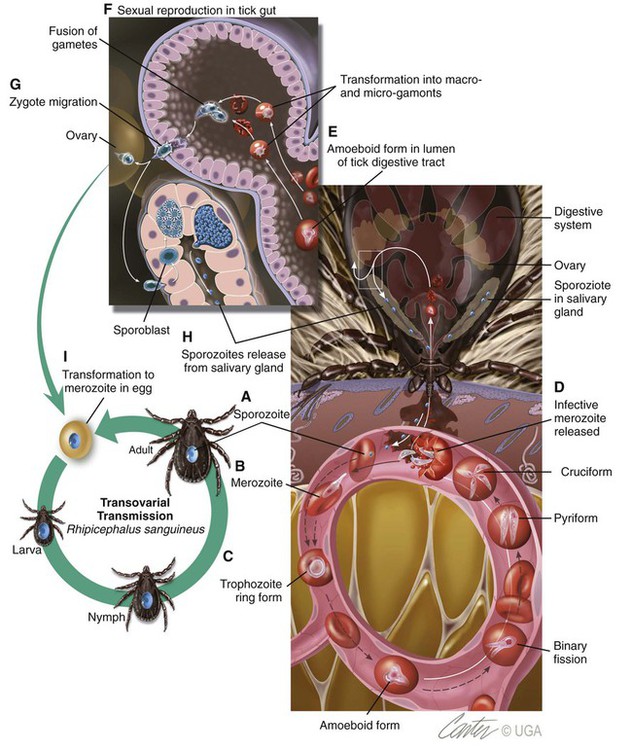
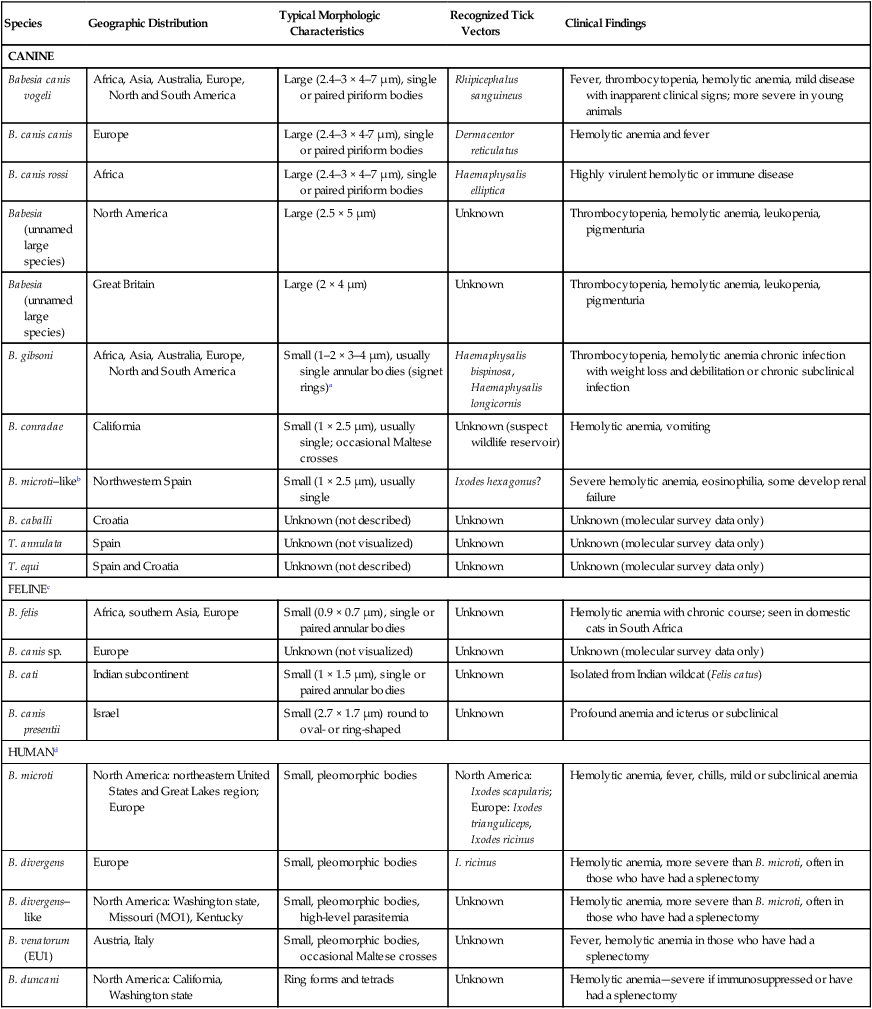
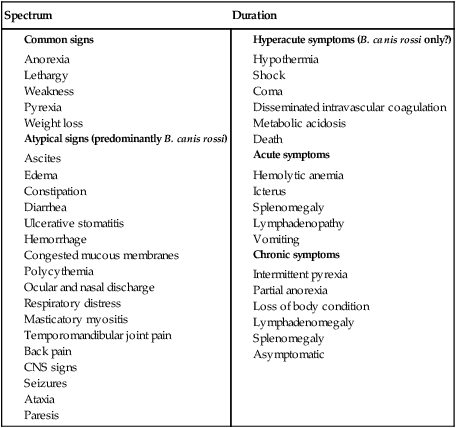
Babesiosis, caused by infection with organisms with the genus Babesia, is characterized by hemolytic anemia, fever and, splenomegaly. Babesia infections can also be subclinical or cause severe life-threatening illness. Babesia spp. are intraerythrocytic protozoan parasites of the phylum Apicomplexa that are frequently transmitted by ticks. More than 100 species of Babesia have been described, and with the advent of molecular techniques such as the polymerase chain reaction (PCR), many new species and genotypes are identified each year.137 Historically, species have been named and identified based on the vertebrate host and the size of the parasite. Based on morphologic phenotype, they are divided into two categories, large and small. Large Babesia spp. tend to be 3 to 7 µm in length, whereas small Babesia spp. tend to be 1 to 3 µm in length (Table 76-1). TABLE 76-1 Common Babesia Species, Vectors, and Distribution ?, Association as a tick vector has not been proved but is suspected. aSome B. gibsoni isolates are larger and have a heterogenous appearance resembling B. canis, so PCR testing gives the most reliable differentiation. bTheileria annae and Babesia annae have been proposed as names for this organism.42,260 cAlso includes B. pantherae, which has been isolated from a leopard cat (Panthera pardus) in Kenya, and B. leo. dPeople are thought to be accidental hosts for babesias of reservoir animal hosts (e.g., B. microti [rodents], B. divergens [cattle]). Canine babesiosis is a disease of worldwide importance. Early descriptions of intraerythrocytic parasites in dogs with signs consistent with babesiosis were made in Africa in 1896, and the first documented case of canine babesiosis in the United States was in 1934. Initially, two species of Babesia infecting dogs were recognized; however, at least nine genetically unique canine piroplasms have been described (see Table 76-1). The observed species of Babesia in dogs varies with geographic region and has been subject to change due to movement of infected animals, movement of tick vectors, and reclassification with evolving diagnostic techniques. The understanding of the true geographic range of nearly all canine Babesia spp. is probably incomplete and limited by lack of reporting and surveys that do not use microscopic, serologic, and molecular techniques. As newer species have been recognized, it has become more important for the clinician to understand their differences in diagnosis, prognosis, and treatment for each species. Although Babesia spp. share some common features, the biology, virulence, and pathophysiology associated with each organism can vary widely. Therefore, information gained from the study of one Babesia sp. may not be directly applicable to others. Feline babesiosis has not been studied as extensively as the canine disease. Only a few feline Babesia organisms have been characterized at the genetic level, which brings much of the proposed phylogeny and nomenclature under question. Babesia felis is a small, highly pathogenic strain that infects domestic cats in southern Africa and the Sudan.187 Infection of domestic cats primarily has been identified in the strip along the coast of South Africa.113,188 The other small species, Babesia cati, is less pathogenic and found primarily in India, but no genetic characterization has ever been reported. Another small piroplasm (Babesia leo) similar to but serologically and genetically distinct from B. felis, was isolated from lions (Panthera leo) in Kruger National Park.146 B. leo DNA was amplified from a single domestic cat in a molecular survey.33 A small piroplasm was visualized in blood smears from feral cats in Rio de Janeiro, Brazil, but no molecular data were available to confirm the identity of these organisms.165 Genetic sequences, most similar to Babesia canis canis and a Babesia microti–like parasite, have been amplified from the blood of three infected cats with retroviral infections from Spain and Portugal, but no organisms were visualized. Unfortunately, these sequences were from a fairly conserved region of the 18S ribosomal RNA gene, so the exact identity of the B. canis species is in question.57 Another Babesia sp., B. canis presentii, was identified in two cats in Israel.15 One of these cats was clinically ill and was co-infected with feline immunodeficiency virus. Another B. canis–like parasite has been identified in a naturally infected cat from Poland.7 A parasite genetically similar to B.canis vogeli was found in stray cats in metropolitan Bangkok, Thailand.209a Babesia herpailuri and Babesia pantherae are large Babesia organisms of wild Felidae in Africa and have been transmitted experimentally to the domestic cat.133 No cases of feline babesiosis have been reported in the United States. Babesia spp. can be transmitted in nature via the bite of the tick vector (Fig. 76-1) or directly between vertebrate hosts.50 While the tick is feeding, sporozoites are released from its salivary glands, and they enter the bloodstream of the vertebrate host. They then attach to and are endocytosed by erythrocytes. Once they are in the erythrocytes, they undergo asexual reproduction and merogony, and the daughter cells can infect new erythrocytes. Infected erythrocytes are ingested by a naïve tick. It is unclear whether or not the transformation from merozoite to gamete (gametocyte) begins in the vertebrate host or in the tick. In the tick midgut, the sexual phase of reproduction occurs when the gametes fuse to form a zygote. The zygote invades the epithelial cell of the tick gut, and an asexual form of reproduction, sporogony, occurs (see Fig. 76-1). The resultant forms, ookinetes, leave the epithelial cell and invade either the salivary gland or the ovary, where they participate in transstadial and transovarial transmission, respectively. In most parts of the world, tick vectors are the most important means of transmission. However, for some Babesia species, such as Babesia gibsoni in North America and Europe, it is believed that non–vector-associated transmission is the primary means of infection. Direct transmission between dogs, via fighting contact or via congenital transplacental transmission, is believed to be the most common route of transmission for B. gibsoni where competent tick vectors are absent.3,70,70 Variations in modes of transmission in these regions could result in different clinical outcomes. Because sexual reproduction of Babesia spp. occurs in the tick, it has been proposed that non–vector-associated transmission can lead to the expansion of genetically similar clonal infections and, therefore, potential widespread drug resistance in a population of animals.258 B. canis is the most common large Babesia sp. and has three distinct subspecies: B. canis vogeli, B. canis canis, and B. canis rossi. There are characteristic differences in genotype, geographic distribution, pathogenicity, and vector specificity, and some have proposed that these are in fact three distinct species: B. vogeli, B. canis, and B. rossi.233 B. canis vogeli is transmitted by the brown dog tick (Rhipicephalus sanguineus) and shares its nearly worldwide distribution. It is most commonly diagnosed in warm, humid regions of the world, and the disease has been diagnosed throughout the year in endemic regions. The presence of B. canis vogeli has been confirmed by DNA sequencing in Africa, Asia, Australia, Europe, and the Americas.* In the United States, it is most commonly diagnosed in the southern regions. B. canis vogeli reported prevalence has ranged from 3.8% to 59%.222 The prevalence of serum antibody reactivity is higher in adult dogs than in dogs younger than 1 year.30 In an antibody serosurvey of dogs in Florida, 46% of 393 greyhounds had positive test results.222 The prevalence of dogs with reactive antibody titers within these kennels ranged from 17% to 100%; the lower prevalence was noted in kennels with more intensive tick control. None of 50 non-greyhound, individually housed adult pet dogs within the same geographic area had reactive serum antibody titers, implicating environment and breed susceptibility as factors in determining seroprevalence in endemic areas.222 Outbreaks may occur and are often localized to a relatively small geographic area or to an individual kennel. Veterinarians in one practice may see affected dogs often, whereas neighboring practices in the same area may not see any affected dogs.220 Transplacental transmission of B. canis vogeli infections is strongly suspected but unproven in an experimental setting.78 B. canis canis is transmitted by the ornate cow tick, Dermacentor reticulatus, but there is some molecular evidence that R. sanguineus may also be a vector.48 The presence of B. canis canis has been confirmed in Europe and Africa by DNA sequencing.* The incidence of infection is highest in the fall and spring. Infection with this subspecies is most commonly diagnosed in France with 45% to 70% of practices reporting confirmed infections each year. Higher prevalence rates are most commonly found in rural or suburban areas that are adjacent to prairies or woodlands that provide suitable habitat for D. reticulatus.34 Infections are being reported more frequently from other European countries such as Croatia, Poland, and Germany, and this may be associated with changes in the distribution of D. reticulatus.265 There is one report of infection of a dog in Norway.179 Outbreaks of B. canis canis infection in Polish sled dogs share many similarities with those of B. canis vogeli infection in North American greyhounds.246 B. canis rossi is transmitted by the yellow dog tick, Haemaphysalis elliptica (formerly Haemaphysalis leachi).13 Reports of B. canis rossi infection have been limited to the African continent, with the vast majority of reports coming from South Africa, where over 10% of the dogs evaluated in some veterinary hospitals may be affected.51,207 The incidence of infection is highest during the summer months. Breed predispositions for infection have not been well studied, but the traditional fighting breeds (American pit bull terriers, Staffordshire bull terriers and bull terriers) are more likely to die when they are diagnosed with “severe” babesiosis.195 A large Babesia sp. has been isolated from dogs in North America that have been splenectomized or that were undergoing chemotherapy for cancer. The infection has been confirmed by DNA sequencing of isolates from dogs in North Carolina, New Jersey, New York, and Texas.29,90,90 A tick vector has not been identified. A different novel large Babesia sp. was reported in a dog from Great Britain that never traveled outside of that country. The dog died as a result of infection, and no tick vector was identified.89 B. gibsoni is transmitted by Haemaphysalis bispinosa and Haemaphysalis longicornis.219 There is limited evidence that R. sanguineus can be a potential vector, but transmission has never been convincingly demonstrated.85–88,206 Infections with B. gibsoni occur throughout the world, and the insidious nature of this infection has allowed the inadvertent transport of infected dogs from Asia to other areas. The presence of B. gibsoni has been confirmed, by DNA sequencing, in Africa, Asia, Australia, North and South America, and Europe.11,116,122,155,230 Depending on the availability of suitable vectors, there appear to be two distinct epidemiologic scenarios for B. gibsoni: tick transmission and direct transmission between dogs.126,157 In its original endemic area of Asia, the geographic range of B. gibsoni correlated with that of the vector tick, H. bispinosa,133 and tick infestation was a risk factor for infection in nonfighting breeds. In regions such as the United States where competent tick vectors are not endemic, B. gibsoni infections have been mostly limited to fighting breeds.21,157 In the fighting breeds, transmission has been associated with a fight or bite by an infected dog or having been born to an infected bitch. There has been strong experimental evidence for perinatal transmission.3,70,70 However, an increased prevalence of infection has even been noted in fighting breeds in areas such as Japan, where Haemaphysalis spp. are endemic. Some confusion exists, as this organism has been referred to by several names, including B. gibsoni, B. gibsoni (USA), “the California isolate,” and the “western piroplasm.”121 B. conradae has only been isolated from dogs in southern California, and tick vectors have not been identified.121,123 Transmission studies attempting to prove vector competence for R. sanguineus and Dermacentor variabilis were unsuccessful or inconclusive.254 Various stages of the parasite were found in the salivary glands of engorged R. sanguineus; however, infection could not be transovarially or transstadially transmitted to other dogs.254 The author of this chapter (AJB) has identified R. sanguineus as well as soft-bodied ticks, Ornithodoros coriaceus, that were removed from B. conradae infected dogs. A B. microti–like parasite (also referred to in the literature as Babesia annae or Theileria annae) has been identified in domestic dogs from Europe and North America. The majority of these dogs have lived in or traveled to the northwestern region of Spain.41,42,75,260 The report in a North American domestic dog involved a single American pit bull terrier confiscated from a suspected dog-fighting operation.258 A high percentage of European and North American foxes are infected with a parasite that is genetically identical to the this B. microti–like parasite.19,56 A tick vector has not been definitively identified, but in Spain an association between Ixodes hexagonus infestation and B. microti–like infection has been observed.44 The pathogenicity of Babesia organisms is determined primarily by the species and strain involved.198,233,233 Host factors, such as the age of the host and the immunologic response generated against the parasite or vector tick, are also important.235 Infected erythrocytes incorporate parasite antigens into their surface and induce host opsonizing antibodies, which leads to removal of infected erythrocytes by the mononuclear-phagocyte system. Additionally soluble parasite antigens may adhere to the surface of some noninfected erythrocytes and platelets. This may lead to their opsonization by antibodies, with or without complement, and account for the hemolytic anemia and thrombocytopenia that is often not correlated with the level of parasitemia. Hosts may develop anti–erythrocyte membrane antibodies against self antigens and have increased erythrophagocytic activity of macrophages, which can contribute to the immune-mediated anemia.* Splenectomy makes the parasitemia and resultant anemia more severe.43 In addition to immune-mediated destruction, multiple mechanisms appear to account for the hemolysis seen with babesiosis. Parasitemia results in osmotically fragile erythrocytes, hemolysis, and subsequent anemia.148 Direct parasitic injury during penetration and occupation of the cell contributes to the hemolytic process. Serum from infected dogs inhibits erythrocyte 5′-nucleosidase, which can lead to the accumulation of cyclic nucleotides and may contribute to erythrocyte damage.93 Oxidative stress is another possible cause of damage to erythrocytes that also results in increased susceptibility to phagocytosis.176 Increased production of superoxide has been demonstrated in erythrocytes infected with B. gibsoni, which may relate to oxidative damage from lipid peroxidation.181 Increased urinary methemoglobinemia levels, as a result of hemoglobin oxidation followed by hemolysis, have been found in dogs with naturally occurring B. canis infections.144 Lipid peroxidation occurring during Babesia infection also increases rigidity of parasitized and nonparasitized erythrocytes and slows their passage through capillary beds. Soluble parasite proteases activate the kallikrein system and induce fibrinogen-like protein formation. The fibrinogen-like proteins make erythrocytes more “sticky,” leading to additional erythrocyte sludging in the capillaries. Vascular stasis from sludging of parasitized erythrocytes and their stroma within capillary beds is thought to contribute to acute anemia and many of the other potential clinical signs. The most severe sludging appears to occur in the central nervous system (CNS) and muscles.250 Thrombocytopenia alone is observed in many cases of babesiosis and may relate to immune or coagulatory consumption of platelets from hemolytic or vascular injury. Despite severely decreased platelet counts, bleeding is very rarely observed in dogs with babesiosis alone, and other abnormal coagulation test results are uncommon.73 However, overt disseminated intravascular coagulation (DIC) can be a devastating complication of the severe forms of canine babesiosis caused by B. canis rossi. Babesia proteases may induce increases in plasma kallikrein levels, which can activate the intrinsic cascade at factor XII. Tissue hypoxia is an important contributor to many of the clinical signs caused by the most pathogenic Babesia strains. Causes of hypoxia in dogs infected with Babesia include anemia, shock, vascular stasis, excessive endogenous production of carbon monoxide, parasitic damage to hemoglobin, and decreased ability of hemoglobin to off-load oxygen.108,144 Hypoxia appears to be more important than hemoglobinuria in damaging the kidneys of experimentally infected dogs.145 Lactic acid generation from tissue hypoxia is considered the main reason for metabolic acidosis that develops in animals with babesiosis.136 Respiratory alkalosis from hyperventilation results partly in the body’s attempt to compensate for metabolic acidosis, but more directly for hypoxemia. Many atypical signs or complications, unrelated to hemolysis, can develop in animals with babesiosis caused by virulent B. canis rossi and B. canis canis infections. Both of these organisms can induce an profound systemic inflammatory response.129 A syndrome similar to septic shock has been described in a low percentage of dogs infected with a large Babesia species presumed to be B. canis canis.152 Resultant tissue damage from the infection probably causes the release of cytokines, which support widespread inflammation and additional damage to multiple organs.108 Multiple organ dysfunction syndrome complications resulting from the systemic inflammatory response syndrome (SIRS) (see Chapter 36) include acute renal failure, hepatopathy, immune-mediated hemolysis, pulmonary edema, rhabdomyolysis, and cerebral dysfunction.247 Pulmonary, CNS, and renal complications were associated with a higher rate of mortality. These atypical signs or complications are almost never reported with infections by B. gibsoni or B. canis vogeli, suggesting differences in their virulence or pathogenesis. Other possible complications include membranoproliferative glomerulonephritis, which may have an immune-mediated pathogenesis.190,217,217 Azotemia and proteinuria are common in dogs infected with B. microti–like parasites, and their presence has been associated with increased mortality.41 The clinical manifestations of Babesia infections are difficult to generalize. The spectrum of clinical signs can vary as much within the same species of Babesia as it does among Babesia species (Tables 76-1 and 76-2). Dogs infected with typically “avirulent” species such as B. canis vogeli may have severe clinical disease, and dogs infected with typically “virulent” species such as B. canis rossi may be subclinically infected without overt clinical or laboratory findings. However, some features that should routinely trigger clinicians to suspect babesiosis include fever, thrombocytopenia, hemolytic anemia, and splenomegaly. The fever often waxes and wanes and may not be present during the initial examination. Dogs often have nonspecific signs such as lethargy, anorexia, and weakness. Occasionally owners will note jaundice, pale mucous membranes (Fig. 76-2), or discoloration of the urine caused by bilirubinuria or hemoglobinuria. TABLE 76-2 Clinical Findings in Dogs with Babesiosis Data from Ref. 220. Because of similarities in their pathogeneses, canine babesiosis has been characterized using clinical severity schemes developed for malaria.108 In a contemporary classification, babesiosis has been categorized as being either “uncomplicated” or “severe.”107 Animals with uncomplicated babesiosis have the typical clinical signs of hemolysis, including fever, anorexia, depression, pale mucous membranes, splenomegaly, and “water-hammer” pulses. Severe babesiosis is characterized by complicating factors such as acute renal failure, CNS dysfunction, coagulopathy, icterus and hepatopathy, immune-mediated hemolytic anemia (IMHA), pulmonary edema, hemoconcentration (“red biliary”), and shock. Although this scheme is pertinent to infections caused by B. canis rossi, and to a lesser degree B. canis canis, it is not useful for those caused by B. gibsoni and B. canis vogeli. This is because the vast majority of signs/features of “severe” babesiosis have not been described or recognized with the North American Babesia spp. Additionally, this terminology can be confusing because so-called uncomplicated babesiosis can still be life-threatening. In Spain, disease caused by the B. microti–like agent is associated with pale mucosae, weakness, hemoglobinuria, tachycardia, tachypnea, and elevated rectal temperature caused by regenerative hemolytic anemia and thrombocytopenia that in 30% to 40% of cases were accompanied by renal failure.42,43,77,260 Nonregenerative anemia, azotemia, and proteinuria with high urine protein/creatinine ratios were found in animals with renal failure.41 As with B. canis canis, the range of clinical signs with B. canis rossi is highly variable. The majority of dogs have uncomplicated babesiosis and can be treated as outpatients. However, up to 31% of the dogs examined at a university clinic required hospitalization, and approximately 10% of those hospitalized dogs did not survive.195 Many of these hospitalized dogs have “severe” babesiosis (see Severe or Complicated Babesiosis, next). The development and severity of many clinical or laboratory abnormalities often correlate with a greater degree of parasitemia; however, there is substantial overlap in the degree of parasitemia between survivors and nonsurvivors.31 Rare complications include gastrointestinal (GI) disturbances, myalgia, ocular involvement, upper respiratory signs, cardiac involvement, necrosis of the extremities, and fluid accumulation. Overlap among the different clinical categories and complications can also occur. These include signs of anuria or oliguria despite adequate rehydration, but these are uncommon complications. Although evidence of renal damage, reflected in urinalysis findings by the presence of proteinuria, casts, and renal tubular epithelial cells, is common in complicated and uncomplicated cases, it does not necessarily predict renal failure. An elevated serum urea concentration alone is an unreliable indicator of renal insufficiency in animals with babesiosis, because a disproportionate rise in serum urea (compared with creatinine) concentration has been related to catabolism of lysed erythrocytes.59 Renal failure is suspected on the basis of hyposthenuria or isosthenuria of prehydration urine, if it is obtainable, or with reduced or absent urine production after rehydration. After rehydration and diuresis, the subsequent urine volume produced and the degree of azotemia can offer more definitive confirmation. Acute intrinsic renal impairment without overt acute renal failure (ARF) occurs in humans with malaria—a clinical situation very similar to canine babesiosis. Renal impairment has been observed in dogs with B. canis rossi infections.145 ARF was also documented in numerous dogs in the same study. Cerebral babesiosis is defined as the concurrent presence of neurologic signs in an animal with babesiosis. Hypoglycemia, which has been documented in severe B. canis rossi infections, or other unrelated causes of CNS dysfunction should be ruled out and treated, when possible, before arriving at a diagnosis of cerebral babesiosis. The signs, typical of peracute onset, include a combination of incoordination, pelvic limb paresis, muscle tremors, nystagmus, anisocoria, intermittent loss of consciousness, seizures, stupor, coma, aggression, paddling, or vocalization.108 Pathologic changes in the brain that cause these signs are congestion, macroscopic and microscopic hemorrhages, sequestration of parasitized erythrocytes in capillary beds, and pavementing of parasitized cells against the endothelium (see the earlier discussion under Pathogenesis). The most consistent hemostatic abnormality in complicated and uncomplicated cases of babesiosis is profound thrombocytopenia; however, clinically apparent hemorrhages are relatively rare. DIC has been reported in animals with babesiosis; however, complete confirmation of DIC in animals with babesiosis may be difficult because of the nature of the underlying disease process and the reported unreliability of the human fibrin degradation product test for evaluating canine specimens.108 Clinical signs of DIC are difficult to recognize until hemorrhages develop in the hypocoagulable phase. In the hypercoagulable phase, signs are related to microthrombus-induced organ dysfunction. In some cases of babesiosis, elevated bile acid levels develop, which may indicate hepatic dysfunction.168 Serum liver enzyme activities may be increased in severe disease. Whether the insult is caused by inflammatory cytokines, hypoxic damage, or a combination of these is not known. In some cases icterus appears disproportionate to the degree of hemolysis or hepatic outflow obstruction. Therefore, hepatocellular dysfunction appears to be at least contributory. Histologic changes usually associated with icterus include diffuse and periportal lesions, whereas icteric dogs with babesiosis have a centrilobular lesion. However, it is possible that the liver has a diffuse, mild or moderate lesion that does not cause histologic changes but is severe enough to cause a functional change. Hypoxic insults are known to cause diffuse hepatocellular swelling; thus the hypoxia in severe babesiosis may be severe enough to cause a transient hepatopathy. IMHA is an increased destruction of erythrocytes caused by erythrocyte-membrane–associated antibodies. This destruction can either be primary, in which the membrane is normal, or secondary (innocent bystander), in which the membrane is altered by the parasite or its antigens and recognized as foreign. Secondary destruction is assumed to occur in babesiosis.229 The cardinal feature of babesiosis-associated IMHA is continuing hemolysis despite successful antibabesial treatment. Diagnosis is suspected by finding autoagglutination with saline dilution of blood, detection of spherocytosis, or both. Coombs’ test cannot be used to confirm whether primary erythrocyte autoantibody is responsible because both primary and secondary forms with IMHA have a positive test result. Acute respiratory distress syndrome (ARDS) is a severe and frequent catastrophic complication of babesiosis. Typical clinical signs are a sudden increase in respiratory rate (which may be caused by other factors, such as pyrexia and acidosis), dyspnea, moist cough, and blood-tinged frothy nasal discharge. The diagnosis of ARDS is based on the presence of diffuse pulmonary infiltrates on thoracic radiography, hypoxemia from ventilation-perfusion mismatch, normal pulmonary capillary wedge pressure, and reduced pulmonary compliance.65 In most clinical situations, pulmonary wedge pressure, blood-gas analysis, and compliance cannot be measured. Thus, diagnosis depends on the recognition of risk factors for ARDS, thoracic radiographs, and exclusion of other causes of pulmonary edema, particularly cardiogenic causes and fluid overload. Excluding fluid overload is particularly important in animals with oliguric renal failure. Fluid loads that can be tolerated by clinically healthy dogs may fatally exacerbate pulmonary edema in dogs with ARDS. The paradoxical phenomenon of severe intravascular hemolysis combined with hemoconcentration constitutes the syndrome “red biliary.” The clinical features are congested mucous membranes, visible hemoglobinemia, hemoglobinuria, or all of these and high-reference-range or elevated hematocrit (HCT) levels.108 Hemoconcentration has been associated with other complications, such as cerebral babesiosis, DIC, ARF, and ARDS. Hemoconcentration in babesiosis is thought to be a result of reduction in blood volume as a result of fluid shifts from the vascular to the extravascular compartment. Because plasma protein concentrations are within reference limits, plasma—rather than a filtrate of plasma—shifts from the vasculature. The widespread increase in capillary permeability that occurs in SIRS may play an important role in the pathogenesis. Concurrent hypoalbuminemia may relate to a loss of albumin into the interstitium because of lost endothelial integrity associated with SIRS. Dogs with severe and complicated babesiosis are frequently in a state of collapse and shock. This can resemble the hyperdynamic phase of septic shock (see Sepsis, Chapter 36). In a study, it was shown that hypotension occurs frequently in dogs with babesiosis, and the presence and severity of hypotension increase with increased disease severity.111 The presence of hypotension in a large proportion of dogs with complicated babesiosis is consistent with the hypothesis that inflammatory mechanisms play a major role in this disease and can result in a sepsis-like state. It is likely that hypotension in animals with babesiosis is a combination of vasodilation, reduced vascular volume caused by increased vascular permeability, dehydration, or all of these and myocardial depression. Hypotension can play a role in the pathophysiologic symptoms of the disease because it has been hypothesized to facilitate parasite sequestration. A syndrome similar to septic shock was diagnosed in Croatian dogs with babesiosis presumptively caused by B. canis canis.152 In one study, dogs with complicated and concurrent IMHA and babesiosis had significantly higher serum cardiac troponin I and T concentrations.141 In this study, dogs with babesiosis developed important electrocardiographic changes such as heart blocks, ventricular premature complexes (VPCs), and prolonged QRS changes and ST segment changes. However, most of the changes were not associated with severity, outcome, and cardiac troponin levels. The exception was the presence of VPCs, because a correlation was found between serum troponin concentrations and VPCs. Cardiac histologic changes reported in the study were hemorrhage, necrosis, inflammatory infiltrate, and fibrosis. A retrospective study reported acute pancreatitis as a complication of canine babesiosis.169 The prevalence of pancreatitis in dogs with babesiosis was 1.8% compared to 0.04% of other hospital cases. In addition to pancreatitis, 80% of dogs had other babesial complications, namely icterus, ARDS, IMHA, renal failure, hemoconcentration, and cerebral syndrome. In this study, 4 dogs had histologic evidence of pancreatitis, and another 16 dogs had serum amylase elevations, lipase activity elevations, or both, in a magnitude that supported a diagnosis of acute pancreatitis. The median time of diagnosis was 2.5 days postadmission, with primarily young (median age 3 years), sexually intact dogs being affected. The development of pancreatitis was unrelated to the degree of anemia at time of admission. Acute pancreatitis may represent the previously reported “GI form” of babesiosis. Dogs with severe B. canis rossi infection have an arterial pH that varies from acidemia to alkalemia.136 A high anion-gap metabolic acidosis is present in many dogs, whereas almost all have concurrent metabolic acidosis and respiratory alkalosis. The severity of these abnormalities could not be linked to clinical outcome. Lactic acidemia is common in hospitalized cases. Persistent lactate concentrations above 40 mg/dL are a poor prognostic indicator for survival. Reports of clinical infection in domestic cats have been predominantly from South Africa. Cats with naturally occurring babesiosis usually are younger than 3 years and have no breed or sex predilection. Affected cats generally have lethargy, anorexia, weakness, a rough haircoat, or diarrhea.113 Fever and icterus are less common. Anemia can be severe and is the underlying reason for the clinical signs. The disease is chronic, and signs may not be apparent until a later stage of illness. Cats usually adapt to the anemia and may have only mild clinical signs until they experience the stress of a physical examination or diagnostic evaluation.170 Complications of the hemolytic anemia included hepatopathy, pulmonary edema, renal failure, CNS signs, and concurrent infections. The primary rule outs for hemolysis typical of acute uncomplicated babesiosis are hemolytic states such as metabolic, parasitic, immune-mediated, oxidative, osmotic, and traumatic insults to erythrocytes. Compared to these disorders, the clinical pathologic changes of babesiosis are nonspecific; the primary hematologic abnormalities are anemia and thrombocytopenia.2,103,162,180 The prevalence of thrombocytopenia is higher than that in dogs with ehrlichiosis, and thrombocytopenia is generally a feature of canine babesiosis, regardless of whether concurrent anemia is present.231 A mild, normocytic, normochromic anemia is generally noted in the first few days after infection, and the anemia then becomes macrocytic, hypochromic, and regenerative as the disease progresses. The reticulocytosis is proportional to the severity of the anemia later in the disease process. Uncommonly with B. canis rossi infections, a relative polycythemia may be noted with plasma protein concentration within reference limits.108 Leukocyte abnormalities are inconsistently observed but may include leukocytosis (with or without a left shift), neutrophilia, neutropenia, lymphocytosis, eosinophilia, or leukopenia.103,180 Studies of European dogs, presumably infected with B. canis canis, reported a high proportion (36% to 74%) of those with neutropenia.74,266 A leukemoid response similar to that observed with IMHA is occasionally seen.140 Autoagglutination of erythrocytes in saline was noted in 21% of 134 dogs with babesiosis in one study, and almost 85% of infected dogs had positive direct antiglobulin (Coombs’) test results in another.108 There are no pathognomonic biochemical or urinalysis findings in dogs with babesiosis. Common biochemical findings observed in North American dogs with babesiosis include hyperglobulinemia and mildly increased liver enzyme activities and less commonly hyperbilirubinemia. Hyperbilirubinemia is a consistent finding during acute disease caused by B. canis canis and B. canis rossi but not by B. gibsoni.103,240 Hemoglobinemia is a relatively rare finding in dogs in North America and is most commonly documented in dogs infected with B. canis rossi. Some dogs will have pigmenturia consisting most commonly of bilirubin, and occasionally hemoglobin. Urinalysis abnormalities may include bilirubinuria, hemoglobinuria, proteinuria, and, in rare cases, granular casts. Hypokalemia may be found in severely affected animals but is probably nonspecific because of decreased potassium intake. Hyperkalemia and hypoglycemia were noted in severe B. canis rossi infections in one study.103 The hypoglycemia of Babesia infected dogs was not associated with increased insulin concentrations.193 Dogs with babesiosis caused by B. canis rossi have low total serum protein and albumin levels, albumin/globulin ratios, and α-globulin levels. Dogs with B. canis infections also have an acute-phase response characterized by elevated α1-acid glycoproteins,143 C-reactive protein, and serum amyloid A. C-reactive protein concentrations, however, were not useful in predicting the outcome of B. canis rossi infections. A study of dual infections with B. canis and Ehrlichia canis showed that the prevalence of hyperglobulinemia was higher in dogs with dual infections than in dogs with a single infection caused by either organism.162 Azotemia and metabolic acidosis are common in dogs infected with B. canis rossi that have severe intravascular hemolysis and appear to contribute to morbidity and mortality. In some dogs, the increase in serum urea does not correlate with decreased glomerular filtration rates. Therefore, serum creatinine is preferable to serum urea nitrogen concentrations to assess glomerular filtration rate in dogs with babesiosis.197 In B. canis rossi infections, anemic dogs were more likely to have increased hepatic enzyme activity and profound leukocytosis with a left shift.195 Nonanemic dogs with B. canis rossi infection may have severe azotemia, marked electrolyte changes, and, in some cases, leukopenia. In feline babesiosis, which is caused by B. felis, the anemia is typically macrocytic, hypochromic, and regenerative.205 No characteristic change in total or differential leukocyte counts occurs, and thrombocytopenia is an inconsistent finding. As with dogs, the saline-diluted blood agglutination test result may also be positive.205 Cats infected with B. felis typically have elevated serum hepatic aminotransferase enzyme activity and total bilirubin concentrations. Serum protein values are usually within reference limits, but polyclonal hyperglobulinemia can occur. Renal parameters are unaffected. Although various electrolyte abnormalities were reported, no consistent pattern has been found.205
Babesiosis
Etiology
Species
Geographic Distribution
Typical Morphologic Characteristics
Recognized Tick Vectors
Clinical Findings
CANINE
Babesia canis vogeli
Africa, Asia, Australia, Europe, North and South America
Large (2.4–3 × 4–7 µm), single or paired piriform bodies
Rhipicephalus sanguineus
Fever, thrombocytopenia, hemolytic anemia, mild disease with inapparent clinical signs; more severe in young animals
B. canis canis
Europe
Large (2.4–3 × 4-7 µm), single or paired piriform bodies
Dermacentor reticulatus
Hemolytic anemia and fever
B. canis rossi
Africa
Large (2.4–3 × 4–7 µm), single or paired piriform bodies
Haemaphysalis elliptica
Highly virulent hemolytic or immune disease
Babesia (unnamed large species)
North America
Large (2.5 × 5 µm)
Unknown
Thrombocytopenia, hemolytic anemia, leukopenia, pigmenturia
Babesia (unnamed large species)
Great Britain
Large (2 × 4 µm)
Unknown
Thrombocytopenia, hemolytic anemia, leukopenia, pigmenturia
B. gibsoni
Africa, Asia, Australia, Europe, North and South America
Small (1–2 × 3–4 µm), usually single annular bodies (signet rings)a
Haemaphysalis bispinosa,
Haemaphysalis longicornis
Thrombocytopenia, hemolytic anemia chronic infection with weight loss and debilitation or chronic subclinical infection
B. conradae
California
Small (1 × 2.5 µm), usually single; occasional Maltese crosses
Unknown (suspect wildlife reservoir)
Hemolytic anemia, vomiting
B. microti–likeb
Northwestern Spain
Small (1 × 2.5 µm), usually single
Ixodes hexagonus?
Severe hemolytic anemia, eosinophilia, some develop renal failure
B. caballi
Croatia
Unknown (not described)
Unknown
Unknown (molecular survey data only)
T. annulata
Spain
Unknown (not visualized)
Unknown
Unknown (molecular survey data only)
T. equi
Spain and Croatia
Unknown (not described)
Unknown
Unknown (molecular survey data only)
FELINEc
B. felis
Africa, southern Asia, Europe
Small (0.9 × 0.7 µm), single or paired annular bodies
Unknown
Hemolytic anemia with chronic course; seen in domestic cats in South Africa
B. canis sp.
Europe
Unknown (not visualized)
Unknown
Unknown (molecular survey data only)
B. cati
Indian subcontinent
Small (1 × 1.5 µm), single or paired annular bodies
Unknown
Isolated from Indian wildcat (Felis catus)
B. canis presentii
Israel
Small (2.7 × 1.7 µm) round to oval- or ring-shaped
Unknown
Profound anemia and icterus or subclinical
HUMANd
B. microti
North America: northeastern United States and Great Lakes region; Europe
Small, pleomorphic bodies
North America: Ixodes scapularis;
Europe: Ixodes trianguliceps, Ixodes ricinus
Hemolytic anemia, fever, chills, mild or subclinical anemia
B. divergens
Europe
Small, pleomorphic bodies
I. ricinus
Hemolytic anemia, more severe than B. microti, often in those who have had a splenectomy
B. divergens–like
North America: Washington state, Missouri (MO1), Kentucky
Small, pleomorphic bodies, high-level parasitemia
Unknown
Hemolytic anemia, more severe than B. microti, often in those who have had a splenectomy
B. venatorum (EU1)
Austria, Italy
Small, pleomorphic bodies, occasional Maltese crosses
Unknown
Fever, hemolytic anemia in those who have had a splenectomy
B. duncani
North America: California, Washington state
Ring forms and tetrads
Unknown
Hemolytic anemia—severe if immunosuppressed or have had a splenectomy
Dogs
Cats
Epidemiology
Geographic Distribution
Life Cycle
Transmission
Specific Epidemiologic Features
Babesia canis
Unnamed Large Babesia spp
Babesia gibsoni
Babesia conradae
Babesia microti–Like Organism
Pathogenesis
Clinical Findings
Dogs
General Features
Babesia Species-Specific Clinical Features
Babesia microti–Like Parasite
Babesia canis rossi
Severe or Complicated Babesiosis
Acute Renal Failure and Renal Involvement
Cerebral Babesiosis
Coagulopathy
Hepatopathy
Immune-Mediated Hemolytic Anemia
Acute Respiratory Distress Syndrome
Hemoconcentration
Hypotension
Cardiac-Related Alterations
Acute Pancreatitis
Acid-Base Disturbances
Cats
Diagnosis
Clinical Laboratory Findings
Dogs
Cats
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine