response to infection or organ dysfunction may be detected. Dogs and cats with bacterial pneumonia and pyothorax can have abnormalities in their leukograms.58,109,109 Leukogram results do not correlate with the severity of the underlying infection, however, and leukocyte counts within or below the reference range are found with some frequency. Whereas infected dogs often have leukocytosis due to a neutrophilia with a left shift,58,188 infected cats may not have abnormalities on their complete blood cell count13,58,58 or they may have either a leukocytosis or leukopenia.12,13,58,133,232 Because bacterial pneumonia can result from hematogenous spread of bacterial infection originating elsewhere in the body, sepsis and multiple organ dysfunction can contribute to morbidity and mortality, making a serum biochemical profile and urinalysis useful to determine extent of disease. Gas exchange and acid-base balance have the potential to be disrupted with bacterial respiratory disease (in particular pneumonia), making arterial blood gas analysis useful to determine the severity of respiratory compromise.245 Although pulse oximetry may provide a less sensitive indication of hypoxemia in a simple, noninvasive fashion, it does not provide information on ventilatory or acid-base status. Serial use of arterial blood gas measurements can help in determining what treatment to use (e.g., supplemental oxygen, mechanical ventilation) as well as provide information on response to treatment. Other infectious agents affecting the respiratory tract may be important differentials and screening for them with fecal testing (parasites) or blood testing can be part of the diagnostic work-up. For example, in cats with chronic nasal discharge, cryptococcal antigen testing of serum is helpful because it will detect this disease before more invasive diagnostic tests (see Chapter 59). Radiography, ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) have been used to image the respiratory tract. Radiographic evaluation of the nasal cavity can be readily performed because of widespread equipment availability; however, it is ideal to obtain multiple views and perform this procedure under general anesthesia to obtain the best quality films possible. Primary bacterial rhinitis is believed to be rare, with only 1 of 80 dogs in a retrospective study having primary bacterial rhinosinusitis.140 Bacterial infection secondary to another nasal disease is more likely, and imaging is often necessary to determine this underlying etiology. Thoracic radiography should be performed first, with, a minimum of two required views (lateral and ventrodorsal or dorsoventral); however, both right and left lateral views are recommended, whenever possible, to fully appreciate lesions on both sides of the respiratory tract. Bronchial patterns can be seen in cases of acute or chronic bronchitis, and interstitial, alveolar, and mixed patterns are commonly seen in patients with pneumonia.133 The presence of pleural fluid in cases of pyothorax can also be evaluated via thoracic radiography. Radiographic signs of free pleural fluid include increased hazy density of the lung fields (ventral portions of lung fields on lateral view) that obscures the cardiac silhouette; retraction of the lobar borders from the chest wall; visibility of the interlobar fissures; and rounding or filling of the costophrenic angles. It is recommended to repeat thoracic radiographs after evacuation of the pleural space to enhance visualization of the pulmonary parenchyma. This may aid in determining the cause of the pleural effusion, which at least in cats is thought to be primarily from parapneumonic spread.12 The etiology of pyothorax in dogs is not always clear; however, local extension from the thoracic wall, tracheobronchial tree, pulmonary parenchyma, or esophagus has been documented, and abnormalities of these structures may be appreciated on thoracic radiography. Ultrasonography has been employed to evaluate the lungs and pleural space in cases of lung lobe consolidation, suspect pulmonary abscess, masses, and pleural effusion.82,183,183 Ultrasonography can be used to guide fine-needle aspiration and pleural fluid collection; there is also some preliminary evidence that it can be useful to estimate the volume of pleural fluid in the chest cavity.154,203,203 CT provides excellent images of the nasal cavity and detailed information regarding soft tissue densities and extent of nasal cavity involvement (Fig. 87-1).128 MRI has been used in evaluation of canine nasal disease and appears to be an alternative to CT imaging.142 Thoracic CT can be performed to obtain further information regarding radiographic abnormalities such as extent and exact location of a lesion. In the scope of this chapter, thoracic CT could be indicated in the case of suspected pulmonary abscess or other pulmonary parenchymal lesions and to investigate the cause of pyothorax (once the thoracic cavity is evacuated). It can also be a useful tool for surgical planning. Endoscopic examination allows direct visualization of the nasal passages, pharynx, larynx, trachea, and bronchi and facilitates collection of specimens for culture, cytology, and histology. Rhinoscopy can be performed using flexible or rigid endoscopes and is described in detail elsewhere.64 Because primary bacterial infection of the nasal cavities is rare, rhinoscopy is most valuable for investigating the underlying cause of secondary bacterial rhinitis.140 Pharyngoscopy and laryngoscopy can be accomplished with the use of a flexible endoscope, and use of this equipment for retrograde rhinoscopy allows visualization of the nasopharyngeal region. Tracheobronchoscopy allows guided collection of samples using brush catheters, transbronchial needles, bronchoalveolar lavage (BAL), and biopsy forceps for culture or microscopic examination from locally affected areas of the lung.96 In medium- to large-sized dogs, inserting a flexible fiberoptic bronchoscope through an endotracheal tube is preferred to maintain airway patency. An endotracheal tube T-adapter allows oxygen and anesthetic gas to flow without leakage to the patient while allowing placement of the flexible endoscope through the endotracheal tube. In smaller dogs and in cats in which the bronchoscope will not fit through the endotracheal tube, the endoscope is directly passed into the airways. The procedure can be performed by use of injectable anesthetic agents and either supplying supplemental oxygen through the biopsy channel of the bronchoscope or tracheal intubation along side the bronchoscope with a red rubber catheter. Culture of the nasal cavity is not routinely warranted because primary bacterial rhinitis is considered rare140; it is more critical to find the primary cause of the secondary bacterial rhinitis and address the former element directly. Bacterial culture is used more commonly for bacterial infections of the tracheal and bronchial airways, pulmonary parenchyma, and pleural cavity where specific organism isolation and antibacterial susceptibility testing are critical for selection of appropriate therapy. Specimens obtained by fine-needle aspiration or transtracheal or bronchoalveolar lavage should be cultured for aerobic and anaerobic bacteria. In some cases, mycoplasma may be a causative agent, and this organism can either be cultured (the laboratory must be notified specifically with this request as it requires special media to optimize growth) or polymerase chain reaction (PCR) can be performed. A Gram stain can help make an early diagnosis of bacterial infection, and an acid-fast stain may be of use in dogs with suspected Mycobacterium, Actinomyces, or Nocardia infections. Bacterial colonization versus infection must be differentiated by use of cytology and quantitative bacterial cultures.174 The threshold for clinically relevant bacterial growth in BAL specimens from dogs is proposed to be over 1.7 × 103 CFU/mL of BAL fluid.174 Bacterial infection can also be diagnosed with microscopic examination of 50 oil-immersion fields on a Gram-stained slide revealing more that two intracellular bacteria.174 False-negative results may be obtained if antimicrobial therapy has not been discontinued for at least 1 week before sampling. Blood cultures have been recommended in humans with bacterial pneumonia to help isolate the infectious agent.190 No studies in animals have been done that document the value of blood cultures in establishing a prognosis or isolating the bacterial agent in cases of pneumonia. Up to 50% of dogs with experimentally induced streptococcal pneumonia have had positive blood culture results within 48 hours of onset of clinical signs.149 Although cats with histologically confirmed infectious pneumonia frequently developed pneumonia from hematogenous spread, blood cultures were not performed in these cats, so their diagnostic value is unknown.133 Blood cultures should be considered in patients with respiratory infections that demonstrate signs of bacterial sepsis (see Chapter 36). Principles and limitations of PCR have been discussed in detail elsewhere (see Chapter 1). This technology has been used in veterinary medicine as a diagnostic modality and as an epidemiologic tool. PCR has been used to screen dogs and cats with respiratory illness for pertinent bacterial infections such as Mycoplasma spp., Chlamydophila felis, and Bordetella bronchiseptica, among others.63,99,176,177,231 It has also been used to identify bacterial isolates, to detect virulence factors, and to determine relatedness between strains in outbreak situations.* Several reports of outbreaks of fatal hemorrhagic pneumonia in dogs or necrotizing pneumonia in cats demonstrate the usefulness of PCR in identifying causative bacterial agents such as Streptococcus equi ssp. zooepidemicus and extraintestinal pathogenic Escherichia coli (ExPEC).28,117,117 A point-source clonal outbreak was documented in dogs with S. equi ssp. zooepidemicus and in cats with ExPEC, further demonstrating the utility of PCR for epidemiologic investigation.177 In several studies, correlation of PCR and bacterial culture results were evaluated. One study of 20 cats found agreement in detection of Mycoplasma spp. in nasal wash and nasal biopsy samples of 89% and 100%, respectively, with PCR identifying an organism that did not grow in culture.110 However, cats were not required to have respiratory disease as entry criteria into that study, so the pathologic significance of Mycoplasma spp. detection is unclear. Another study in cats with upper respiratory tract disease evaluated agreement of PCR and bacterial culture results from nasal and pharyngeal swabs for detection of Mycoplasma spp. and reported an overall agreement of 64.3%.231 The lack of complete agreement could be explained by the increased sensitivity of PCR compared to culture. Considering the increased sensitivity of PCR, it is important to consider results in conjunction with the clinical signs and other diagnostic findings. PCR may be detecting the presence of organisms at low levels that are not causing the observed disease. Studies evaluating association between positive PCR results and disease have been performed with mixed results.36,97,173,244 PCR methods can be completed in a short time frame compared to culture of some organisms (e.g., Mycoplasma spp.).110,180,180 Other advantages of PCR include the ability to discriminate among bacterial species, to provide information regarding clonality of isolates, to detect organisms that are difficult or impossible to culture, and to detect small numbers of organisms.29,63,177,210,244 However, a major disadvantage is that PCR may detect DNA from nonviable organisms, which could confound the interpretation of results.110,208 Sample acquisition and handling should minimize contamination, and the PCR environment must be strictly maintained and monitored for contamination to prevent unreliable results.110,208 Because bacterial organisms have been identified by PCR in specimens from clinically healthy patients,36,87 one must consider the results in the context of the clinical picture to determine whether organisms identified via PCR are related to the clinical disease.208 A 16- to 19-gauge through-the-needle intravenous catheter is used for the procedure. The animal is allowed to sit or is placed in sternal recumbency with its neck extended. The area over the ventral neck inclusive of the larynx and proximal trachea is clipped and scrubbed as for surgery. The cricoid cartilage, identified by palpating the tracheal rings from the midcervical region toward the larynx, is the first prominent ventral ridge at the larynx. The cricothyroid ligament is cranial to the cricoid cartilage and between the thyroid cartilages. From 0.5 to 1 mL of 2% lidocaine is injected intradermally and subcutaneously over the cricothyroid ligament. The needle and catheter are then directed caudoventrally through the skin and cricothyroid ligament into the larynx in a single motion (Fig. 87-2, A). The catheter is passed into the trachea, and the needle is withdrawn from the cricothyroid ligament (see Fig. 82-7, B). The needle guard is then applied to prevent the catheter from being cut by the needle. Paroxysms of coughing can be expected as the catheter is passed into the trachea. This technique is performed using general anesthesia without an endoscope.96 The animal is placed in lateral recumbency and intubated. The cuff of the endotracheal tube is inflated, and a syringe adapter is connected at the end of the tube. Aliquots (5 mL/kg) of warmed sterile 0.9% saline are infused via syringe. Immediately after each aliquot, mild suction is applied to the syringe. The syringe is immediately disconnected after the fluid is taken, and 100% oxygen is given through the endotracheal tube for 5 minutes. Alternatively (and preferred by the authors of this chapter), a 7-French red rubber catheter can be introduced in cats and small dogs through the lumen endotracheal tube until it is wedged (i.e., until gentle resistance is felt). Saline is lavaged through a syringe attached to the red rubber catheter and gently aspirated (Fig. 87-3). The cytologic preparations collected by bronchoalveolar lavage through the endoscope are considered superior to those obtained by transtracheal or endotracheal washings because larger volumes of cells and proteinaceous materials are obtained from the deeper portions of the lungs.94 Septic suppurative inflammation with or without intra- or extracellular bacteria would be supportive of bacterial infection. Quantifying bacteria obtained by airway washings is needed to distinguish lower airway contamination or transient colonization from actual infection. In addition to bronchoalveolar lavage, brush catheters can be passed through the biopsy channel of the endoscope to obtain specimens. Given the expense of a catheter system that can be used only once, the authors of this chapter feel there is no advantage of using brush catheters compared with the bronchoalveolar lavage technique for identification of bacterial infections. Transbronchial or transthoracic fine-needle aspiration and thoracocentesis can be used to procure material for microbial culture and cytologic examination directly from the lung. Transbronchial needle aspiration can be performed with specialized needles that pass through the biopsy channel of the bronchoscope. These needles can be particularly helpful in aspirating enlarged hilar lymph nodes or discrete masses. Transthoracic fine-needle aspiration may have a higher diagnostic yield than lavage (especially tracheal washings and blind BAL) when focal lesions are present. Fine-needle aspiration should be considered before employing more aggressive diagnostics, such as a thoracotomy. The transthoracic fine-needle aspiration techniques (blind, ultrasound-guided, fluoroscopically guided, or CT-guided), indications, contraindications, and complications have been reviewed elsewhere.56,222,222 The fine-needle method has much less risk compared with transthoracic “tru-cut” needle biopsy, which can cause severe pulmonary hemorrhage or pneumothorax. Using the fine-needle method, specimens can generally be classified as being neoplastic, inflammatory (infectious or noninfectious inflammatory) or nondiagnostic. The recommended size of the needle is 25 or 27 gauge and 1.5 inches in length but may vary depending on patient size. The needle is advanced into the lung, and gentle suction is applied with a 6-mL syringe, taking care not to move or redirect the needle. Thoracocentesis can be both diagnostic and therapeutic. The procedure can easily be performed in any veterinary facility and requires readily available equipment (needle and extension set or butterfly catheter, 3-way stopcock, and large syringe). An area over the seventh to eighth intercostal space, approximately two-thirds of the way down the chest, is clipped and sterilely prepped, the needle is inserted cranial to the rib using sterile technique, and aspiration of fluid is performed with a large syringe. Fluid is submitted for appropriate cytology and culture. This procedure has been described in detail elsewhere.225 Preparation of material for cytologic evaluation can be done using several methods. Visible strands of exudate may be teased onto a microscope slide, smeared, and stained. Small quantities of material can be centrifuged and smears made of the sediment. New methylene blue wet mounts and Wright-Giemsa or Gram stain of air-dried smears can be used for identification of cellular elements and bacteria. Bacterial infections are typically associated with degenerate neutrophils and intracellular bacteria; in addition, excess mucus, proteinaceous material, and activated alveolar macrophages can be present. Bacteria are demonstrable only in a fraction of washings from pneumonic animals, and their absence in cytologic specimens, especially if the patient has been placed on antibacterial therapy, does not rule out bacterial pneumonia (Fig. 87-4). Cytologic evaluation of pleural fluid is usually consistent with a highly cellular and proteinaceous fluid (Table 87-3). Degenerate neutrophils and mixed populations of bacteria are often observed. Nondegenerate neutrophils can be present with low-grade bacterial infection or prior antibacterial therapy. Neutrophil degeneration may be observed in severe cases; however, the neutrophil concentration can vary and does not always correlate with the severity of infection. Aerobic and anaerobic cultures of pleural fluid should be performed, regardless of whether bacteria are seen cytologically. TABLE 87-3 Characteristics of Pleural Fluid from Pyothorax LDH, Lactate dehydrogenase. aThese changes have presently been documented in cats and humans. In cats, the correlation of gross rhinoscopic mucosal abnormalities with histologic evidence of inflammation has been weak, underscoring the need for histologic evaluation of biopsy specimens whether or not lesions are observed via rhinoscopy.109 It is likely that this is true for other sites within the respiratory tract. Histopathologic evaluation of pharyngeal, laryngeal, and lung tissue is the ultimate test to confirm invasion of bacteria with subsequent inflammatory reactions within the tissue. This assessment must be coupled with culture or histochemical tests to determine causative agents. Lung biopsy is considered a relatively aggressive diagnostic technique, because definitive diagnosis is often reached with fine-needle aspiration cytology or bronchoalveolar lavage fluid analysis. However, biopsy can provide complementary and additional information in complicated cases of pulmonary parenchymal disease.158,159,159 Bacteria typically stain as blue rods or cocci with hematoxylin and eosin (H&E) stain. Special stains that may be required for evaluation of tissue for underlying bacterial causes include Brown and Brenn staining for gram-negative organisms and protocols using carbol-fuchsin for acid-fast bacilli such as Mycobacterium infections.47,226 Primary bacterial rhinitis is rare in the dog and cat.* This is in part demonstrated by persistence of nasal discharge after empiric treatment with (often multiple) courses of antibacterials. Presence of bacteria in nasal cultures is not synonymous with primary bacterial infection, because bacteria can be isolated from nasal passages of cats without nasal disease and cats with other nonbacterial nasal diseases.59 B. bronchiseptica is the main primary nasal bacterial pathogen in cats, affecting mostly young kittens and cats in overcrowded, stressful environments.209 Bacterial rhinitis is most commonly secondary to nasal trauma; allergic rhinitis; lymphoplasmacytic rhinitis; inhalation of foreign material; reflux of liquids or food into the nose caused by pharyngeal, esophageal, or gastric dysfunction; viral, fungal, or parasitic infections; neoplasia; dental disease; oronasal fistula; nasopharyngeal polyps; and bacterial bronchopneumonia.59,244 Common clinical signs seen in dogs and cats with nasal disease include sneezing, mucopurulent nasal discharge, ocular discharge secondary to nasolacrimal duct obstruction, and cough with gagging or retching.59,244 Epistaxis occurs infrequently with bacterial rhinitis but may be associated with underlying disease such as fungal rhinitis or neoplasia.151 Pawing at the face or nose indicates severe nasal irritation, often caused by foreign bodies lodged in the nasal cavity. Ulceration of the external nares and accumulation of crusted exudate occur in severe or chronic cases. Chronic bacterial sinusitis is uncommon in the dog, although mucous accumulation does occur when nasal diseases occlude normal drainage of the frontal sinus through the sinus ostium. Chronic sinusitis in cats most often occurs as a result of mucosal and bone damage secondary to feline viral respiratory infections (see Chapter 14). Severe mucosal ulceration and turbinate destruction damage local immune defense mechanisms and allow secondary bacterial infection of the nasal passages and frontal sinuses. Acute sinusitis is postulated to accompany a large number of inflammatory conditions of the upper respiratory tract caused by inhaled irritants, allergens, and viral agents, although acute sinusitis is poorly characterized in small animals. Very few studies have evaluated the types of bacteria involved in chronic rhinosinusitis (with most reports focusing on nasal cultures in chronic rhinitis). In one study, mean total bacterial detection rates from nasal flushes were not significantly different between control cats and cats with chronic rhinosinusitis; however, Mycoplasma species and anaerobic bacteria were only detected in cats with chronic rhinosinusitis.111 Sinus trephination may be required in severe, chronic cases to obtain samples for culture and susceptibility testing and to obtain biopsy specimens to look for an underlying cause. Cultures taken during surgery from the frontal sinus in clinically affected cats showed that Pseudomonas organisms were most commonly isolated.162 Pasteurella may also be a common isolate in cats.31 There is a paucity of information on bacterial frontal sinus cultures in dogs. Results of bacterial cultures in dogs differ when sampled from the rostral versus caudal nasal cavity1; the authors of this chapter are not aware of studies correlating nasal and frontal sinus culture results in dogs. Treatment of bacterial sinusitis is often frustrating because an underlying cause may remain elusive or difficult to eradicate (e.g., feline herpesvirus); in addition, many patients fail to respond to all forms of symptomatic therapy. A course of broad-spectrum antibacterial (anaerobic and aerobic) therapy with good penetration into bone and secretions is recommended. Identification and treatment for underlying diseases such as fungal infection and neoplasia should be attempted. There is concern regarding drug penetration of antibacterials into the sinus; penetration is speculated to increase with inflammation, but actual levels are unknown. Broad-spectrum antibacterials are sometimes recommended for prolonged periods (2 to 4 months) after bacterial culture and susceptibility testing, especially with bony involvement. There are inadequate data in the veterinary literature to assess the efficacy of nasal or sinus lavage or irrigation with or without antibacterials or other antiseptic solutions. Therapy with nasal decongestants has been empirically advocated, although there is concern for exacerbation of the condition by drying the exudate. Surgical turbinectomy and sinus trephination can establish drainage of the sinuses and remove inspissated pockets of exudate. More aggressive surgical approaches have included sinus obliteration and reconstruction of apertures into the frontal sinuses. In human medicine, novel approaches using agents that inhibit bacterial biofilms are being studied.39 The tonsils comprise a portion of the peripheral (secondary) lymphoid organs and are responsible for collecting local antigen and mounting an adaptive immune response. Tonsillar tissue can also become infected, and in humans, tonsillitis is caused by bacterial and viral infections. Both have been reported in dogs.18,126,126 Overall, there is a striking paucity of information about this condition in the primary literature, likely reflecting that it is rare. Primary tonsillitis usually occurs in young, small-breed dogs that exhibit clinical signs of malaise, cough with retching, fever, and inappetence.126 Tonsillitis is usually bilateral but may occasionally occur as a unilateral disease when a foreign body is trapped in the tonsillar tissue or crypt. Inspection will often reveal a bright-red tonsil with an associated pharyngitis. Punctate hemorrhages on the tonsil and purulent exudate in the tonsillar crypt may also be visible. The tonsil will be friable and will easily bleed on manipulation. Tonsillitis is believed to occur most commonly secondary to an underlying disease process. Diseases associated with a secondary tonsillitis include chronic vomiting or regurgitation, chronic gingivitis or periodontitis, tracheobronchitis, and nasopharyngeal irritation caused by rhinitis. Tonsillar enlargement is not synonymous with tonsillitis, even in the presence of respiratory signs, and tonsillar lymphoid hyperplasia (a normal immune response to antigens) needs to be considered as a possible cause. Tonsillar lymphoid hyperplasia was reportedly more common than tonsillitis in racing greyhounds with enlarged tonsils and a history of poor racing performance.147 Many bacteria associated with tonsillitis are consistent with resident flora of the oral and pharyngeal regions. The organisms most frequently isolated in cases of tonsillitis include E. coli, and various species of Staphylococcus, hemolytic streptococci, diplococci, Proteus, Pseudomonas, Enterococcus, Streptococcus, and Pasteurella; Listeria monocytogenes has also been isolated in dogs.126,147 Inflamed, swollen tonsils are not an absolute indication for treatment. Elimination of preexisting problems usually results in resolution of the tonsillitis if it is secondary. If clinical signs are severe or persistent, then broad-spectrum antibacterial therapy for 10 to 14 days can be considered. Tonsillectomy may be indicated when primary tonsillitis is a recurrent problem, when purulent tonsillitis is unresponsive to antimicrobial therapy, or when hyperplastic tonsils protrude from the crypts causing mechanical interference with breathing and swallowing. For further information on diagnosis and treatment of this disorder, see Tonsillitis, Chapter 88. The pharynx can be subdivided into three anatomical areas: nasopharynx, oropharynx, and laryngopharynx. In one study, dogs with nasopharyngeal, oropharyngeal, and laryngopharyngeal disorders were evaluated, and only 2 of 67 cases were diagnosed with bacterial disorders, a nasopharyngeal abscess and a tonsillar abscess.18 In a study of 53 cats with nasopharyngeal disease, none had a primary bacterial infection.4 Therefore, bacterial infection of these areas also appears rare. Generally, pharyngitis arises secondary to local or systemic disease. Bacterial pharyngitis often accompanies viral or bacterial upper respiratory tract infections, pharyngeal foreign bodies, and retropharyngeal abscesses. Clinical signs depend on the anatomic portion of the pharynx affected. For nasopharyngeal disease, clinical signs are usually referable to the respiratory tract, whereas for oropharyngeal and laryngopharyngeal disease, clinical signs are referable to either the respiratory or upper gastrointestinal (GI) tracts.18 Treatment is aimed at underlying diseases, such as removal of foreign bodies, and broad-spectrum antibacterial therapy may be used for 7 to 14 days. If an abscess is identified and is not responsive to antibacterial therapy, surgical drainage may be useful. For further information on this topic, see Gingivostomatitis and Pharyngitis, Chapter 88. Laryngitis usually occurs as part of a widespread bacterial or viral respiratory infection such as canine infectious tracheobronchitis or feline viral rhinotracheitis.46 B. bronchiseptica in dogs is one of the primary bacterial pathogens associated with laryngitis, tracheobronchitis, and pneumonia (see Chapter 6). Laryngeal foreign bodies have also been reported18 and can lead to a nidus of infection. Differential considerations for bacterial laryngitis include noninfectious inflammatory disease (often granulomatous inflammation), trauma to the larynx during endotracheal intubation or surgery, inhalation of toxic substances, insect bites, and prolonged barking (dogs) or coughing.46 Clinical signs vary between species, with dogs exhibiting cough, voice change, gagging, or stridor and cats having more severe local and systemic signs.46 Treatment is aimed at the coexisting infectious problem, although in many dogs, the disease is self-limiting. The trachea and bronchi serve as conducting airways to ultimately allow gas exchange in the lung; inhaled air is not sterile, so there must be protective defenses in place to prevent infection. These include reflexes (cough, sneeze), the mucociliary apparatus, innate immunity, and adaptive (predominantly mucosal) immunity.43 Acute primary or secondary bacterial infections of the airways are relatively common in dogs (canine infectious tracheobronchitis), whereas chronic bacterial infections are usually secondary in both dogs and cats and are not nearly as common. Canine infectious tracheobronchitis (“kennel cough” or “canine infectious respiratory disease complex”) is a highly contagious respiratory disease associated with a wide variety of viral, mycoplasmal, and bacterial agents. Clinical signs are referable to nasal, tracheal, bronchial, and pulmonary parenchymal involvement and can include nasal discharge, sneezing, cough, and sometimes systemic signs of lethargy, anorexia, and fever. The diagnosis is usually made based on history and physical examination alone. Chapter 6 discusses this syndrome in detail. B. bronchiseptica can be a primary respiratory pathogen in dogs without accompanying viral or Mycoplasma infection.16,116,116 Other bacterial species that have been identified include Pasteurella spp. and S. equi ssp. zooepidemicus,37 although especially in conjunction with primary viral infections, a number of bacteria can become involved secondarily. In cats, controversy exists about whether B. bronchiseptica is a primary or secondary pathogen, and although the organism has been associated with tracheobronchitis, conjunctivitis, rhinitis, and pneumonia, many cats harboring the organism are clinically healthy (see Chapter 14). One study showed the prevalence of B. bronchiseptica infection increases with the number of cats in the cattery and with the presence of other cohoused cats having upper respiratory tract disease.99 Of interest, an association has been identified between higher carriage rate in cats (usually displaying minimal clinical signs) and contact with dogs having kennel cough.19,69,69 This association leads to speculation of interspecies transmission and the role cats can serve in maintaining the organism in the environment. One report of B. bronchiseptica infection spreading from dogs to a pair of cats residing in the same household strongly suggests transmission between these species; in this instance, both the dogs and cats were clinically ill.54 Human infections with B. bronchiseptica have been reported,69 although the organism has a preference for infecting animals, whereas Bordetella pertussis and Bordetella parapertussis cause whooping cough in humans (see Public Health Considerations, Chapter 6 and Bordetella bronchiseptica Infection, Chapter 99).164,247 Many cases of infectious tracheobronchitis are self-limiting. Cough suppressants (contraindicated with concurrent pneumonia) and taking measures to minimize cough by decreasing exercise, excitement, or other triggers are often helpful. Widespread resistance of B. bronchiseptica isolates to cephalosporins and ampicillin has been reported;69 therefore, routine, indiscriminant use of antibacterials for all dogs with kennel cough should be avoided to minimize antibacterial resistance. Antibacterial therapy should only be considered for dogs with complicating lower airway disease with persistent clinical illness. For guidelines on antibacterial therapy, see Chronic Bronchitis, later, and Therapy in Chapter 6. There has been interest in examining the host immune response to B. bronchiseptica to see if high antibody levels correspond to protective immunity. An enzyme-linked immunosorbent assay was evaluated to measure antibodies to B. bronchiseptica lipopolysaccharide as a means to determine which dogs were susceptible or would be more likely to develop severe disease in a large kennel with endemic kennel cough.37 Unfortunately, serum antibodies against B. bronchiseptica LPS were not predictive of susceptibility of severity of disease. Secretory IgA concentrations, which may be more predictive, were not examined. Because this is a highly contagious disease in dogs, vaccination of at-risk animals should be considered. Although more commonly given to dogs, a vaccine is also available for cats. The intranasal modified-live bacterial vaccine for cats has been evaluated and found to induce a protective response within approximately 3 days with immunity lasting 1 year.238 The role of bacterial infections in initiating chronic bronchitis in both dogs and cats has not been determined, although it is frequently listed as a major cause of this disease.15,124 Definitively proving that bacterial infections lead to chronic bronchitis is challenging, because the disorder is generally diagnosed in its advanced stages, often long after the inciting cause is gone. Secondary bacterial infections may also complicate established chronic bronchitis. Active bacterial infection versus bacterial colonization or translocation of flora from the upper airways must be distinguished, because the former is not as common.62,174 The hallmark clinical sign of chronic canine bronchitis is a cough of 2 or more months in duration. Importantly, in contrast to bacterial pneumonia, bacterial airway infections in dogs lack systemic signs of inappetence, weight loss, or lethargy. In cats, because chronic bronchitis and asthma are often lumped together as one syndrome, clinical signs of cough, wheeze, and episodic expiratory respiratory distress have been described.152 There are many differential considerations for cough, which can broadly be divided into cardiac disease or respiratory disease, with the latter being further subdivided into upper airway, lower airway, and pulmonary parenchymal disorders. Along with a thorough history and physical examination, thoracic radiographs are very useful in initial stages to focus the diagnostic work-up on the heart or respiratory tract. With chronic lower airway disorders, radiographic abnormalities most commonly consist of interstitial or peribronchial infiltration, or both. Bronchiectasis, an irreversible pathologic dilation of the airways due to destruction of elastic and muscular components, is a severe outcome of chronic airway inflammation or infection and can be appreciated radiographically.93,161 A lack of thoracic radiographic abnormalities has been noted in up to 23% of cats with lower airway disease.2 Definitive diagnosis of chronic bronchitis is generally made by cytologic examination of airway lavage fluid with increased nondegenerate neutrophils being prominent.98 The presence of degenerate neutrophils with or without intracellular bacteria suggests active infection. Quantitative bacterial cultures of BAL fluid are strongly recommended to differentiate between airway colonization and infection. In dogs, chronic bronchitis is not commonly associated with clinically relevant bacterial growth when quantitative bacterial cultures and cytologic examination of lavage fluid are performed.174 The role of infection in feline bronchial disease is poorly defined. Culture results of tracheal and bronchial washes from clinically healthy cats often reveal a mixed pattern of low numbers of organisms (fewer than 5 × 103 organisms/mL), which probably reflect airway contamination rather than infection when more than 105 organisms/mL are expected (see Table 87-2).166 A variety of bacteria have been isolated from cats with chronic lower respiratory tract disease, some of which are similar to what is found in healthy cats (see Table 87-2). The role of Mycoplasma is intriguing because these organisms can be isolated in airway washings from cats with chronic bronchial disease,38,170 but not in those from healthy cats.170 Mycoplasma spp. have been associated with increased airway hyperreactivity by degrading neural endopeptidase and prolonging the effect of substance P, a potent bronchoconstrictor localized to airway C-fibers.206 It is unknown if this mechanism of smooth muscle constriction contributes to clinical signs in cats with naturally developing asthma. Aside from its role in potentially causing or complicating chronic bronchitis, bacterial infection of the airways may also cause or complicate bronchiectasis and bronchiolitis. Although rare, cycles of repeated infection and inflammation from chronic bronchitis, neoplasia, and bronchopneumonia in cats can lead to bronchiectasis.161 In dogs, bronchiectasis may occur secondary to congenital diseases such as primary ciliary dyskinesia or acquired disorders such as chronic bronchitis, eosinophilic bronchitis, or bronchopneumonia.157,174 Bronchiectasis is more prevalent in certain breeds such as the American cocker spaniel, West Highland white terrier, miniature poodle, Siberian husky, English springer spaniel, and dogs over 10 years of age.93 Bronchitis and bronchiolitis caused by cilia-associated respiratory bacillus-like organisms has been reported in a cat.184 These silver-staining organisms were found in close parallel arrangement in association with mucosal cilia and the presence of airway inflammation. Bronchiolitis obliterans with organizing pneumonia (technically an interstitial lung disease, not an airway disease) results from injury to the distal airways; the injury can be due to infectious causes.186 Bronchiolitis obliterans with organizing pneumonia has been documented to naturally develop in both dogs and cats160 and can be experimentally induced in dogs with Mycoplasma infection.120 Although antibacterial therapy is not indicated for treatment of self-limiting tracheobronchitis, it is often considered for animals with chronic bronchial inflammation. The blood-bronchus barrier represents a potential impediment to effective antimicrobial therapy. Penetration of antimicrobial drugs into airway secretions and pulmonary tissues is favored by high lipophilicity and low molecular weight. Drugs enter normal bronchial secretions at a fraction of their serum concentrations. Trimethoprim, clindamycin, quinolone, erythromycin, and doxycycline enter in highest proportions. Penicillins have the lowest penetration; cephalosporins and aminoglycosides have intermediate distribution. With inflammation, penetrance is increased, but antibacterial distribution through the airway may be impaired in exudates. Avoidance of antibacterials that are not active against B. bronchiseptica (see previous discussion) and those achieving low concentrations in airway tissues and secretions (e.g., amoxicillin-clavulanate and gentamicin administered parenterally) should be considered.116 Culture and susceptibility testing should be used to guide therapy. A negative culture result would support viral infection, in which case antibacterials would not be useful.
Bacterial Respiratory Infections
Resident Bacterial Flora
Upper Respiratory Tract
Dogs8,40,205
Cats167
GRAM-POSITIVE AEROBIC OR FACULTATIVE
GRAM-NEGATIVE AEROBIC OR FACULTATIVE
OBLIGATE ANAEROBES
Lower Respiratory Tract
Clinically Healthya,104,170
Chronic Bronchial or Lower Airway Diseaseb,c
GRAM POSITIVE
GRAM NEGATIVE
OTHER
Respiratory Diagnostics
Ancillary Diagnostics
Medical Imaging Findings
Endoscopic Examination
Bacteriologic Culture
Polymerase Chain Reaction
Cytologic Examination
Transtracheal Aspiration
Bronchoalveolar Lavage: Blind Technique
Bronchoalveolar Lavage: Bronchoscopic Technique
Fine-Needle Aspiration
Cytologic and Microbiologic Examination
Parameter
Reference Characteristics
Pyothorax
Physical characteristics
Color: clear, transparent
Color: variable yellow to reddish-brown, opaque, flocculent; sometimes granules can be odorous
Cells
<500/µL
>7000/µL
Protein
<1.5 g/dL
>3 g/dL
Specific gravity
<1.017
≥1.025
Biochemistry of fluid
LDH activity and glucose concentration in reference blood range
LDH activity increased; glucose concentration decreased compared with blooda
Histopathologic Examination
Upper Respiratory Tract Infections
Bacterial Rhinitis
Etiology
Clinical Findings
Chronic Sinusitis
Etiology and Differential Diagnoses
Therapy
Tonsillitis, Pharyngitis, and Laryngitis
Lower Respiratory Tract Infections
Acute Tracheobronchitis
Chronic Bronchitis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine