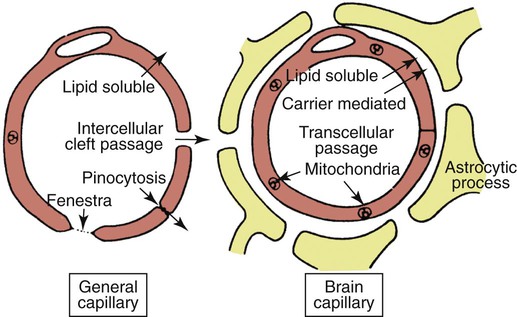
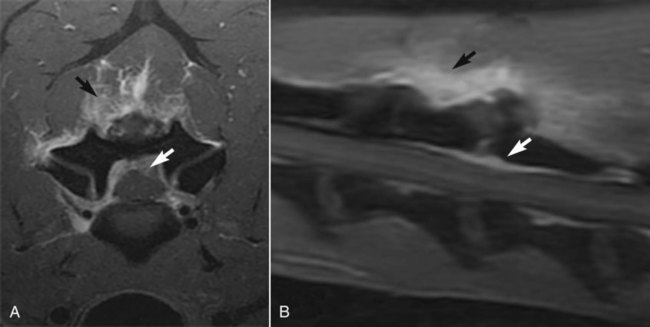
Central nervous system (CNS) inflammation is described as encephalitis, myelitis, and meningitis based on whether the process affects the brain, spinal cord, or meninges, respectively. Inflammation in the CNS may be the result of an infectious process, immune-mediated inflammation in the absence of infection, neoplasia, trauma, or infarction. The underlying pathogenesis of immune-mediated disease is often unknown. Granulomatous meningoencephalitis (GME) is a classic example of an immune-mediated process without a determined cause.30 Other immune-mediated diseases preferentially affect certain breeds. Breed-specific inflammatory disease has been identified in the pug dog,53 Maltese terrier,236 Yorkshire terrier,249 Pekingese,41 and greyhound,38 and Chihuahua.106 Other immune-mediated diseases such as steroid-responsive meningitis-arteritis typically affect young patients, usually under the age of 2 years.160 See Chapter 82 for more information on the inflammatory diseases described previously. Alternatively, immune-mediated disease is sometimes categorized based on the principal inflammatory reaction, GME,30 or eosinophilic meningoencephalitis.227 Chemical meningitis can be induced by the subarachnoid injection of contrast agents as is seen with myelography.42 Finally, some neoplasms such as meningioma have been associated with an inflammatory response.14,43,43 Infectious diseases that affect the CNS include viral, protozoal, fungal, rickettsial, and bacterial organisms.28,88,96,135,172 Some viruses (canine distemper virus [CDV; Chapter 3] and rabies virus [Chapter 20]) are neurotropic, as are some protozoans such as systemic amebic infections (Chapter 78) and Toxoplasma gondii and Neospora caninum (Chapter 79). These disorders produce primarily parenchymal inflammation.238 Others agents that are nonneurotropic become microembolized in the meningeal vasculature as a result of their systemic spread to many tissues. In this somewhat immunoprivileged site, they cause disease as a consequence of their induction of vasculitis and meningitis with secondary parenchymal injury, for example, feline infectious peritonitis virus (FIPV; Chapter 10), Ehrlichia and Anaplasma (Chapter 26), Rickettsia rickettsii (Chapter 27), Brucella canis (Chapter 38), Bartonella spp. (Chapter 52), Blastomyces dermatitidis (Chapter 57), Cryptococcus neoformans (Chapter 59), and Coccidioides immitis (Chapter 60). The main focus of this chapter is on bacterial infections of the CNS. Bacteria by their nature are nonneurotropic. They cause infection by producing meningitis or abscess formation. Bacterial meningitis is an inflammatory response to bacterial invasion of meninges and cerebrospinal fluid (CSF). Such infections can rapidly disseminate and, based on the virulence of the pathogen, lead to serious illness and death.152 Bacterial meningitis, which can be localized or diffuse, occurs when pathogenic organisms overcome (or elude) host defense mechanisms and reach the subarachnoid CSF. Meningitis typically causes diffuse or multifocal lesions in dogs and cats, although the clinical signs may not reflect the diffuse nature of the disease.248 Brain abscesses, which result from a focal pyogenic infection, appear to result from contiguous spread of infections, such as progression of otitis interna, more than from hematogenous spread of organisms. Brain abscesses are uncommon in the dog and cat.35,153,153 This finding may be caused in part by the inability of the CNS to form scar tissue, which restricts the spread of pyogenic infections in other tissue. Because epidural abscess (spinal empyema) develops outside the parenchyma of the CNS, true abscess formation can occur.63,173 Intracranial and spinal empyema has been reported in the dog and cat but occur less commonly than meningitis.* In humans, staphylococci or streptococci are the most commonly implicated agents, and skin infections are a frequent source. Spinal empyema is commonly the result of diskospondylitis in dogs.2,61,61 Other sources include paraspinal abscess, epidural administration of analgesics, and migrating foreign material.45,89,89 Spinal epidural abscesses are more common than cranial epidural abscesses because of the greater available space in the spinal canal. The veterinary literature details numerous examples of isolated cases and a few retrospective reviews of a limited number of cases of bacterial CNS infections.50,190,209,248 Consequently, much of the information regarding bacterial CNS infections in veterinary patients must be gleaned from experimental studies and the literature pertaining to cases in people. In humans, bacterial meningitis is caused by several distinct microorganisms that demonstrate neurotropism. These microorganisms include Haemophilus influenzae type b (Hib), Neisseria meningitidis, and Streptococcus pneumoniae serotype C.132 In the past 15 years, the epidemiology of bacterial meningitis in humans has changed dramatically. Hib infection has virtually disappeared as a result of immunizations, and there has been a decrease in N. meningitidis and a slight increase in S. pneumoniae infections.218 In addition, occurrence of clusters of cases of bacterial meningitis and septicemia in adolescents has increased as a result of contact in schools and universities.86 Several risk factors have been identified in human cases. Age, socioeconomic factors, and cigarette smoking have all been associated with bacterial meningitis.86 In contrast to humans, bacterial meningitis in veterinary patients does not seem to be caused by microorganisms with a predilection for the nervous system. Consequently, bacterial CNS infections in small animals are relatively uncommon. A wide variety of bacterial organisms have been reported in small animals. These organisms have included Staphylococcus spp., Streptococcus spp., Pasteurella spp., Escherichia coli, Klebsiella sp., Proteus spp., Salmonella spp., Actinomyces spp., and Nocardia spp.* Anaerobes, such as Prevotella oralis, Fusobacterium spp., Bacteroides spp., Peptostreptococcus spp., Eubacterium spp., Flavobacterium breve, and Propionibacterium sp., have been identified in the CNS.4,35,66,97,190 Infection in the CNS requires numerous interactions between the pathogen and the host immune system. Bacterial organisms can spread to the CNS in a wide variety of ways. The most common route of infection is through hematogenous spread from a distant focus. Other less common routes into the CNS include direct penetration (blunt trauma, bite wound, or penetrating objects), contiguous spread (nasal cavity, paranasal sinuses, and otitis media and interna), and invasion along nerves and nerve roots (Table 91-1). In humans, the first step in the pathogenesis of hematogenously spread infections involves mucosal colonization by bacteria, then evasion of local host mucosal defenses, and eventually entrance into the intravascular space. This process has been well defined in humans.138 Bacteria such as S. pneumoniae, N. meningitidis, and H. influenzae are known to secrete proteases, which degrade mucosal IgA, allowing for colonization.261 Once beyond the mucosal barrier, bacteria can enter the subepithelial vasculature. Systemic infections, which may ultimately lead to CNS bacterial infections, arise from splenic abscesses, pleuritis, lung abscesses, vegetative endocarditis, bite wounds, urinary tract infections, pneumonia, infected cranial sinuses, and middle ear infections.† In some instances, immunosuppression likely plays a role in the development of abscess formation in the CNS.8,182,182 Intravascular survival depends on the ability of bacteria to circumvent immune system activation. The ability to evade major host defenses against bacteremia, complement activation, circulating antibodies, and neutrophil phagocytosis has been attributed to bacterial capsular polysaccharides.138 To gain entry into the CNS from the vascular space, pathogens must traverse a final impediment. TABLE 91-1 Source and Localization of Nonneurotropic Infections of the Central Nervous System This last obstacle is composed principally of two barriers: the blood-brain barrier (BBB) and the blood-CSF barrier.179 The BBB is formed primarily by the brain endothelium. The brain endothelium is unique in that it lacks the fenestrae found in other capillary beds (Fig. 91-1). Brain endothelial cells are interconnected by tight junctions composed of zona occludens and zona adherens. These tight junctions form a seal at the apical region of brain endothelial cells that result in the lack of permeability of the BBB, effectively segregating the brain from the vascular space. Of note is that the development and maintenance of this aspect of the BBB depends on astrocytes, perivascular microglia, and the basal laminae.1 In addition, the endothelial cells in the brain are relatively devoid of pinocytotic vesicles, suggesting little transcellular movement of substances. An analogous barrier is constructed between adjacent epithelial cells of the choroid plexus, making up the blood-CSF barrier, which prevents entry into the CSF.179 For pathogens to cross the BBB, they must first adhere to the brain endothelium or the epithelium of the choroid plexus. For E. coli K1, fimbrial components have been identified that preferentially bind brain endothelial cells.237 Attached to the endothelium, pathogens can traverse the BBB through paracellular pathways, through transcellular transport, or intracellularly within leukocytes as they undergo diapedesis. Bacteria traversing the BBB can lead to infection of the CNS parenchyma, resulting in encephalitis or myelitis; the leptomeninges via blood vessels of the pia mater, causing meningitis; or entering across the choroid plexuses, leading to ventriculitis.198 Regardless of the site of penetrance, infection can disseminate through the brain parenchyma to the CSF or from the CSF to the brain parenchyma because the extracellular fluid in the brain is contiguous with the CSF. Alternatively, the BBB can be circumvented by direct penetration. Normal anatomic structures such as the vertebral column, calvaria, and meninges help protect the nervous system. However, with blunt impact trauma or penetrating objects, this aspect of the protective barrier around the CNS tissue can be overcome (Fig. 91-2). Migrating foreign material has been associated with the development of intracranial infection.60,153 Microorganisms can also enter the CNS during infections in adjacent structures containing penetrating nerves.174 Nasal aspergillosis can result in significant osteolysis of the nasal turbinates and cribriform plate,221 which may allow for direct invasion into the brain. Encephalopathy and suppurative meningoencephalitis have been reported secondary to topical treatment of nasal aspergillosis in the dog.154 Similarly, otitis interna and media can invade the adjacent brainstem, usually along cranial nerves VII and VIII, which penetrate the petrous temporal bone on their course to the brainstem, producing meningitis, with or without abscess formation of the neuroparenchyma (Figs. 91-3 and 91-4, see Otitis Media and Otitis Interna, Chapter 85).52,180,233,238 Although hematogenous spread predisposes the patient to meningitis or parenchymal infarction, or both, contiguous spread into the CNS along nerve access often causes subdural abscesses.256 Despite the close proximity, diskospondylitis rarely progresses to invade the adjacent spinal cord parenchyma.75 Microorganisms can also invade the CNS by traveling within axons from peripheral nerves. In ruminants, Listeria monocytogenes likely gains access to the CNS along axons of the trigeminal nerve.238 Concurrent CNS and sinonasal infections with C. neoformans has been documented.20,37 Whether CNS involvement is related to direct penetration through a damaged cribriform plate, the result of extension along the olfactory nerves, or through hematogenous spread is unclear. Once inside the BBB, the host defenses are severely limited. The CSF is largely devoid of complement, opsonizing proteins such as immunoglobulins and neutrophils, which, in part, allow for bacterial replication to occur largely unchecked.138 With increased bacterial replication and autolysis, concentrations of the bacterial cell wall products, lipopolysaccharides, teichoic acid, and peptidoglycans are increased, which stimulate the host inflammatory response.138 Unlike infections in extraneural sites, in CNS infections, the damage is largely the result of the inflammatory response rather than the pathogen itself.242 The inflammatory response involves the production numerous cytokines and chemokines (tumor necrosis factor-α [TNF-α], interleukin [IL]-1, IL-6, IL-8, and IL-10), matrix metalloproteinase (MMP), reactive oxygen species (ROS), and nitric oxide synthetase (NOS).138 Cytokines and chemokines are small proteins that are crucial in the development and modulation of inflammation. In general, cytokines are factors that bind to cell membrane receptors and modulate leukocyte function, whereas chemokines bind to transmembrane receptors and function as chemoattractants.39 Many different cells possess the capability of producing these various proinflammatory molecules. Brain cells such as endothelial cells, ependymal cells, resident macrophages, astrocytes, and glial cells can express cytokines in response to stimuli in bacterial meningitis.215 Among the cytokines, IL-1 and TNF-α play a crucial role early in the inflammatory process in the CNS.193 In bacterial meningitis, IL-1 and TNF-α concentrations increase early in the disease course. Both IL-1 and TNF-α induce the generation of IL-6, IL-8, and IL-10. IL-6 induces the release of acute-phase reactants, fever, and leukocytosis. IL-8 is important in mediating neutrophil chemotaxis, adhesions, and migration into the subarachnoid space. IL-10 is largely anti-inflammatory, causing a reduction in the levels of IL-1, IL-6, IL-8, and TNF-α. TNF-α also causes the production of MMPs. MMPs are a family of proteinases that help break down the extracellular matrix, thereby allowing for the migration of leukocytes. MMP-8 and MMP-9 are elevated in meningitis and contribute to the breakdown of the BBB.215 ROS and NOS also play prominent roles in the inflammatory process. As a result of their free-radical actions, ROS are cytotoxic. The brain is particularly sensitive to free-radical injury owing to a lack of antioxidants, relative high oxygen consumption, and high concentration of unsaturated fatty acids.138 Nitric oxide (NO) also plays a substantial role in perpetuation of inflammation, the alterations in cerebral vascular perfusion, and the production of peroxynitrite, a potent free radical responsible for lipid peroxidation.215 Meningeal inflammation is largely responsible for the clinical signs seen in meningitis more than the direct effects of organisms. The direct toxic effects of a pathogen contribute to neuronal injury; however, the indirect effects of the inflammatory mediators are more important factors in neuronal loss. Experimental models and autopsy findings in humans reveal two distinct histologic forms of injury: extensive cortical damage and selective injury to the dentate gyrus of the hippocampus.120 TNF-α may be responsible for apoptotic loss of neurons in the hippocampus.241 ROS and NO likely cause the ischemic injury to the cortex through their regulation of cerebral blood flow.241 Excitatory amino acids such as glutamate also contribute to neuronal loss.137 In addition, the production of cytokines and other proinflammatory molecules results in endothelial damage, disrupting the BBB and allowing plasma components and leukocytes to traverse into the CNS, which plays a part in the development of vasogenic edema.241 If the endothelial damage is severe enough, thrombosis can occur, producing ischemia and necrosis. Additionally, cerebral edema contributes to intracranial hypertension, which alters autoregulation of the cerebral vasculature and cerebral perfusion, ultimately contributing to ischemia and neuronal loss.11 Ependymal inflammation from bacterial meningitis may lead to obstructed CSF flow with secondary hydrocephalus,62 a phenomenon also found with FIPV meningoencephalitis of cats (see Chapter 10) and parainfluenza virus 5 infection (see Chapter 7). Patients with CNS infections can show a wide variety of clinical signs, ranging from those indicative of both systemic and neurologic dysfunction to those strictly referable to the CNS.248 If the primary source of infection is located in another organ system, clinical signs may reflect dysfunction of that particular organ. Thorough ocular, funduscopic, and otoscopic examinations should be performed on every patient suspected of having meningitis. The optic nerves are a direct extension of the diencephalon. Abnormalities such as papilledema, optic neuritis, chorioretinitis, retinal hemorrhages, and uveitis can be seen with inflammatory CNS disease.225 Similarly, the ear canal can provide a portal of entry of organisms into the CNS. Fever may result from the inflammatory process or the consequence of components of bacterial cell walls. Alternatively, elevated body temperature (hyperthermia) may be secondary to muscular activity, seizures, or environmental factors. Severe systemically affected patients may have signs consistent with shock, hypotension, and disseminated intravascular coagulation.159 Abnormalities in the cardiac rate or rhythm or a newly diagnosed murmur may be supportive of bacterial endocarditis as the primary source of sepsis. Additionally, bradycardia with concurrent systemic hypertension (Cushing’s reflex) may be the consequence of intracranial hypertension.11 Nausea and vomiting can also occur with meningitis.159 In advanced disease, with severe brain edema or mass effect, brain herniation with acute respiratory insufficiency may be seen. As with all other aspects in neurology, the key to accurate diagnosis relies heavily on a precise neuroanatomic diagnosis. Classically, infectious diseases of the CNS have been associated with multifocal neurologic signs.176 However, focal signs do not exclude the possibility of infection. Hyperesthesia is a cardinal feature of inflammatory or compressive diseases involving the meninges. In the cranial region, hyperesthesia may be demonstrated by compression of the cranial vault across the temporalis muscles or by opening the jaw. Photophobia or nuchal rigidity may also be present. Two-thirds of patients with inflammatory disease processes of the CNS have focal neurologic deficits.248 Focal inflammation of cranial or spinal nerves occurs in the subarachnoid space as the roots of these nerves enter and exit the CNS and are bathed by CSF. Focal signs of hyperesthesia, lower motor neuron dysfunction, or involuntary twitching of muscles may occur. Depending on the anatomic site affected, inflammation of the meninges, parenchyma of the brain, or of the spinal cord is termed meningitis, encephalitis, or myelitis, respectively. Similarly, inflammation in the CSF is referred to as meningitis. Prosencephalic (cerebrum and thalamus) lesions may alter mentation, cause seizures, and result in abnormal postural reactions with a preservation of gait. Brainstem lesions can also alter mentation. Moreover, brainstem lesions frequently result in cranial nerve deficits and gait abnormalities. Signs associated with spinal cord lesions depend largely on the level at which the spinal cord is affected. In general, proprioceptive ataxia and paresis are the hallmarks of spinal cord dysfunction. Further assessment of reflexes helps localize the affected spinal cord segments. Spinal meningeal inflammation may result in paraspinal discomfort, paraspinal muscle rigidity, and decreased vertebral mobility.229 The epidural space is in the area around the spinal cord from the base of the skull to the sacrococcygeal region. Pus can accumulate in the region outside the CNS, causing signs that mimic those of true CNS infection. Cranial epidural empyema is less common, because the dura is adherent to the skull bones forming the cranial vault, leaving little space for accumulation of pus. Because of its similar clinical presentation to neural infections, spinal empyema is discussed here, although it has been rarely reported in the dog and cat.61,63,89,136 Most of the cases have involved large-breed dogs. Clinical signs consist of evidence of systemic inflammatory response such as fever, lethargy, and anorexia. Additionally, spinal pain and neurologic deficits are common. Neurologic signs typically reflect a T3-L3 myelopathy. Clinical laboratory abnormalities often consist of neutrophilic leukocytosis in blood and a neutrophilic pleocytosis in CSF. However, bacteria are rarely identified in CSF, reflecting the fact that the infection is outside the dura and CNS. In conjunction with consistent signalment, history, and clinical laboratory data, presumptive diagnosis is supported with magnetic resonance imaging (MRI) findings of extradural lesions that are hyperintense on T2-weighted images and display peripheral or diffuse contrast enhancement (Fig. 91-5).61 Spinal empyema is commonly the result of diskospondylitis in dogs.2,61,61 Coagulase-positive staphylococci are the most commonly isolated organisms from cases in humans, and the same would be suspect for those in dogs. Other sources include paraspinal abscess, epidural administration of analgesics, and migrating foreign material.45,89,89 CSF analysis and culture are the sole methods of providing a definitive diagnosis of CNS bacterial infection. Because of the BBB, the absence of fever or leukocytosis does not eliminate meningitis from consideration. However, establishing a definitive diagnosis is complicated by the difficulty of identifying an underlying etiologic agent. Ancillary laboratory tests such as a complete blood cell count, biochemical profile, and urinalysis provide a minimal database. When present, abnormalities in the complete blood cell count, leukocytosis (neutrophilia with or without a left shift), or leukopenia largely reflect a systemic inflammatory response rather than providing a specific diagnosis. Biochemical profile abnormalities may provide insight into a possible source of sepsis or concurrent disease process. Alternatively, results of these tests may be within reference limits.248 In postoperative neurosurgical patients, distinguishing surgically induced bacterial infections of the meninges from irritant meningitis caused by contrast agents, surgical trauma, and lavage performed at the time of surgery can be difficult. In humans, an overlap exists of CSF test results for patients with chemical meningitis and infectious meningitis determined by bacterial isolation or identification.33 Therefore, administration of antibacterials may rely on detecting surgical wound infections, additional neurologic signs such as mental changes or seizure disorders, high rectal temperatures, or otorrhea or rhinorrhea. Given the close physical relationship that exists between the CSF and CNS parenchyma, disease affecting the parenchyma may be reflected as changes in the CSF. Furthermore, the CNS has limited ways to respond to disease. Therefore, most disease processes in the CNS result in inflammation. As a consequence, even using CSF analysis, definitive diagnosis may be difficult, if not impossible, to obtain in at least one third of patients with inflammatory CNS disease.248 Despite this problem, CSF analysis is necessary for determining that inflammatory CNS disease is present and obligatory for the diagnosis of bacterial CNS disease. CSF is often obtained from the cerebellomedullary cistern (CMC). Additionally, CSF can be collected from the dorsal or ventral subarachnoid space at the L5-L6 intervertebral spaces. Alternatively, CSF can also be obtained from the L6-L7 intervertebral space in some patients. In cases of hydrocephalus, CSF can also be collected from the lateral ventricles.175 One mL per 5 kg of body weight can safely be taken from patients.47 Little specialized equipment is needed for collecting CSF. In general, a 22-gauge, 1.5- or 2.5-inch spinal needle is used. For small patients (cats and dogs less than 5 kg), a 23-gauge, 0.5-inch scalp-infusion needle (“butterfly needle”) can be used, which allows for better control. Sterile surgical gloves are worn by the person performing the procedure. CSF should be collected in sterile containers without preservatives such as EDTA. EDTA can artificially lower cell counts and alter protein measurements.44 With the patient in right lateral recumbency, the head is flexed to a 90-degree angle with respect to the axis of the vertebral column.93 The axis of the head must remain straight (parallel to the table) to ensure that the vertebral column is not rotated. Positioning is done by an assistant who is situated across the table from the person performing the procedure. Once proper positioning is attained, the assistant should be instructed not to move the patient until the spinal needle is withdrawn from the skin when the procedure is completed. CSF from the CMC is obtained by inserting a needle between the occipital bone of the calvaria and the dorsal lamina of the first cervical vertebra. Landmarks for this site include the external occipital protuberance and the cranial aspect of the wings of the atlas.58 An imaginary line is drawn from the external occipital protuberance caudal along the midline. Similarly, a second imaginary line is drawn between the cranial aspects of the wings of the atlas. These lines bisect at approximately the level of needle insertion. The needle is inserted parallel to the surface of the table and directed perpendicular to the long axis of the vertebral column (Fig. 91-6). Blood contamination is often encountered. If the CSF flows readily, mild blood contamination may clear with additional drops or by replacing the stylet and waiting 1 minute before collecting additional, less contaminated CSF. Correction factors have been used to predict the leukocyte count in blood-contaminated samples. These correction formulas are inaccurate.267 Moreover, mild blood contamination does not significantly alter the number of leukocytes in CSF samples.108,267 If substantial blood or “pure” blood is encountered, the needle is withdrawn without replacing the stylet, and a new needle is used for another attempt. Many times, obtaining pure blood means that the needle was misdirected laterally. More serious and devastating complications include penetration of the needle into the CNS parenchyma and brain herniation. With lumbar punctures, needle penetration into the nervous tissue is often unavoidable because, in many cases, CSF cannot be collected from the dorsal subarachnoid space. However, at the level of the low lumbar vertebral column, this problem rarely results in clinical deficits. Despite this, hematomyelia secondary to lumbar CSF acquisition can occur.185 In stark contrast, injury at the level of the CMC can be devastating. In cases with confirmed or suspected intracranial hypertension, CSF collection can result in brain hemiation.118 By removing CSF, a pressure gradient is created whereby intracranial contents can shift from an area of high pressure to one of lower pressure (i.e., the site of CSF collection). Brain herniation can result in alterations in mentation and pupil size and symmetry, abnormal nystagmus, tetraparesis, abnormalities in respiration and cardiac rhythms, apnea, and death. Brain herniation can also be clinically silent. Herniation has been associated with anesthesia and CSF collection.129 Care should be exercised when considering CSF collection in patients with suspected or confirmed intracranial disease or intracranial hypertension. After collection of CSF, analysis should consist minimally of the following tests: gross visual inspection; total cell count, as well as leukocyte and erythrocyte counts; differential leukocyte count; and total protein analysis. Further biochemical, microbiologic, and serologic testing should be performed based on the results of the aforementioned tests. Normal CSF contains a relatively low protein concentration and is therefore hypotonic. As a result, cell lysis can occur quickly. Consequently, analysis should be performed quickly after collection, ideally within 30 minutes of collection.47 If analysis cannot be performed immediately, several techniques can be used to prevent in vitro cell degeneration. Refrigeration can slow degeneration for several hours; however, several improved methods of preserving cellular morphology have been developed. The addition of an equal volume of bovine albumin, fetal calf serum, or hetastarch to the CSF sample preserves cell morphology for up to 4 hours.80,194 Alternatively, an equal aliquot of serum from the patient can be mixed with its CSF, which preserves cell morphology and provides accurate cell counts for up to 48 hours.23 Precise measurements of the amount of CSF and the amount of serum mixed together are needed to calculate final cell counts. A separate aliquot of CSF, not diluted with serum or bovine albumin, is needed for measuring protein content. Mixing CSF with either 40% ethanol or 10% formalin has also been suggested to help preserve cell morphology.126 Normal CSF is clear and colorless. In general, turbidity or cloudiness becomes grossly visible with nucleated cell counts greater than 500 cell/µL. Other authors have suggested that turbidity is seen with nucleated cell counts greater than 200 nucleated cells/mm3 and 400 erythrocytes/mm3.47 Blood contamination or recent hemorrhage into the subarachnoid space will result in a pink to red discoloration of the CSF. Most often, blood contamination is iatrogenic as a consequence of the needle puncture rather than an underlying disease. Xanthochromia, a yellow-orange color in the supernatant, is usually an indication of previous hemorrhage caused by the breakdown of hemoglobin into bilirubin. In some instances, prolonged hyperbilirubinemia can cause xanthochromia. High CSF-nucleated cell counts and protein concentrations are indicative of neurologic disease, even if a moderated number of erythrocytes are present from iatrogenic blood contamination.108 Normal CSF contains a leukocytes in the range of 0 to 6 cells/µL in dogs and 0 to 5 cells/µL in cats, composed primarily of mononuclear cells and few to no erythrocytes.47,222 Further characterization of mononuclear cells is sometimes difficult because of the morphologic alteration induced in leukocytes as a result of preparation techniques.114 An elevation in CSF nucleated cell count is called pleocytosis. Performing differential cell counts is important even in cases that have total cell counts within normal ranges.48 One fourth of low-cellularity samples were cytologically abnormal with cytocentrifuge preparations, with the most common abnormalities being increased phagocytocytic activity, neutrophils, and reactive lymphocytes.48 In general, cell-concentration techniques are needed to improve cytologic evaluation of CSF given that most samples have low cellularity. The most accurate method is cytocentrifugation, in which CSF is spun at 1500 g for 10 minutes in a conventional centrifuge91 or 100 g for 10 minutes in a cytocentrifuge (Cytospin, Shandon Southern Instruments, Sewickley, PA).48 Sedimentation procedures can also be employed whereby CSF is mixed with serum and allowed to sediment for 1 hour. In this procedure, larger amounts of CSF are required. Various techniques can be used in which an aliquot of CSF is allowed to sediment on a glass slide, which is then air dried and stained for interpretation.51,83,83 Another method for CSF cytologic analysis uses membrane filtration; however, this method is more technical and requires different staining techniques (trichome or hematoxylin) and results in poor morphologic preservation of cells.203 The hallmark of bacterial CNS infections is a neutrophilic pleocytosis. Toxic changes can be appreciated with cytologic evaluation of neutrophils in CSF. Occasionally, intracellular bacteria can be seen within neutrophils or other phagocytic cells. However, the significance of organisms observed outside of leukocytes (free, nonphagocytosed) in the CSF should be interpreted with caution. Free organisms can be the consequence of in vitro contamination. The implication of free organisms should be defined based on cytologic evidence of infection and clinical signs. Alternatively, repeat CSF analysis can be performed to validate the existence of bacterial organisms in CSF. Unfortunately, neutrophilic pleocytosis is not specific for bacterial disease. SMRA can result in a pronounced pleocytosis composed mostly of neutrophils.160 Many of these patients will also have fever and cervical hyperesthesia, mimicking the signs of bacterial meningitis.160 Acute viral meningoencephalitis as a result of CDV infection can cause necrosis, resulting in an occasional marked neutrophilic pleocytosis.257 Dogs with CDV infections may have systemic signs of illness, including gastrointestinal and respiratory signs. In cats, infection with FIPV usually results in a mixed pleocytosis; however, in some cases a CSF inflammatory reaction composed predominantly of neutrophils is seen.125 Cats infected with FIPV may also show systemic signs and clinicopathologic changes such as fever, anemia, and hyperglobulinemia. Aberrant Cuterebra larval migration through the CNS can also cause a neutrophilic response in the CSF.84 Prodromal signs often include upper respiratory signs and abnormal body temperature (hyperthermia or hypothermia). Meningioma in the dog has also been associated with neutrophilic pleocytosis, although relationships between patterns of CSF changes and specific histologic tumor types cannot be made.14 Typically, dogs with intracranial neoplasia are older than 5 years and have progressive signs related to focal deficits. Recently performed myelographic procedures can also cause a change in CSF cytology consisting of primarily a neutrophilic pleocytosis. For a summary of CSF findings in various CNS disorders see Table 91-2.42,266 TABLE 91-2 Representative CSF Changes Seen in Various CNS Inflammatory Diseases of Dogs and Cats47,74,195,248
Bacterial Infections of the Central Nervous System
 References
References  Images
Images  Web Tables, Boxes, and Appendices
Web Tables, Boxes, and Appendices
Etiology
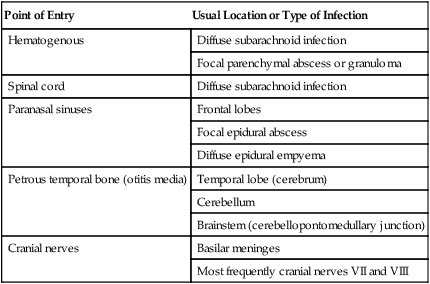
Pathogenesis
Point of Entry
Usual Location or Type of Infection
Hematogenous
Diffuse subarachnoid infection
Focal parenchymal abscess or granuloma
Spinal cord
Diffuse subarachnoid infection
Paranasal sinuses
Frontal lobes
Focal epidural abscess
Diffuse epidural empyema
Petrous temporal bone (otitis media)
Temporal lobe (cerebrum)
Cerebellum
Brainstem (cerebellopontomedullary junction)
Cranial nerves
Basilar meninges
Most frequently cranial nerves VII and VIII
Clinical Findings
Extraneural Signs
Neural Signs
Spinal Epidural Empyema
Diagnosis
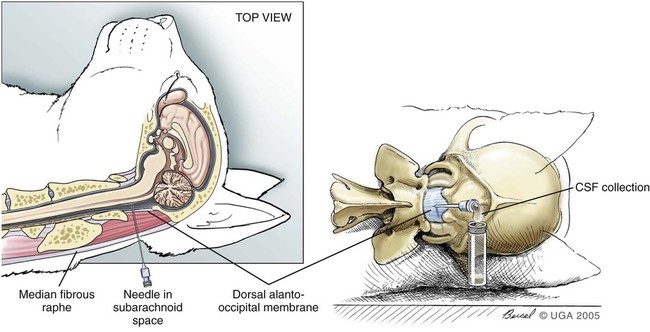
Cerebrospinal Fluid Evaluation
Technique
Complications
Cerebrospinal Fluid Analysis
Disease (Chapter)
WBCa,b Count
WBC Type
Total Protein Concentration (mg/dL)c
Albumin Proportion
Globulin Proportion
CSF Antibodies Detected
Organisms Seen
Bacterial meningitis (91)
++ (+++)
PMN (mixed)
++ (+++)
++
Varies
Yes (varies)
Rarely
Steroid-responsive meningitis/arteritis (82)
+++ (++)
Mixed (mono)
+++ (++)
++
+ (++)
No (IgA)
No
Tumor (e.g., meningioma)-associated pleocytosisd
+ (++)
PMNs (mixed)
+ (++)
+ (++)
WNL
No
No
Granulomatous meningoencephalitis (82)
++ (+++)
Mixed–PMN, mono
++ (+++)
++
++
No
No
Feline infectious peritonitis (10)
+++ (++)
Mixed–PMNs, some mono
+++
++
++
Yes (?)
No
Fungal and algal meningoencephalitis (57, 58, 59, 60, 65, 67, 69)
++
Mixed–PMN, eos, mono
++
++
+ (++)
Varies
Varies
Protozoal meningoencephalitis (72, 73, 75, 76, 78, 79, 80)
+
Mixed–PMN eos, mono
+ (++)
+
+ (++)
Variable
Rarely
Ehrlichiosis (26)
+ (++)
Mono
+
+
+
?
Varies
Rocky Mountain spotted fever (27)
+
Mixed (neutro)
+ (WNL)
+
+
?
No
Eosinophilic meningitis
+ (++)
Eos
+
+
+
No
No
Canine distemper (3)
Inflammatory
+
Mono (lymph)
+
WNL
+
Yes
No
Noninflammatory
WNL (+)
Mono (lymph)
WNL
WNL
WNL (+)
No (varies)
No
Other viral encephalitis (7, 24)
+
Mono
+ (++)
WNL
+
?
No
Necrotizing encephalitis in Yorkshire terriers (82)
+
Mono
+
+
+
No
No ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree