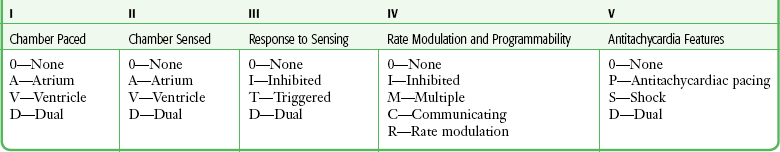
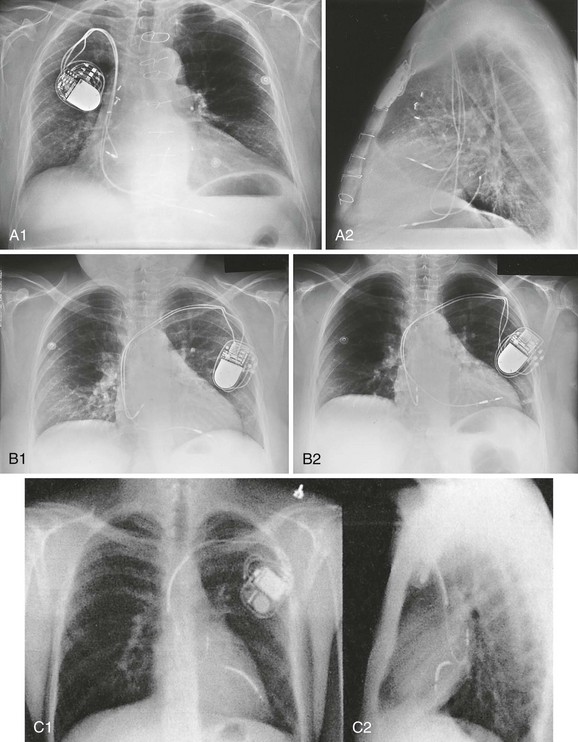
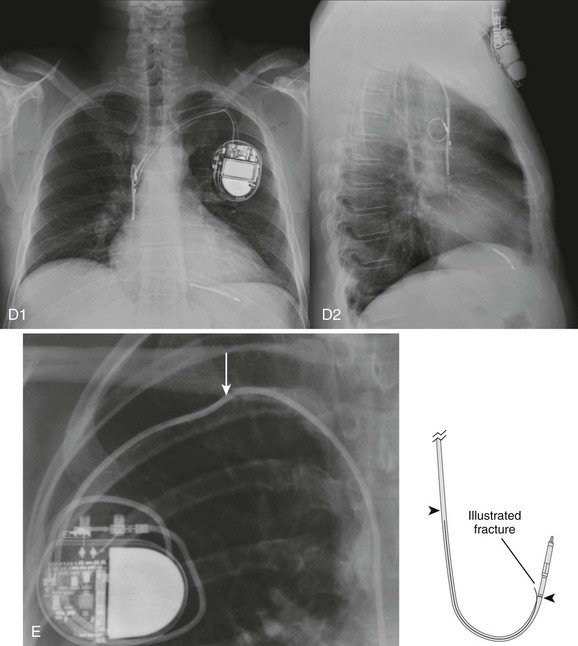
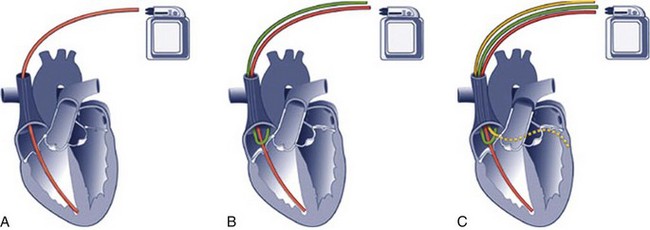
Chapter 13 Patients with implanted pacemakers or automatic implantable cardioverter-defibrillators (AICDs) are commonly seen in the emergency department (ED). Fortunately, the increased reliability of these devices has prevented a marked increase in patients with true emergencies related to device malfunction, but such patients clearly have serious underlying medical problems that must be considered. Pacemaker complications are not uncommon, with rates ranging from 2.7% to 5%.1 Many pacemakers fail within the first year.2 AICD complication rates, including inadvertent shocks, occur in up to 34% of patients with the device.3 The basic evaluation and treatment of patients with cardiac complaints are not substantially different in patients with pacemakers and AICDs than in those without. However, a general knowledge of the range of problems, complications, and techniques for evaluating or inactivating pacemakers or AICDs is important for emergency clinicians. These devices are complicated, so appropriate consultation may be necessary, depending on the clinical situation. In essence, a pacemaker consists of an electrical pulse–generating device and a lead system that senses intrinsic cardiac signals and then delivers a pulse. The pulse generator is hermetically sealed with a lithium-based battery device that weighs about 30 g and has an anticipated lifetime of 7 to 12 years. A semiconductor chip serves as the device’s central processing unit. The generator is connected to sensing and pacing electrodes that are inserted into various locations in the heart, depending on the configuration of the pacemaker. Newer models are programmable for rate, output, sensitivity, refractory period, and modes of response.4 They can be reprogrammed radiotelemetrically after implantation. Pacemakers are classified according to a standard five-letter code developed by the North American Society of Pacing and Electrophysiology/British Pacing and Electrophysiology Group (Table 13-1). Known as the NBG code, it consists of five positions or digits. The first letter designates the chamber that receives the pacing current; the second, the sensing chamber; and the third, the pacemaker’s response to sensing. The fourth letter refers to the pacemaker’s rate modulation and programmability, and the fifth describes the pacemaker’s ability to provide an antitachycardia function. Whereas standard pacemakers generally do not have an antitachycardia function, AICDs do have this capability and overdrive pacing is the device’s first response to tachycardia. In normal practice, only the first three letters are used to describe the pacemaker (e.g., VVI or DDD).5 Pacemaker wires are embedded in plastic catheters. The terminal electrodes, which may be unipolar or bipolar, travel from the generator unit to the heart via the venous system. In a unipolar system, the lead electrode functions as the negatively charged cathode, and the pulse generator case acts as the positively charged anode into which electrons flow to complete the circuit. The pulse generator casing must remain in contact with tissue and be uninsulated for pacing to occur. In the case of bipolar systems, both electrodes are located within the heart. The cathode is at the tip of the lead, and the anode is a ring electrode roughly 2 cm proximal to the tip. Bipolar leads are thicker, draw more current than unipolar leads, and are commonly preferred because of several advantages, including a decreased likelihood of pacer inhibition as a result of extraneous signals and decreased susceptibility to interference by electromagnetic fields.6 The typical entry point for inserting the leads is the central venous system, which is generally accessed via the subclavian or cephalic vein. The terminal electrodes are placed either in the right ventricle or in both the right ventricle and the atrium under fluoroscopic guidance. Proper lead placement and sensing and pacing thresholds are assessed with electrocardiograms (ECGs).7 The typical radiographic appearance of an implanted pacemaker is shown in Figure 13-1. The pacemaker is typically programmed to pace at a rate of 60 to 80 beats/min. A significantly different rate usually indicates malfunction. When the battery is low, the rate generally begins to drop and gets slower as the battery fades. Sensing of intracardiac electrical activity is a combination of recognizing the characteristic waveforms of P waves or QRS complexes while discriminating them from T waves or external interfering signals, such as muscle activity or movement. The pacing electrical stimulus is a triphasic wave consisting of an intrinsic deflection, far-field potential, and an injury current, which typically delivers a current of 0.1 to 20.0 mA for 2 msec at 15 V.8 Several innovations in rate regulation have been incorporated into some pacemakers. When present, the hysteresis feature causes pacing to be triggered at a rate greater than the intrinsic heart rate. When the hysteresis feature is used in a single-chamber ventricular pacemaker, it is designed to maintain atrioventricular (AV) synchrony at rates that are lower than what would be normal for a ventricular-paced rhythm alone. To illustrate, were the hysteresis feature of the pacemaker set at 50 beats/min, an intrinsic rate lower than 50 beats/min would trigger ventricular pacing. Unlike a standard ventricular pacemaker, the hysteresis feature might be set to offer a ventricular pacing rate at 70 beats/min or greater once the pacer is triggered.9 Rate modulation by sensor-mediated methods is an additional feature triggered and mediated by a sensed response to various physiologic stimuli.9 The primary application for this rate modulation feature is in patients with pacemakers who continue to engage in vigorous physical activity. When present, the rate regulation feature is engaged and modulated through motion sensors installed within a pulse generator device, with a corresponding increase or decrease in the pacing rate depending on the degree of motion sensed by the pacemaker device. Other physiologic sensors that may be installed as part of the pacemaker system include those designed to sense minute ventilation, the QT interval, temperature, venous oxygen saturation, and right ventricular contractions. The latter sensors generally require that additional leads be placed. The basic components of an AICD, including sensing electrodes, defibrillation electrodes, and a pulse generator (Fig. 13-2), can be seen on a chest radiograph. Transvenous electrodes have obviated the previous need for surgical placement. They are inserted into the pectoralis muscle. Many transvenous systems consist of a single lead containing a distal sensing electrode and one or more defibrillation electrodes in the right atrium and ventricle.10 Leads are inserted through the subclavian, axillary, or cephalic vein into the right ventricular apex. The left side is preferred because of a smoother venous route to the heart and a more favorable shocking vector.11 In an effort to improve the efficiency of defibrillation, an additional defibrillation coil may be used.11 Various placements of AICDs are demonstrated in Figure 13-3. The pulse generator is a sealed titanium casing that encloses a lithium–silver–vanadium oxide battery. It has voltage converters and resistors, capacitors to store charge, microprocessors and integrated circuits to control analysis of the rhythm and delivery of therapy, memory chips to store electrographic data, and a telemetry module.12 Whereas a pacemaker can draw the voltage required for function from its component battery, the energy needed for defibrillation requires a battery that is prohibitively large.6 To circumvent this problem, an AICD contains a capacitor that maximizes the voltage required by transferring energy from the battery before discharge. To achieve the energy required, AICDs use capacitors that are charged over a period of 3 to 10 seconds by the battery and then release this energy rapidly for defibrillation.10 The maximal output is 30 J in most units and 45 J in higher-energy units.6 This energy is high enough that a discharge is very obvious and often distressing to the patient. Most AICDs use a system in which the pulse generator is part of the shocking circuit, often described as a “can” technology, and most of them have a dual-coil lead with a proximal coil in the superior vena cava and a distal coil in the right ventricle.13 Current flows in a three-dimensional configuration from the distal coil to both the proximal coil and the generator.14 This dispersion of the electrical field increases the likelihood of depolarizing the entire myocardium at once, thereby leading to successful defibrillation.14 AICDs may have the same programming capabilities as pacemakers and can be single chambered, dual chambered, or used with cardiac resynchronization therapy (CRT) .15 Single-chamber devices have only a right ventricular lead. They often have difficulty identifying atrial arrhythmias, which can result in inappropriate defibrillation of atrial tachycardias. Dual-chamber AICDs have right atrial and right ventricular leads and improved ability to discriminate rhythms. In most studies, dual devices have been found to offer improved discrimination between ventricular and supraventricular arrhythmias, thus decreasing inappropriate shocks as a result of rapid supraventricular rhythms or physiologic sinus tachycardia.16 Approximately 50% of AICDs implanted in the United States are dual-chamber devices.17 CRT devices add an additional left ventricular lead that is placed in the coronary sinus or epicardium. In patients requiring both AICD and pacemaker functions, both these devices are placed together. The advent of technology has allowed placement of a single device that can perform both pacemaker and defibrillator functions. AICDs use a combination of antitachycardia pacing, low-energy cardioversion, defibrillation, and bradycardiac pacing in a combination also known as tiered therapy. They are programmed with specific algorithms that identify and treat specific rhythms. Ventricular arrhythmias may initially be converted (or undergo attempts at conversion) with antitachycardiac pacing as opposed to immediate defibrillation. This overdrive pacing may terminate the rhythm without the need for electrical defibrillation in up to 90% of events. It is most successful for terminating monomorphic ventricular tachycardia with a rate of less than 200 beats/min.1 Overdrive pacing is better tolerated by patients than cardioversion and reduces the risk for inducing atrial fibrillation.18 These events may be silent, not felt by the patient, and discovered only by interrogating the device. If unsuccessful, the next intervention may be low-energy cardioversion (<5 J). The device may be programmed to very low levels of electricity that, again, are better tolerated by the patient. This works best for ventricular rates higher than 150 and lower than 240 beats/min.14 This may be followed by a high-energy defibrillation. Traditionally, the energy level of the first shock is set at least 10 J above the threshold of the last defibrillation measured.12 If the first shock fails, a backup shock may be required, but this may induce or aggravate ventricular arrhythmias (see the later section “Pacemaker-Mediated Tachycardia”). Unlike the proarrhythmic effects of medication, these arrhythmias are almost never fatal, although they may be associated with increased morbidity.11 Currently used biphasic waveforms have improved defibrillation thresholds.12 This tiered approach obviates the need for unnecessary energy requirements. The devices also have antibradycardiac pacing that allows these patients to have one device instead of separate units. Additional complications associated with AICDs that have antibradycardiac pacing algorithms include a tendency toward oversensing, increased current drain, potential detection problems, and an increased incidence of hardware and software design problems.1 At the time of insertion the amount of energy required for various AICD functions, such as the defibrillation threshold, is determined for any given patient, and output and sensing functions can be adjusted by reprogramming as needed. The most common indication for placement of a cardiac pacemaker is for the treatment of symptomatic bradyarrhythmias.19 Roughly 50% of pacemakers are placed in such patients for the treatment of sinus node dysfunction (sick sinus syndrome). Other diagnoses include symptomatic sinus bradycardia, atrial fibrillation with a slow ventricular response, high-grade AV block (including Mobitz type II and third-degree AV block), tachycardia-bradycardia syndrome, chronotropic incompetence, and selected prolonged QT syndromes. Though not classified as absolute indications, pacemakers are sometimes placed for the treatment of severe refractory neurocardiogenic syncope, paroxysmal atrial fibrillation, and hypertrophic or dilated cardiomyopathy. In recent years, CRT has emerged as a primary approach for patients with severe diastolic dysfunction and a low left ventricular ejection fraction (LVEF).19 Commonly, such patients manifest low-grade AV blocks and left bundle branch block.20 The resultant delay in left ventricular conduction often results in corresponding biomechanical delays in ventricular contraction, which in turn causes a further decrement in cardiac output and worsening congestive heart failure. Such prolongation may occur in as many as 33% of patients with advanced heart failure.20 This electromechanical “dyssynchrony” has been associated with increased risk for sudden cardiac death.21 CRT comprises atrial-synchronized, biventricular pacemaking, which overcomes the atrial and ventricular blocks while optimizing both preload and LVEF.22 Clinical trials and systematic reviews have confirmed the efficacy of CRT, with decrements in mortality of 22% to 30%, as well as improved LVEF and quality of life.23,24 It is therefore likely that emergency physicians will see the CRT configuration with increasing frequency in patients with implanted pacemakers and AICDs. The 2008 American Heart Association guidelines for implantation of a cardiac pacemaker are summarized in Box 13-1.19 AICD technology is used principally for both primary and secondary prevention in patients at risk for sudden death. Primary prevention is an attempt to avoid a potentially malignant ventricular arrhythmia in patients identified as being at high risk.25 Secondary prevention is for patients who have already had a ventricular arrhythmia and are at risk for further events. In addition, AICDs are implanted for a number of other congenital or familial cardiac conditions. Box 13-2 is a summary of class I indications for the placement of AICDs.26
Assessment of Implantable Devices
Pacemaker Characteristics
Characteristics of AICDs
Indications for Placement of Implantable Pacemakers and Aicds
Assessment of Implantable Devices