Artificial Insemination of Llamas and Alpacas
The development and application of artificial insemination (AI) has been slow in llamas and alpacas and is still in its infancy. Several technical reasons exist for this, including lack of standardized protocols for semen collection, handling, extending, and thawing. In addition, very few fertility trials have been conducted to evaluate frozen-thawed semen.1 This chapter discusses the state of our knowledge regarding this biotechnology in alpacas and llamas as well as the challenges facing its implementation.
Semen Collection and Initial Preparation for Dilution
Semen collection techniques used in alpacas and llamas have been discussed in detail elsewhere in this text. Electroejaculation and artificial vagina are the methods of choice to obtain ejaculates that are suitable for semen evaluation and processing.2–4
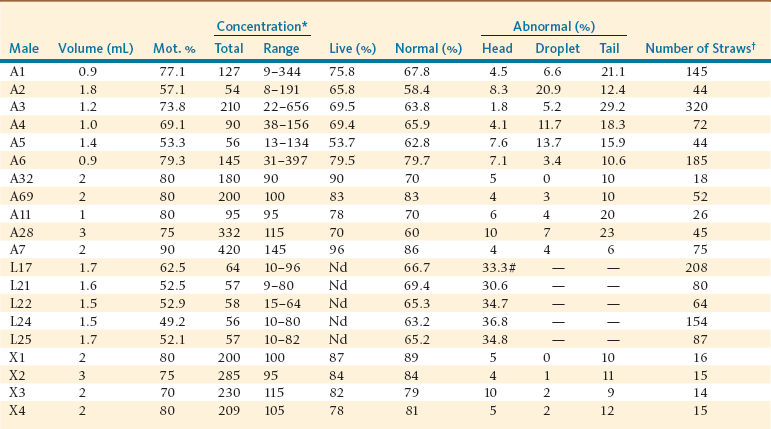
Regardless of the method of semen collection, the first step in semen handling is the elimination of its viscosity.5–9 This is achieved by addition of proteolytic enzymes (i.e., collagenase) to the extender, as has been described previously in this text. Once the ejaculate is liquefied, seminal parameters are determined by using standard techniques. Semen parameters to be determined include motility, concentration, percentage of live spermatozoa, and evaluation of morphology. It is important to note that even with the use of artificial vagina, not all ejaculates are suitable for processing. Semen quality may reflect individual variation as well as management factors. Table 27-1 shows typical ejaculate characteristics and frozen semen straws produced in recent years at the La Raya research station in Peru. The majority (92%) of ejaculates collected are suitable for preservation and artificial insemination.10,11 Herd sires that are being used for breeding with a large number of females often produce poor-quality semen (low concentration, total sperm in ejaculates, or both) or refuse to service the dummy. The minimum standards for semen parameters for ejaculate suitable for preservation and artificial insemination have not been yet established.
Extenders and Semen Dilution
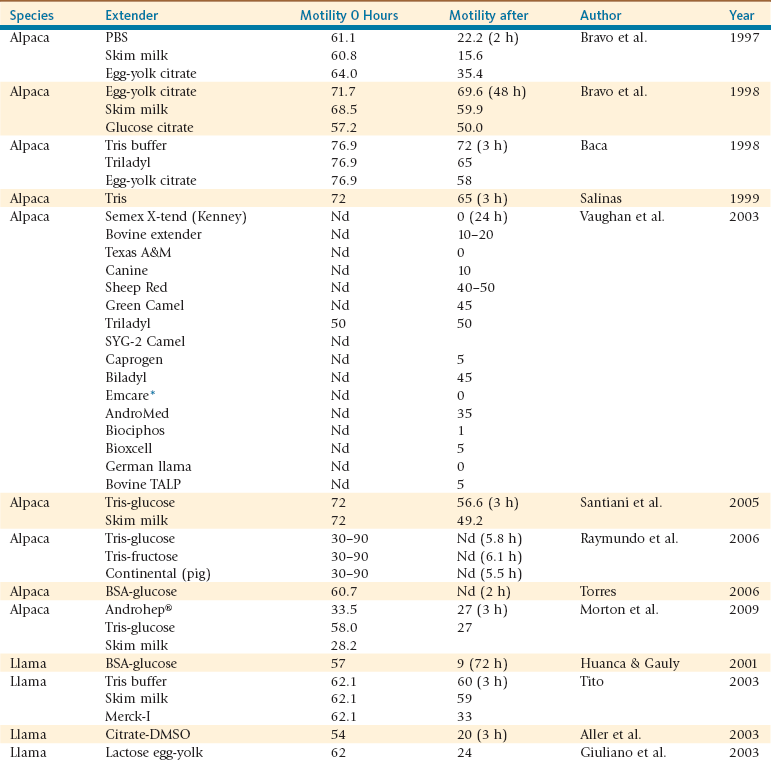
Several extenders commonly used for ruminant semen preservation have been used for alpaca and llama semen cryopreservation. The only commercial extender specifically labeled for camelid semen (Camel Buffer, IMV Technologies, France) has been pulled out of the market recently. Studies comparing the effects of the extender and of the condition of the storage on the viability of semen after dilution remain limited. Table 27-2 summarizes results obtained with some of the extenders tested in alpaca and llama semen. Viability and motility after dilution vary greatly among studies. In general, Tris-buffered extenders seem to be more suitable for preservation of llama and alpaca semen compared with other commonly used extenders.
TABLE 27-2
Percentage of Motile Spermatozoa of South American Camelids Using Different Extenders and for Different Times (in parenthesis)
*Semen samples were kept refrigerated at 4°C.
Santiani A, Huanca W, Sapana R, Huanca T, Sepulveda N, Sanchez R. 2005. Effects on the quality of frozen-thawed alpaca (Lama pacos) semen using two different cryoprotectants and extenders. Asian J Androl 7:303-309.
Giuliano SM, Pinto M, Director A, Miragaya M. 2003. Implementación de un protocolo de criopreservacion de semen de llama (Lama glama) a 5 °C durante 24 horas. 26avo Cong Argentino de Prod Anim. RF 13 Abstr
Raymundo FT, Huanca WL, Huanca TM, Huerta SO, Cordero AR. 2006. Efecto de tres dilutores en la conservación del semen de alpacas. RIVEP 17:125-130
Alpaca semen shows better viability (% live spermatozoa) after 2 hours of incubation at 35°C following dilution in egg-yolk citrate (36.4%) compared with dilution in phosphate-buffered saline (PBS) (22.2%) or skim milk (15.6%).12 Alpaca semen extended and stored at 4°C for up to 72 hours showed better viability with egg-yolk glucose citrate than with skim milk or glucose citrate extenders. The percentage of live spermatozoa was nearly 70% in egg-yolk glucose citrate for up to 48 hours. Other studies have shown Tris-buffered extenders to be superior to the commercial extender Triladyl or egg-yolk citrate in alpacas and to skim milk and Merck-I extenders in llamas.
Semen Cryopreservation
Trials on preservation of alpaca and llama semen have been conducted in the last decade. However, detailed studies on the effect of various factors (i.e., dilution rate, extender, type of cryoprotectant) are scarce. Except for treatment to eliminate viscosity, protocols used for cryopreservation of alpaca and llama semen follow closely those used for bull semen. The critical steps for cryopreservation of alpaca and llama semen are collection of ejaculate with good initial quality, liquefaction, dilution, cooling, glycerolization, equilibration, and freezing.13–26
Following collection and treatment to eliminate viscosity, semen is diluted with an extender and cooled down slowly from 37°C to 4°C over 1 hour. No controlled studies have been conducted on the rate of initial cooling. Addition of the cryoprotectant is performed at the end of the cooling period. Although ethylene glycol and dimethyl sulfoxide (DMSO) have been used for alpaca and llama semen, respectively, glycerol remains the most used cryoprotectant in semen preservation of both species. Glycerol concentration of 7% was found to be superior in terms of viability and post-thaw motility compared with concentrations below 4%. The addition of glycerol is generally done in three equal volumes at 15-minute intervals.
Freezing and Thawing
Cryopreservation of South American camelid (SAC) semen has been reported since 1978. Following equilibration at 4°C, semen is packaged in 0.25-milliliter (mL) or 0.5-mL plastic straws and frozen in liquid nitrogen vapors.18,20–27 No controlled studies on the effects of freezing rates on post-thaw quality have been performed.
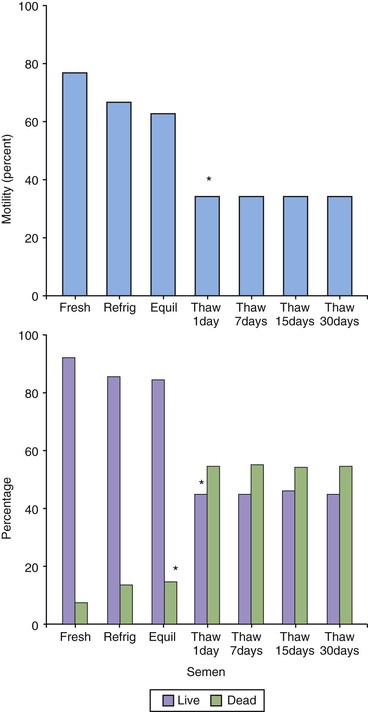
No controlled studies on the appropriate thawing rate for straws have been performed. The thawing protocol consists of placing straws in water bath at 35°C for 8 to 10 seconds or at 37°C for 20 to 60 seconds. In a study on alpaca semen, post-thaw motility was similar (50%) for straws thawed at 35°C or 37°C for 1 minute but was significantly lower (38%) when straws were thawed at 37°C or 40°C for 5 or 10 minutes. Post-thaw motility and viability of alpaca semen was not different following storage in liquid nitrogen for 1, 7, 15, or 30 days (Figure 27-1). Reported post-thaw motility varies between 5% and 50%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree