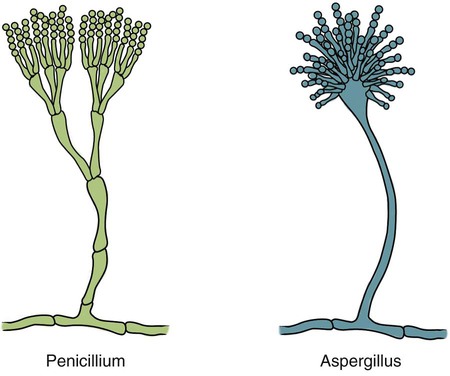
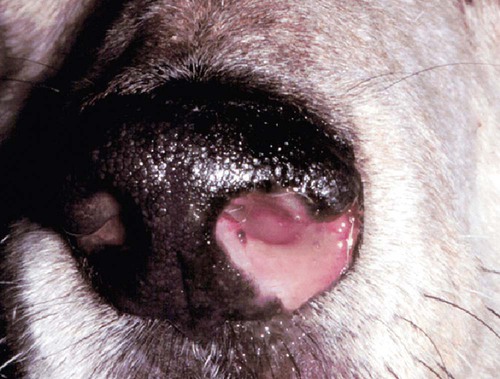
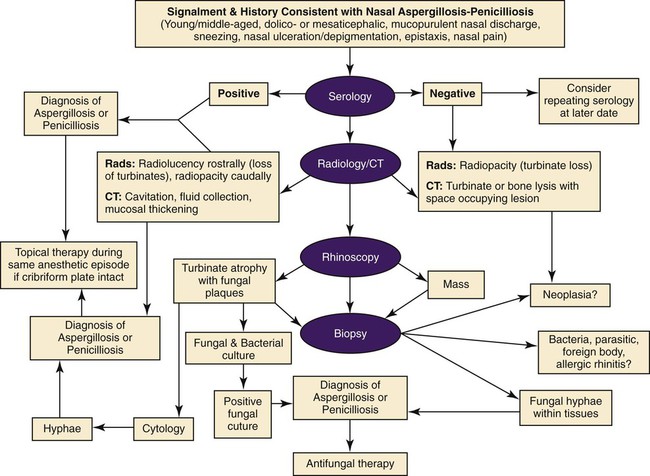
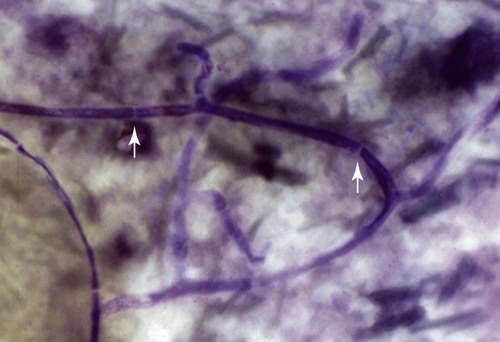
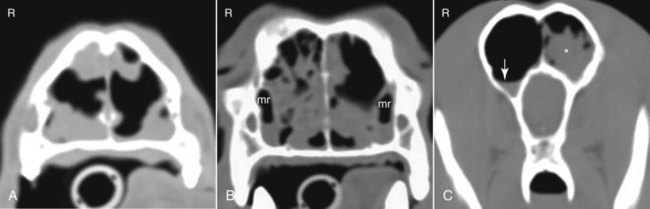
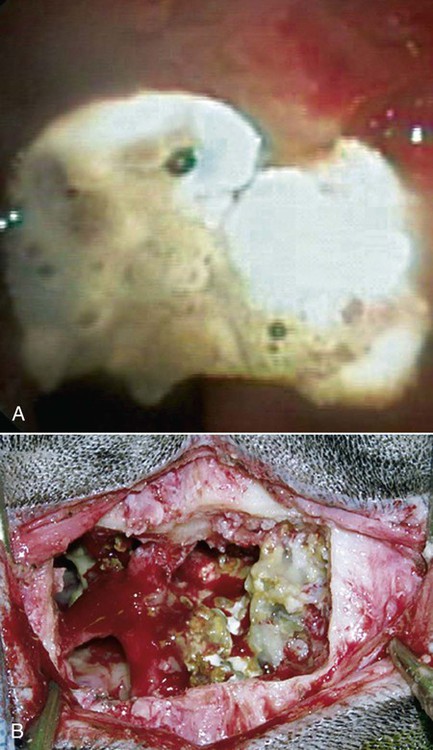
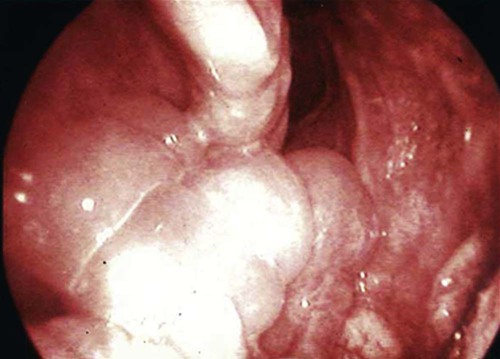
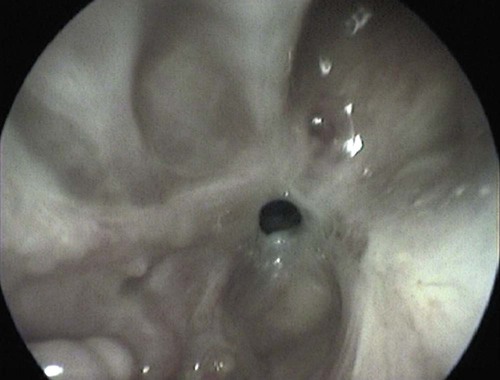
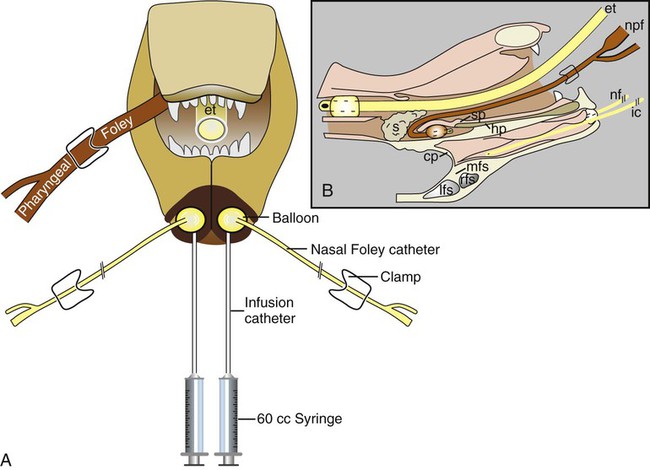
These fungal spores are components of aerosols where they disperse over varying distances to deposit on solid or liquid surfaces. Everything in the environment, even pet food, has the potential of having high levels of contamination.20 Should the organisms locate in appropriate conditions of temperature, humidity, and nutrients, they germinate. Aspergillus and Penicillium are common contaminants of body or mucosal surfaces and of the respiratory tract; therefore mere culture or identification by polymerase chain reaction (PCR), without either histologic or cytologic evidence of associated inflammation or tissue invasion, can be misleading. Because of their similar gross and microscopic appearance, Penicillium spp. are often confused with Aspergillus (e.g., Neosartorya) spp., culture or molecular analysis is required to distinguish these fungi. Solitary lesions most often arise within the nasal passages, and although they may extend locally in cats, sinonasal infections do not disseminate to distant locations.75 Aspergillus and Penicillium are generally opportunistic pathogens, and host immunocompetence is thought to be an important determinant in the development of these infections. Cell-mediated immunity (CMI) is probably the major factor in limiting systemic spread of infection. Immunosuppressive conditions such as diabetes mellitus, persistent neutropenia, cytotoxic chemotherapy, glucocorticoid therapy, concurrent infection, and hereditary cell-mediated immunodeficiency may be associated with disseminated disease. German shepherd dogs are predisposed to opportunistic infections where CMI is needed for protection, especially systemic aspergillosis (as described herein) and rickettsial diseases (see Chapters 26 and 27). For a discussion of infection with other opportunistic saprophytic fungi, see Chapter 65. Michael J. Day, Dominique Peeters, and Cecile Clercx Sinonasal aspergillosis (SNA) is encountered much more frequently than is sinonasal penicilliosis. The two organisms are indistinguishable other than by the microscopic appearance of their conidiophores (Fig. 62-1) or by molecular characterization. Aspergillus fumigatus is the most common species encountered, although Aspergillus niger, Aspergillus nidulans, and Aspergillus flavus are occasionally involved. The species of Penicillium causing sinonasal penicilliosis have not been well characterized, but Penicillium is considered a rare cause of canine rhinitis/sinusitis. Aspergillus and Penicillium branch dichotomously (branches are approximately equal in diameter to the stem) at 45-degree angles and form septate, nonpigmented hyphae of approximately 3 to 8 µm diameter in culture and in tissue specimens.96 Both groups of fungi are ubiquitous saprophytes and are regarded as opportunistic pathogens. Aspergillus spp. are found in the environment living and growing on organic debris. Their airborne conidia of 2 to 3 µm are released in such large numbers that they land on most inanimate and animate objects. Their small size keeps them airborne indoors and out, with most hosts inhaling hundreds of conidia daily.73 In immunocompetent individuals, these organisms are eliminated by innate immune mechanisms. To invade the host, Aspergillus must adhere to and penetrate the respiratory epithelium, kill surrounding cells, and resist phagocytosis.98 Conidia bind to various surface proteins; however, only a few of the adhesion systems, such as hydrophobins, have been identified. A. fumigatus also produces a toxic metabolite known as gliotoxin, which inhibits macrophage phagocytosis and has a broad range of immunosuppressive actions. Other metabolites can impair mucociliary action and prolong the organism’s epithelial residence. Enzymes such as proteases allow for tissue invasion. SNA is a common disease in dogs that affects primarily young to middle-aged mesaticephalic and dolichocephalic breeds. In one report of 60 dogs with SNA, the age of affected animals ranged from 3 months to 11 years (mean 3.3 years).104 In contrast, nasal neoplasia tends to occur in older animals.70 A history of prior nasal trauma is uncommon, but has been reported in some dogs.68 Rarely, SNA can occur concomitantly with, and probably secondary to, nasal tumors and nasal foreign bodies such as grass awns. Other differential disorders include extension of dental disease and cleft palate, as well as lymphoplasmacytic, bacterial, and allergic rhinitis. Sinonasal infection with Cryptococcus neoformans is less common but has also been reported in dogs and should be considered as a potential differential disorder (see Chapter 59).102 Dogs with disseminated aspergillosis do not generally have any clinically overt nasal involvement (see Canine Disseminated Aspergillosis, later). It is suggested that many dogs with SNA have an underlying immunodeficiency that predisposes them to infection. However, despite impaired CMI, as measured by low lymphocyte stimulation responses, few studies have confirmed immunocompromise, and most patients are systemically healthy.5,160 Impaired CMI may be either a cause or a result of infection, because A. fumigatus products have been shown to inhibit lymphocyte transformation in vitro.32 Impaired CMI responses can also persist long after the organism has been eliminated.160 In immunocompromised people, receiving low-dose itraconazole (ITZ) for prevention of aspergillosis, infection developed with non-Aspergillus fungi that were less susceptible to the drug.117 SNA may be associated with extensive osteolysis of the cranium. This most often affects the nasal turbinates; however, occasionally there may be extension to the cribriform plate, palatine, or orbital bone. It has been proposed that these destructive changes are caused by direct fungal invasion; however, histopathologic studies indicate that fungal plaques are generally restricted to the mucosa and that underlying tissue damage is mediated by extensive infiltration of mixed inflammatory cells.126 A. fumigatus produces an endotoxin that is both hemolytic and dermonecrotic.177 This toxin may also contribute to local tissue destruction. The immunopathologic features of canine SNA have been characterized. The mucosal inflammatory infiltrates are a mixed pyogranulomatous and lymphoplasmacytic response with numerous granulocytes and macrophages expressing the MAC387 antigen and class II molecules of the major histocompatibility complex (MHC). There is a dominance of IgG plasma cells over IgA and IgM cells, and a mixture of CD4+ and CD8+ T lymphocytes. The former T-cell population predominantly expressed the αβform of the T-cell receptor.126 These infiltrating leukocytes have upregulation of genes expressing a range of proinflammatory and regulatory cytokines including interleukin (IL)-6, IL-8, IL-10, IL-12, IL-18, IL-23, tumor necrosis factor (TNF)-α, transforming growth factor-β, and interferon (IFN)-γ. Genes encoding the chemoattractant molecules (chemokines) monocyte chemotactic protein-1, -2, -3 and -4, and the eosinophil chemoattractant eotaxin-2 are also upregulated.127,128 These observations allow formulation of a model for immunoregulation in this disease that involves elements of Th1- and Th17-mediated pathology that is partially controlled by Treg cells that may serve to limit tissue pathology but permit the establishment of chronic infection.43 Pulmonary aspergillosis is the primary form of localized disease observed in people. The long nasal passages of dogs may more effectively trap Aspergillus spores before they reach the lower airways. Pulmonary aspergillosis is relatively uncommon in the dog.2,91 Pulmonary cavitary lesions of a chronic nature have been observed in German shepherd dogs, without evidence of dissemination.92b,187a Clinical and historical signs consistent with SNA in dogs include mucopurulent nasal discharge, sneezing, signs of nasal discomfort (pawing at the face or pain on palpation), epistaxis, decreased appetite, lethargy, and depigmentation or ulceration of the nostrils (Fig. 62-2). This last sign is almost exclusively seen in SNA. Rarely is open-mouth breathing ever observed, and if so, it is likely due to concomitant nasal occlusion by other concurrent disease processes. Nasal discharge is initially unilateral in most dogs but often progresses to bilateral involvement caused by destruction of the midline nasal septum. With time, paranasal extension may result in facial swelling; nasolacrimal duct obstruction or destruction may result in epiphora. Orbital invasion can result in oculonasal discharge, exophthalmos, discomfort on opening the mouth, corneal injury, or strabismus. Enophthalmos and phthisis bulbi may be found in the healing stages.195 Erosion of the cribriform plate, if it occurs, usually does not result in clinical signs but may preclude topical therapy. On rare occasions, an animal will show seizures or dullness that are caused by extension of nasal or frontal sinus infection to the forebrain.121 Physical examination should include facial palpation for evidence of pain or asymmetry, evaluation of airflow through each nostril, and an oral examination. There is no single gold standard for diagnosis, and a combination of diagnostic procedures may be required (Fig. 62-3). These include visualization of fungal material on rhinoscopy, characteristic changes on imaging examinations (radiography, computed tomography [CT] or magnetic resonance imaging [MRI]), detection of fungal hyphae on cytologic or histopathologic examination of biopsy material, culture of the causative organism, serologic investigations (for antibody or antigen detection), or molecular characterization of causative agents by PCR. Samples for cytologic evaluation may be collected by (1) a direct smear of nasal discharge, (2) blind swabbing under anesthesia, (3) brushing of the nasal lesions under rhinoscopy, and (4) imprint or squash preparations of biopsy samples (Fig. 62-4). Findings of a study evaluating these techniques were detection of fungal hyphae in all situations where squash preparations were made, in 93% of cases of brush cytology, but in only 13% of discharge smears and 20% of blind swabs.47 Rhinoscopically obtained biopsy samples of nasal mucosa may be evaluated for the presence of fungal hyphae. Periodic acid–Schiff or Grocott staining may be required to highlight these structures, but if the biopsy samples include nasal mucosal tissue without overlying fungal plaque, the histologic confirmation of fungal infection may be missed. Aspergillus spp. can be isolated from cultures of the nasal passages of affected dogs, and a positive culture result in association with characteristic clinical signs is highly suggestive of SNA. However, because Aspergillus spp. are often part of the endogenous flora of the nasal cavity, positive culture results should never be interpreted in a causal relationship without complete consideration of all the diagnostic evidence (see Fig. 62-3).69 Culture should be performed on biopsy specimens of healthy and diseased nasal and sinus mucosae and bone adjacent to areas of necrosis. Culture of tissue samples or fungal plaques taken by endoscopic visualization is more reliable than that of swabs of nasal secretions taken blindly; culture was more reliable when it was performed at 37° C than at room temperature.9 Laboratory culture is also needed to differentiate Aspergillus or Penicillium infections from other saprophytic infections, such as those caused by Mucorales or Alternaria spp. In addition, fungal culture results may be negatively affected by secondary bacterial overgrowth. Pseudomonas spp. and Enterobacteriaceae may cause secondary infections of necrotic tissue within the nasal chamber. Numerous techniques for determination of serum Aspergillus-specific antibody titers have been evaluated, including agar gel double diffusion (AGDD), counterimmunoelectrophoresis (CIE), and enzyme-linked immunosorbent assay (ELISA) techniques. Historically, AGDD and CIE tests have been reported to be highly sensitive and specific, whereas ELISA appears to be less reliable.90,94,94 However, negative AGDD results should be viewed with some skepticism if the clinical, radiographic, and rhinoscopic findings are consistent with fungal rhinitis. Serologic (AGDD) testing has been compared with fungal culture of nasal biopsy tissue in a study of 21 dogs with SNA, 25 dogs with nonfungal rhinitis, 12 dogs with nasal neoplasia, and 26 healthy control dogs. Culture had greater sensitivity (81%) than serologic testing (67%), but the specificity of both methods was similar (culture 100% vs. serologic testing 98%).132 An investigation has compared AGDD and ELISA test results using a purified Aspergillus-antigen preparation selected by screening a number of commercially available extracts. Serum samples from 17 dogs with SNA were tested in parallel with samples from 33 clinically healthy dogs, 18 dogs with nasal neoplasia, and 11 dogs with lymphoplasmacytic rhinitis. Results of testing from 76.5% of dogs with aspergillosis were positive using the AGDD test, but results from control sera or samples from dogs with other nasal diseases were negative. By ELISA, 88% of serological testing results from dogs with aspergillosis were positive, but results from 18% of samples from dogs with lymphoplasmacytic rhinitis were also positive. AGDD therefore has higher specificity (100% vs. 96.8%) but lower sensitivity (76.5% vs. 88.2%) than ELISA.11 Sinonasal penicilliosis, which is clinically indistinguishable from SNA, can result in a negative AGDD test if only Aspergillus antigens are used.69 In addition, a positive result does not eliminate the possibility of a concurrent disease process such as neoplasia. Request for an Aspergillus titer, followed by general anesthesia, a radiographic evaluation or a CT scan, and rhinoscopy with the intent to perform biopsy and culture, is a logical diagnostic approach (see Fig. 62-3). In humans with invasive aspergillosis, efforts to detect Aspergillus antigens in body fluids have focused on detection of Aspergillus galactomannan (GM) or other carbohydrates in serum, cerebrospinal fluid, respiratory washings, or urine by latex agglutination or immunoblot methods.76,153,153 This component of the polysaccharide cell wall of the organism is released in variable quantities in body fluids during fungal growth in tissues. False-negative results in serum correspond to low test sensitivity, whereas false-positive results in urine correspond to reaction to other fungi and organisms causing urinary tract infections.85,174,174 In people, ELISA tests are more sensitive and reproducible than are latex methods. Furthermore, the presence of serum Aspergillus-specific antibody may interfere with the ELISA test.71 Increases in GM levels have been associated with treatment failures in patients with invasive aspergillosis.16 Application of tests for GM for the diagnosis of canine SNA has been limited.58 In the serologic study described earlier comparing AGDD and ELISA,11 one test kit was evaluated using the same set of serum samples. A positive result was obtained with 24% of sera from dogs with SNA, but also with 11% of dogs with nasal tumors, 9% of dogs with lymphoplasmacytic rhinitis, and 24% of control dogs. Currently available antigen testing cannot therefore be recommended in the evaluation of patients with SNA. The application of PCR methods to diagnose fungal infections is now becoming more widespread in human medicine, and it has been applied to samples of whole blood, serum, urine, cerebrospinal fluid, and bronchoalveolar lavage fluid. A range of target genes are used, including those within the 18S, 5.8S, and 28S rDNA gene complex that are relatively conserved among fungal species, mitochondrial genes, and the variable sequences of the intervening internal transcribed spacer regions.79,134 In human blood samples, positive PCR results correlated with increased GM levels.18 Quantitative (real-time) PCR has been used to monitor infectious load in the blood of human patients with invasive aspergillosis, and the increased sensitivity of this assay permits earlier diagnosis (by days to weeks) compared with serologic methods.83,194 PCR results may be positive in patients that have negative culture results,135 and people with invasive aspergillosis having positive PCR results develop negative PCR results after successful treatment.97 One study has examined the application of PCR to the diagnosis of canine SNA.129 Genus-specific assays were developed to detect all Aspergillus and Penicillium species (PenAsp) and also for individual distinction of Aspergillus species (A. fumigatus, A. flavus, A. terreus, and A. niger) in blood and tissue biopsies from the nasal mucosa of dogs with SNA, nasal neoplasia, lymphoplasmacytic rhinitis, or from control dogs. Overall, these assays were not as useful as serologic detection (see earlier discussion) for the diagnosis of SNA. With tissue specimens, the PenAsp PCR assay had sensitivity of 100% and specificity of 6%, whereas the A. fumigatus-specific PCR had sensitivity of 50% and specificity of 97%.129 Nasal and frontal sinus radiography can be used as a screening tool to evaluate dogs with suspected fungal rhinitis. Rostral radiolucency (cavitary destruction) caused by turbinate lysis or a mixed pattern of caudal lucency and opacity (or both) are usually seen on dorsoventral views, whereas increased radiopacity and frontal bone thickening are typical on rostrocaudal “skyline” views.173 Nonspecific thickening of the mucosa of the inner surface of bones of the frontal sinus, maxillary recess, and nasal cavity is often noted.148 Changes may be asymmetrical because the disease may affect one nasal cavity initially and then spread to the opposite side. CT is becoming more available in veterinary medicine and is now widely used for examination of nasal cavity disorders because it offers several advantages relative to conventional radiography for examination of the nasal cavities and sinuses.22,35 The anatomy of the mesaticephalic canine nasal cavity as seen with CT and MRI has been well described.48,61 CT is superior to radiography for defining the extent of nasal disease, detecting cortical bone lesions, and evaluating the cribriform plate, and for differentiating infectious from neoplastic rhinitis.22,35,35 In two large studies using CT in the diagnosis of SNA, abnormalities of the nasal cavities were present in all dogs, whereas frontal sinus abnormalities were found in 72% to 74% of the dogs.22,35 The most common CT findings were (1) moderate to severe cavitary destruction of the turbinates with a variable amount of abnormal soft tissue in the nasal cavities; (2) a rim of soft tissue along the frontal bone, maxillary recess, and nasal bones; and (3) thickened reactive bone (maxillary, vomer, or frontal bone) (Fig. 62-5). The sensitivity of CT in the detection of SNA has been greater than that of radiography.147 Particularly, CT is superior to radiography for demonstration of soft tissue rims along the nasal and frontal bones, frontal bone lesions, and cavitary processes.147 CT can better detect nasal aspergillosis restricted to the nasal cavities or associated with a foreign body but, in such patients, the diagnosis may still be difficult.147 Moreover, in some cases, CT can demonstrate cribriform plate lysis that is not visible using radiography.147 This finding is not essential for the diagnosis of SNA but may influence the choice of therapeutic protocol.164 MRI is also superior to radiography in the diagnosis of SNA.145 Nevertheless, CT better demonstrates bony changes, particularly cortical changes, and there is no clear advantage of using MRI instead of CT in the diagnosis of SNA. Rhinoscopy is considered to be the most useful diagnostic tool for canine SNA. In most cases, it allows visualization of fungal plaques (Fig. 62-6)81,145,145 and is probably the best method of obtaining meaningful samples for cytologic, histopathologic, or cultural analysis.47,48 Moreover, rhinoscopy allows endoscopically guided debridement that is part of the treatment of the disease (see later discussion). Typical rhinoscopic abnormalities include moderate to severe destruction of turbinates, intranasal mucopurulent secretions, intranasal fungal plaques, and roughening of the mucosa in almost all cases.145,198 Nasal septum destruction is observed less frequently.145,198 Fungal plaques appear as off-white or greenish fuzzy plaques that are adherent to the mucosa (see Fig. 62-6, A and B). An inexperienced observer may confuse these fungal colonies with mucopurulent exudate at an early stage of the disease.107 In most cases of aspergillosis with frontal sinus involvement and destructive rhinitis, the frontal sinus(es) can be explored by an experienced endoscopist via antegrade sinuscopy using a flexible endoscope.145,198 In very rare cases, fungal plaques will not be observed in the nasal cavity and will only be detected in the frontal sinus after sinus trephination and sinuscopy.81 In those instances, these procedures should only be recommended when frontal sinus involvement is evident with CT or MRI, and where fungal infection has not been detected by rhinoscopic, cytologic, or histopathologic examination. Systemic oral treatments are noninvasive but require prolonged administration (more than 4 weeks) because of poor to moderate efficacy. Therefore, this approach is expensive, and side effects such as hepatotoxicosis, anorexia, or vomiting are commonly reported.62 Triazole compounds used orally include thiabendazole, ketoconazole (KTZ), ITZ, and fluconazole (FCZ). See Table 62-1 for recommended dosages. Clinical cure is reported to be obtained in about half of patients treated with oral administration of thiabendazole and KTZ, and as many as 70% of patients treated with ITZ or FCZ,* although no large studies evaluating systemic treatment with ITZ have been done, and cost of nongeneric drug is often prohibitive. TABLE 62-1 Therapy for Canine Nasal Aspergillosisa IV, Intravenous; PO, by mouth; prn, as needed. aSystemic oral therapy has poor to moderate efficacy as compared to topical treatment. Drug toxicity is also greater than with topical treatment. For additional information on individual drugs, see the Drug Formulary in the Appendix. bDose per administration at specified interval. cTherapy to control infection may require months to years, although clinical remissions may be achieved; the infection may reactivate in a variable time period after discontinuing treatment. Topical therapy with azole drugs is generally superior to systemic administration for nasal aspergillosis; see text. dFor a total of 9 to 12 treatments until a cumulative dose of 24 to 27 mg/kg is reached. eThis dose is extrapolated from human studies. No efficacy studies have been conducted on this drug for treatment of aspergillosis in dogs or cats; however, it has been used empirically in nonresponsive cases. Amphotericin B (AMB) has been used in the past to treat SNA in dogs.12,168 The results were generally unsuccessful, and this drug is not recommended. Given that AMB has some efficacy against pulmonary and disseminated infections in humans and disseminated infections in dogs (see Therapy, under Canine Disseminated Aspergillosis), the problem likely relates to insufficient concentrations in the nasal passages achieved by systemic administration. Terbinafine, an antifungal drug used to treat disseminated aspergillosis in humans, has been tried on an empiric basis, alone and combination with azole drugs, to treat refractory cases of nasal aspergillosis. Further studies are needed to determine the efficacy of this drug in SNA. Topical administration of antifungal medications is more effective compared with oral administration; however, for better effect, this approach requires curettage, lavage, and suction, either perendoscopically or by sinuscopy, to remove fungal material before drug application.† Various procedures have been developed to administer medication topically, and these procedures vary in invasiveness and ease of performance. Enilconazole and clotrimazole, the most widely used topical agents, have poor solubility and limited intestinal absorption.157,164 These azole drugs are fungistatic at low concentrations but fungicidal at higher concentrations.109,157 They work by inhibiting synthesis of sterols in the fungal cell wall as well as that of nucleic acids, triglycerides, fatty acids, and oxidative enzymes.109,157 Clotrimazole, at high local concentration, causes direct damage to fungal membranes and while inhibiting fungal ergosterol synthesis.78 Clotrimazole is irritant to the digestive system and is systemically toxic. A 1% formulation of clotrimazole in a polyethylene glycol base (Lotrimin solution, Schering Corporation, Kenilworth, NJ; see the Drug Formulary in the Appendix) is readily available in a 30-mL vial. In mid-sized to large-breed dogs, regardless of head size, 60 mL per side is used, whereas 30 mL per side should be adequate for smaller breeds. Formulations that contain polyethylene glycol, isopropyl alcohol, and propylene glycol (Canesten solution, Miles Canada, Etobicoke, Ontario, Canada; Clotrimazole USP, VET Solutions, Vetoquinol USA, Fort Worth, TX) should be used with caution because propylene glycol that they contain may result in pharyngeal irritation and edema.4a,27,38 Enilconazole (Imaverol, Janssen-Cilag SA, Beerse, Belgium) is less toxic and irritating, especially at low concentrations.157 The only acute adverse effect observed for enilconazole at a dose of 640 mg/kg (290 mg/lb) given orally is emesis.157 Furthermore, enilconazole is also active in the vapor phase within the nasal cavity, over a distance of as much as 1 cm.164 The earlier standard treatment method of canine SNA involved topical enilconazole emulsion infusion twice daily for 7 to 14 days via tubes surgically implanted into the nasal cavities and frontal sinus.163 After sinusotomy and placement of indwelling catheters into the nasal cavities or frontal sinuses, 5 to 10 mL of a 50 mg/mL solution of enilconazole was slowly infused. This regimen led to resolution of the nasal discharge in 80% of the dogs.164 However, this invasive and expensive method was not particularly acceptable to owners and has now been replaced by other procedures. An alternative technique using clotrimazole resulted in the resolution of clinical signs in many affected dogs, with many cases resolving after a single treatment.104 In this technique, a 1-hour infusion of clotrimazole was administered under general anesthesia, after placement of catheter into the frontal sinus during sinusotomy.104 This method reduced hospitalization time and eliminated the complications associated with maintenance of indwelling catheters. Noninvasive techniques using nonsurgically placed catheters have been developed to infuse the drug topically into the nasal cavities and frontal sinus under general anesthesia. The use of these methods eliminates the need for surgical trephination and is associated with fewer complications.104,108,108 Several treatment protocols have been used in order to improve success rate, tolerance by the animal, and owner compliance. Under general anesthesia, tubes are blindly104 or perendoscopically108,198 placed into the nasal cavity or the frontal sinus, and either enilconazole or clotrimazole have been used at various concentrations. The dog receives an infusion that is repeated a second or even a third time (at 3-week intervals), in a variable percentage of cases and depending on the technique used. These methods show improved efficacy, reaching a success rate of up to 80% to 90%.104,108,108 The first noninvasive topical therapy described consisted of intranasal infusion of clotrimazole for 1 hour.104 Results of a preliminary study evaluating the distribution of dye injected into cadaver skulls of clinically healthy dogs were that a noninvasive technique for inrtanasal infusion resulted in better distribution of infusate within the nasal cavity and paranasal sinuses than did techniques using catheters placed via sinusotomy.136 Bilateral administration of 50 mL (100 mL in total) through the nares resulted in excellent distribution of infusate to the entire cavity and frontal sinuses, as determined by evaluation of pre- and post-infusion images on CT in 12 dogs with confirmed fungal rhinitis,105 and with minimal leakage into the pharynx.136 The clinical response to topical administration of 1% clotrimazole in dogs with SNA was evaluated by comparison of the efficacy of surgical versus nonsurgical placement of catheters in 60 dogs.104 The noninvasive procedure is performed under general anesthesia, with the dog intubated with a cuffed endotracheal tube (Fig. 62-7). The tip of a 24-French diameter Foley catheter is inserted through the mouth dorsal to the soft palate at the junction between the hard and soft plate, where the 30-mL balloon is inflated to occlude the nasopharynx. Sponges are placed in the pharynx to avoid leakage of drugs into the trachea, and to help keep the Foley catheter in place. One 10- or 12-French diameter catheter is advanced dorsomedially in each nostril, to the level of the medial canthus of the palpebral fissure. A 12-French diameter Foley catheter is then inserted into each nostril, and 5-mL balloons are inflated just behind the nostrils to occlude them, and all Foley catheters are clamped. The 12-French diameter catheters are the infusion catheters, and each catheter is connected via a T-shaped connecting piece to a manometer tube and a 60-mL infusion syringe, filled with the topical drug. Constant infusion is performed by use of a syringe driver. The dog’s head is rotated and maintained in dorsal, left lateral, right lateral, and ventral recumbency, each for 15-minute intervals to ensure drug contact with all nasal surfaces. At the end of the intranasal infusion, the head is tilted downward at an angle of 30°, the catheters and gauze sponges are removed, and the nasal cavities are allowed to drain for 20 minutes. The pharynx and larynx are examined before the dog is allowed to recover from anesthesia. Complications during the procedure include leakage around the catheters, through the incisive ducts or the nasolacrimal system, and rarely, bleeding at withdrawal of catheters.104 Topical administration of clotrimazole by this method resulted in clinical cure in 65% of dogs after one treatment and in 87% of dogs after two or more treatments. No difference in outcome was noted between surgically and nonsurgically placed catheters.104 An improvement of this noninvasive procedure has been described108 that accompanies the diagnostic rhinoscopy procedure. Infusion catheters are placed under endoscopic guidance with a flexible bronchoscope into the caudal part of the frontal sinus. This modification facilitates placement of the infusion catheters into the dorsal aspect of the frontal sinus, which appears to increase therapeutic efficacy.108 The effectiveness of enilconazole administration by two noninvasive infusion techniques was compared in a study in 26 dogs where a 1% emulsion of enilconazole through blindly placed intranasal catheters was used in 19 dogs, while a 2% emulsion of enilconazole was infused through endoscopically placed catheters into the frontal sinuses in the remaining 7 dogs.198 Results of this study were that infusion of 2% enilconazole emulsion into the nasal cavities and frontal sinuses administered via endoscopically placed catheters increased the chance for success, from 89% up to 100%, after one to three infusions at 3-week intervals. In all dogs, there were two major adverse effects during the immediate posttreatment period: profuse nasal discharge and sneezing.198 These problems mostly resolved within 24 hours. None of the dogs had anesthetic or neurologic complications. Endoscopic intrasinusal administration of 2% enilconazole required fewer infusions for success and appeared to be successful in resolving clinical signs of rhinosinusitis in most dogs. Findings using follow-up rhinoscopy in all dogs were an absence of fungal plaques and the presence of mucosal blebs resulting from the treatment procedure (Fig. 62-8).198 Rare complications included a partial occlusion of one or both nares that needed to be surgically corrected; this occlusion resulted from the formation of scar tissue consecutive to severe ulceration. Fibrotic reactions also occurred at the entrance of the frontal sinus (Web Fig. 62-1), causing fluid retention and recurrent inflammatory sinusitis. There is a report of sinonasal tumors developing after treatment with intranasal clotrimazole for SNA, presumably as a result of inflammatory reaction to the drug.62a However, because pretreatment biopsies were not performed, it is possible that the nasal neoplasia was the primary inciting nasal insult with SNA as a complicating factor. Infusion with either clotrimazole or enilconazole through catheters is contraindicated when the cribriform plate is damaged. However, infusions have been administered in a few dogs with a damaged cribriform plate without any complication and with therapeutic success.198 Potential complications include the development of neurologic signs from chemical irritant–induced meningoencephalitis. Therefore, a CT analysis is recommended to assess the integrity of the cribriform plate before each treatment. The noninvasive topical infusion of antifungal agents is well tolerated and shows a high success rate. However, these procedures are time-consuming and require prolonged anesthesia. A prospective study, evaluated invasively administered 1% clotrimazole cream in the frontal sinus. The intent was to provide a lasting depot effect to minimize anesthesia time while providing an extended period of drug contact.166 Fourteen dogs were treated by frontal sinus trephination and a short (5-minute) flush of 1% topical clotrimazole solution, followed by instillation of 1% clotrimazole cream as a depot agent. During a minimum follow-up period of 6 months, 12 of 14 dogs (86%) responded well to treatment and either had resolved clinical illness or had signs of mild rhinitis.166 Only 1 dog required multiple treatments. Treatment was well tolerated by all patients, with minimal complications. Effect of 1% bifonazole cream, instilled through perendoscopically placed catheters into the frontal sinuses, either as single therapy after debridement or as adjunctive therapy after 2% enilconazole infusion has been evaluated.10 Twelve dogs were treated initially with the combined (enilconazole and bifonazole cream) therapy: 7 and 3 of these dogs were free of disease after one and two procedures, respectively, whereas 2 dogs were cured after single therapy with bifonazole cream was used as second procedure. Five dogs were treated with single therapy with bifonazole cream only: in 3 dogs with moderate disease, cure was obtained after a single procedure, whereas in 2 debilitated patients, cure could not be confirmed. Based on data of 33 cases, use of combined (enilconazole and bifonazole cream) therapy (25 cases) has an excellent success rate (60% after one procedure and 96% after a repeated application), while after a single therapy with bifonazole cream (8 cases), the success rate was 63% after the first procedure, and 100% after a repeated procedure.8a Therefore, topical administration of 1% bifonazole cream appeared to be an effective therapy in SNA, either as an adjunctive therapy to enilconazole infusion, or as a sole therapy in moderately affected patients.10 Results of a multicenter retrospective review of the first treatment outcome in 85 cases given commonly utilized treatment techniques indicated that the reasons for treatment failure are multifactoral.153a Its authors concluded that treatment of mycotic rhinosinusitis remains challenging and multiple treatments are frequently required for a successful outcome. Despite these treatment procedures, some dogs remain refractory to treatment and the prognosis for these patients is poor. Therefore, more invasive surgical procedures, including sinusotomy, in selected poorly responsive dogs, and in dogs in which the cribriform plate is severely damaged, should be considered. Turbinectomy is of no benefit in controlling the nasal discharges and is often detrimental.67,95,164,186,187 An invasive procedure was described in three dogs with mycotic rhinitis, refractory to several previous treatments.74 Temporary rhinostomy was performed followed by topical povidone-iodine dressings, intended to produce a sustained release of povidone-iodine. This treatment was designed because of evidence that topical povidone-iodine had antifungal activity. The use of a slow-release form of dressing was intended to maintain adequate local levels of active iodine, and to reduce the frequency of handling the animal. The affected nasal cavity and/or frontal sinus were exposed via a dorsal approach and partial turbinectomy was performed.75 The dressing was replaced every 2 to 3 days for 15 to 21 days, usually under general anesthesia, until all exposed tissue was covered by healthy granulation tissue, at which time the rhinotomy was closed by soft tissue reconstruction. Although successful, the topical povidone-iodine pack used in this study was more invasive than any other alternative option, which makes it undesirable for routine use. Although no toxic effects were observed, systemic absorption of iodine should be investigated if this treatment should be considered. Another surgical method has been described in seven dogs with severe or recurrent SNA involving rhinotomy and surgical debridement followed by topical administration of 2% enilconazole.33 Intrasinusal administration of enilconazole via endoscopically placed catheters is effective and attractive due to its noninvasiveness, but extensive rhinoscopic debridement before infusion is critical.198 Invasive methods may be needed in some cases, whereas very large fungal plaques and/or abundant necrotic material are present in the frontal sinus. This technique can be advised in diseased animals that have not responded to noninvasive treatment, that have lesions that cannot be debrided, or that have damage to the cribiform plate. In one study,33 the bone flap was either discarded or left attached cranially and attached again at the end of the procedure. Nasal passages were then debrided and irrigated with enilconazole solution for 1 hour. Follow-up rhinoscopy was performed in all dogs. Rhinotomy with removal of the flap combined with 1-hour infusion of 2% enilconazole resulted in satisfactory outcome, whereas nonremoval of the bone flap resulted in 100% persistence of fungal colonies on the flap or at the level of cerclage.
Aspergillosis and Penicilliosis
Canine Sinonasal Aspergillosis-Penicilliosis
Etiology
Pathogenesis
Clinical Findings
Diagnosis
Cytologic and Histopathologic Findings
Organism Cultivation
Antibody Testing
Antigen Detection
Genetic Detection
Medical Imaging Procedures
Endoscopic Evaluation
Therapy
Systemic Therapy
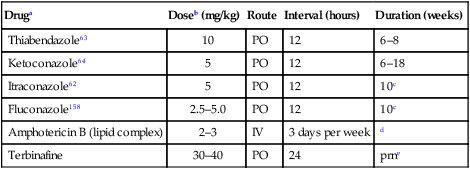
Druga
Doseb (mg/kg)
Route
Interval (hours)
Duration (weeks)
Thiabendazole63
10
PO
12
6–8
Ketoconazole64
5
PO
12
6–18
Itraconazole62
5
PO
12
10c
Fluconazole158
2.5–5.0
PO
12
10c
Amphotericin B (lipid complex)
2–3
IV
3 days per week
d
Terbinafine
30–40
PO
24
prne
Topical Therapy
Surgery
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aspergillosis and Penicilliosis