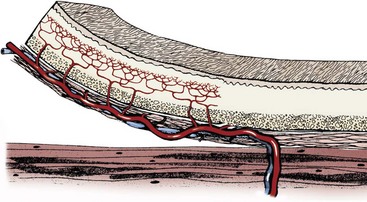
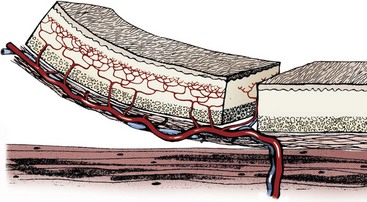
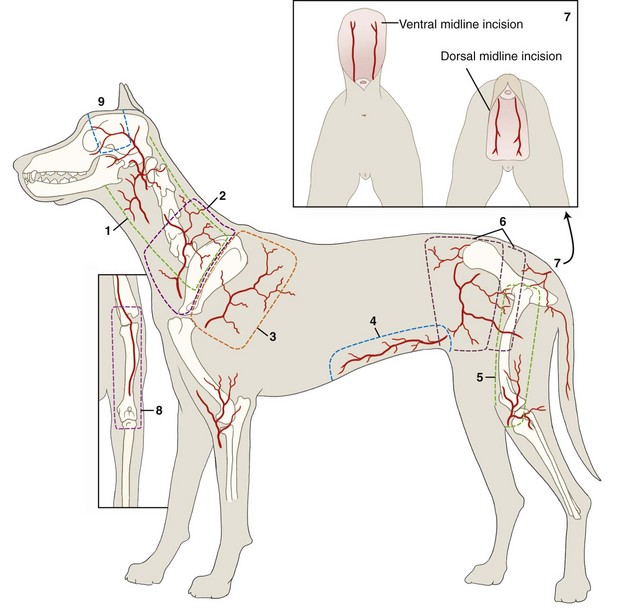
Chapter 79 Axial pattern flaps incorporate a direct cutaneous artery and vein, terminal branches of which supply blood flow and drainage for the subdermal plexus (Figure 79-1). Compared with random or subdermal plexus flaps (see Chapter 78), incorporation of direct cutaneous vessels allows for a larger flap with more consistent survival. A variety of axial pattern flaps have been evaluated in small animal patients; anatomic descriptions of these flaps are listed in Table 79-1. An axial pattern flap can be rotated up to 180 degrees at its base to cover wounds adjacent or distant to the donor site. Rotation greater than 180 degrees may result in venous congestion, leading to flap necrosis. An axial pattern flap can be created as a peninsula or an island. A peninsular flap has intact skin at its base, providing protection to the vascular pedicle during dissection and rotation. The drawback to a peninsular flap is the resulting “dog ear” at its point of rotation. With island flaps, the skin is incised along all edges and the flap is rotated on its vascular pedicle (Figure 79-2). Island flaps provide a more cosmetic appearance because of the absence of a “dog ear”; however, the vascular pedicle is more exposed and can be damaged during dissection or rotation. Composite or compound flaps are specialized forms of an axial pattern flap that include muscle, bone, or cartilage with the overlying skin. A composite flap that is composed of skin and muscle is termed a myocutaneous flap. Composite flaps add bulk for additional protection of underlying tissues. They also provide specific tissue components and may increase local blood supply, potentially improving healing.55 Advantages of axial pattern flaps over other methods of wound closure include the ability to close a large defect without tension, early closure without extended open wound management, coverage of areas with less than optimal wound healing conditions, and excellent flap survival rates.2,55 Many of these flaps are easy to develop and rotate, and no specialized equipment is required. Unlike mesh grafts, axial pattern and myocutaneous flaps can be placed directly over bone, tendons, or ligaments. And unlike long subdermal plexus flaps, they do not require two-stage procedures to enhance circulation. Overall survival rate of axial pattern flaps is 87% to 100%.1,3–6,8,55 Although the tips of the flaps are prone to necrosis, mean survival of axial pattern flaps is at least 50% greater than for subdermal plexus flaps.7 In some patients, cosmesis may be less acceptable than local flaps because of variations in coat length and color. Axial pattern flaps may also be limited for use on distal limb wounds, depending on patient conformation. Another potential disadvantage is regional variability in vascular anatomy. Use of ultrasound and color-flow Doppler can facilitate identification of arterial branches; however, image quality is poor in obese patients. Ease of identification varies with each type of axial pattern flap, which may contribute to higher failure rates of axial pattern flaps in overweight patients. Of direct cutaneous vessels supplying axial pattern flaps, the superficial cervical artery is the most difficult to identify consistently; the caudal superficial epigastric appears to be the easiest to identify.9,10 Cutaneous circulation and location of direct cutaneous vessels in cats are similar to those in dogs. However, comparison of cutaneous angiosomes revealed that dogs have a much higher density of tertiary and higher order vessels than cats, especially on the trunk.65 This translates into less cutaneous perfusion to the uninjured skin in cats.8 The link between tissue perfusion and wound healing is well established; comparative decrease in vascularity may therefore place cats at risk for slower wound healing or greater tissue necrosis with flap development.24,31 Compared with dogs, integument in cats takes longer to heal and has significantly less strength at closure, with lower rates of granulation, epithelialization, and contraction.7,8 At 7 days after wounding, wound strength with first intention healing in cats is half that of dogs.8 In open wounds, granulation tissue takes twice as long to form in cats and begins at the wound edges rather than across the entire bed simultaneously.8 These differences in healing may necessitate altering bandage, drainage, and suturing practices for cats. Cats may require longer care before their traumatic or surgical wounds are strong enough to undertake normal physiologic stress. Removal of the subcutis also reduces the rate of wound epithelialization, particularly in cats.7 Because the subcutis is a major source of precursors for granulation tissue and an important contributor to wound healing, preservation of the subcutaneous tissue when harvesting axial pattern flaps in cats is recommended. Axial pattern flaps allow a much larger segment of skin to be transferred to cover a wound or defect (Figure 79-3) because direct cutaneous vessels increase tissue viability. The primary direct cutaneous axial pattern flaps in dogs and cats are summarized in Table 79-1 and Figure 79-3. The versatility and size of each flap depend on robustness of the respective arterial supply and patient conformation. Distal extremity wounds in patients with long torsos and short legs may be reconstructed using axial pattern flaps.55,59 Wider flaps may be achieved in lean patients and patients with more loose skin. Boundaries for flap development in the dog and cat are summarized in Table 79-1. An axial pattern flap can be rotated as a peninsula or mobilized as an island flap. The middle portion of an axial pattern flap can be tubed to cover a distant, noncontiguous wound; more commonly, a bridging incision is created to connect the donor and recipient beds. Flaps can be cut in an L-shape to cover wide, irregularly shaped wounds. Peninsular flaps are longer than L-shaped, “90-degree” or “hockey stick” flaps, but L-shaped flaps cover wounds with less rotation of the flap pedicle base. The more a flap rotates, the shorter its functional distance becomes and the greater the skin “bunches” or folds at the base. Postoperative analgesia is very important in patients undergoing reconstructive surgery. Skin tension, swelling, and inflammation can decrease flap viability and increase patient morbidity. A multimodal approach (e.g., a nonsteroidal antiinflammatory drug and an injectable opioid) is recommended by the authors to reduce pain and inflammation. Thermal packs can also help reduce swelling when applied properly. Cool packs (15° to 20° C [ i.e., tap water temperature]) can be applied to the flap base and donor site for the first 36 to 72 hours, and then warm packs should be used for 3 to 5 days. Excessive cold temperatures should not be used because they can result in tissue hypoxia, decreased tensile strength, and prolonged wound healing.36 Axial pattern flaps currently used in dogs and cats are summarized in Table 79-1. The most commonly used axial pattern flaps are the omocervical, thoracodorsal, deep circumflex iliac (superficial or deep branches), and caudal superficial epigastric. The thoracodorsal and caudal superficial epigastric axial pattern flaps are the most robust and versatile of these flaps in dogs and cats. The omocervical axial pattern flap is based on the cervical cutaneous (superficial cervical) branch of the omocervical artery and vein (see Figure 79-3). This flap has been used for head, neck, facial, ear, cervical, shoulder, and axillary defects and experimentally for repair of palatal defects. The omocervical flap has also been advocated for chronic axillary wounds when the thoracodorsal flap is involved in the extent of the lesion.19 The patient is placed in lateral recumbency. Gentle traction is placed on the skin around the forelimb and cervical region while the animal is positioned on the surgical table. A hanging leg preparation is performed with the forelimb loosely extended and slightly abducted. The superficial cervical branches of the omocervical artery and vein originate at the level of the prescapular lymph node and course cranial to the scapula in a dorsal direction (Figure 79-4, A). The caudal incision begins at the level of the acromion and courses dorsally along the scapular spine. The distance between the cranial edge of the scapula (cranial shoulder depression) and the scapular spine is measured. This measurement is used to identify a point cranial and equidistant to the cranial edge of the scapula. The cranial incision starts at this point and extends parallel to the caudal incision (see Figure 79-4). Cranial and caudal incisions can be connected on dorsal midline to form a rectangle; alternatively, an inverted L-shaped configuration can be made, pointing caudally (Figure 79-4, B). The peninsular flap can extend to the level of the contralateral scapulohumeral joint; however, the L-shaped flap should be kept shorter.47 The omocervical flap can then be pivoted, rotated, and inserted into a bridging incision or tubed to reach the targeted defect (Figure 79-4, C). The omocervical flap has been described in cats with similar landmarks.53 The primary concern with the omocervical axial pattern flap is distal flap necrosis. Blood supply to the omocervical axial pattern flap is less robust than that of the thoracodorsal artery and supplies a smaller area.47 Additionally, landmarks for the cutaneous branch of the omocervical artery are less consistent and appear harder to find, even with color-flow Doppler.51 Use in palatal defects may be limited by flap length.13 The thoracodorsal axial pattern flap is based on the cutaneous branch of the thoracodorsal artery and vein (see Figure 79-3). This large flap has been used for thoracic, shoulder, forelimb, and axillary defects.47 The patient is positioned in lateral recumbency. Gentle traction is placed on the skin around the forelimb and cervical region to relax the skin during patient positioning. A hanging leg preparation is performed, with the forelimb loosely extended and slightly abducted. The thoracodorsal artery and vein originate caudal to the shoulder at the level of the acromion and course dorsally toward the midline (Figure 79-5). The cranial incision begins at the level of the acromion and continues dorsally along the scapular spine. The distance between the scapular spine and the caudal edge of the scapula (caudal shoulder depression) is measured. This measurement is used to identify a point caudal to and equidistant from the caudal shoulder depression. The caudal incision starts at this point and extends dorsally parallel to the cranial incision. Cranial and caudal incisions can be connected on dorsal midline to form a rectangular flap; alternatively, a flap can be developed in an inverted L-shaped configuration, pointing caudally. A peninsular flap can extend to the level of the contralateral scapulohumeral joint; however, an L-shaped flap should be kept shorter. With longer flaps, the opposite cutaneous branch of the thoracodorsal artery and vein may need to be ligated. Complications include seroma formation, edema, distal bruising, infection, dehiscence, and distant flap necrosis. Experimentally, the area of survival of thoracodorsal axial pattern flaps in dogs was reported to be 98%; however, these flaps were not rotated from their wound beds.47 Clinically, results in dogs are not as successful. Partial tip necrosis is reported in up to 70% of dogs when thoracodorsal flaps are used to repair forelimb defects.2 In one study of dogs, the percentage of flap necrosis ranged from 2% to 53% (mean, 21%). Flap loss may result from tension, vascular compromise from rotation, or pressure on the flap by the olecranon during movement or recumbence. In cats, the area of survival for experimental orthotopic and heterotopic thoracodorsal flaps was 98%.54 Thoracodorsal flaps are particularly useful in cats because of excellent skin mobility and small leg-to-trunk ratio. Because of these characteristics, an island thoracodorsal flap allows coverage of the carpus with one-step reconstructive surgery in this species.53 Successful results in cats have also been described with a one-step procedure using a thoracodorsal flap in combination with an omental pedicle graft for management of chronic nonhealing axillary wounds.35 The deep circumflex artery and vein exit the lateral abdominal wall cranioventral to the wing of the ilium and divide into dorsal and ventral branches, both of which can be used for axial pattern flap development (see Figure 79-3). The dorsal deep circumflex iliac axial pattern flap is based on the dorsal branch (Figure 79-6), which is shorter in length than the ventral branch. The dorsal flap can be used for ipsilateral flank, lateral lumbar, pelvic, and lateromedial thigh defects and areas over the greater trochanter.47 The survival area for this flap has not been reported. The patient is positioned in lateral recumbency, with the hindlimb in a relaxed, extended position. During positioning, abdominal and thigh skin is retracted and released to a natural position. The base of the flap is located at the ventral extent of the cranial edge of the wing of the ilium. The caudal incision begins at this level, midway between the wing of the ilium and greater trochanter, and extends dorsally. The distance between the greater trochanter and cranial edge of the wing of the ilium is measured. The cranial incision is parallel to the caudal incision and equidistant to the iliac wing. The flap length can extend to the contralateral paralumbar or flank fold. The incision can divert on dorsal midline cranially to create an L-shaped flap; however, L-shaped flaps should be shorter in length than the peninsular version (see Figure 79-6). Flaps are elevated below the cutaneous trunci muscle, beginning at the distal border of the flap. Long flaps may necessitate ligation of the contralateral dorsal branch of the deep circumflex iliac artery and vein. The ventral deep circumflex iliac axial pattern flap is based on the ventral branch of the deep circumflex iliac artery and vein (see Figure 79-3), which extends down the lateral flank and craniolateral thigh. This flap has been described for repair of lateral abdominal wall and pelvic defects and, as an island arterial flap, can be rotated 180 degrees to cover a sacral defect.47 To preserve leg function and minimize dehiscence, the donor site must be able to be closed with minimal tension. Flap survival percentages have not been reported. The flank fold flap is a derivation of this flap that is positioned more cranially on the thigh.28 Flank fold skin can be used to cover inguinal defects when the caudal superficial epigastric axial pattern flap cannot be utilized.
Axial Pattern and Myocutaneous Flaps
Anatomy
Advantages and Disadvantages
Species Differences
General Considerations For Reconstructive Flaps
Flap Development
Postoperative Care
Specific Axial Pattern Flaps
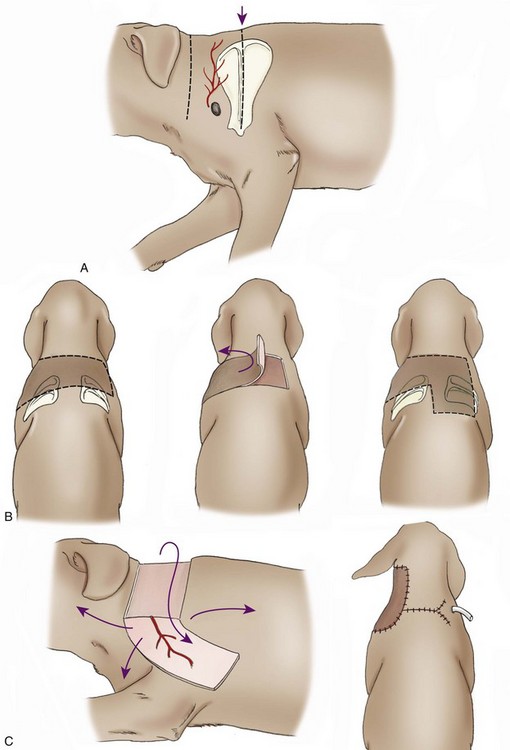
Cervical Cutaneous Branch of the Omocervical Axial Pattern Flap
Surgical Technique
Outcome
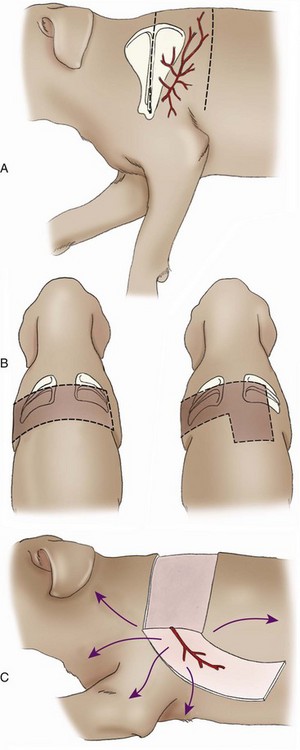
Thoracodorsal Axial Pattern Flap
Surgical Technique
Outcome
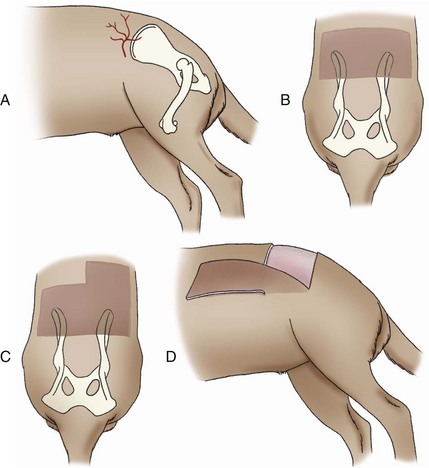
Dorsal Deep Circumflex Iliac Axial Pattern Flap
Surgical Technique
Ventral Deep Circumflex Iliac Axial Pattern Flap
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Axial Pattern and Myocutaneous Flaps
Only gold members can continue reading. Log In or Register to continue