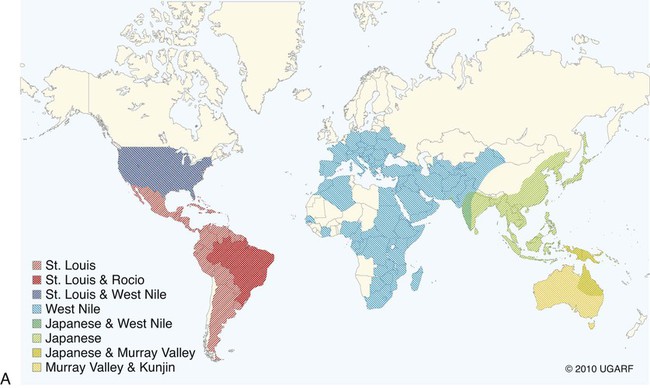
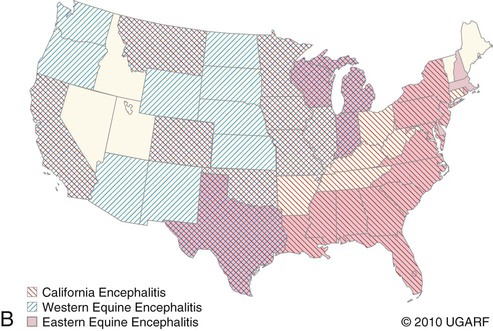
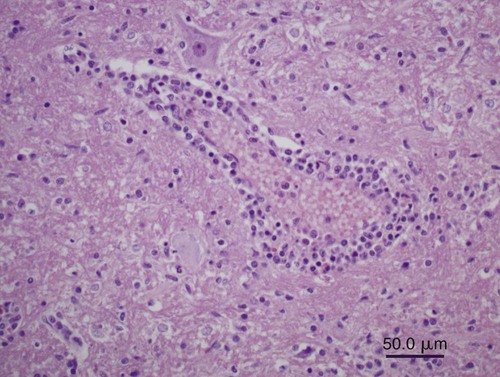
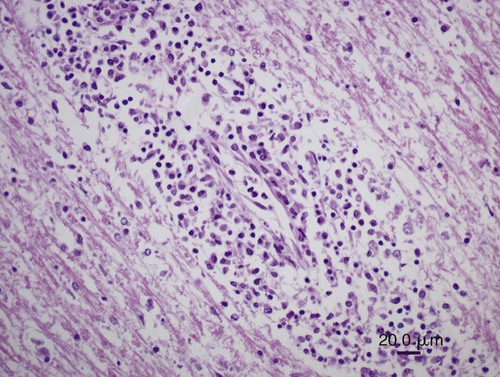
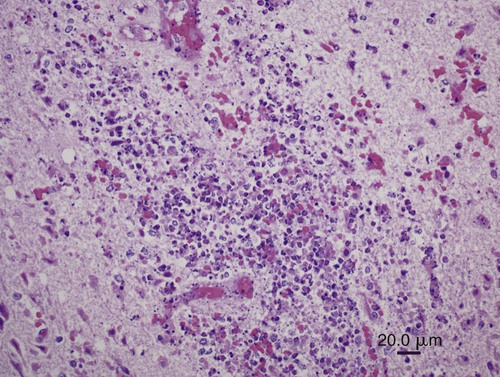
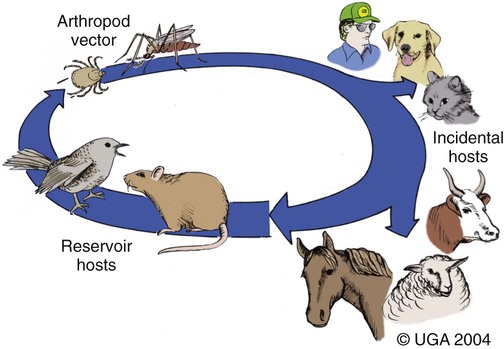
All arthropod-borne viruses (arboviruses) known to infect dogs and cats belong to the families Togaviridae, Flaviviridae, Bunyaviridae, or Reoviridae (Table 24-1). These RNA viruses are usually maintained in nature by a sylvan cycle involving an arthropod vector and a vertebrate reservoir host (Fig. 24-1). The worldwide distribution of the mosquito-borne infections is presented in Fig. 24-2, A and B. Arboviruses usually replicate within their insect vectors, causing minimal or no cell injury. In contrast, vertebrate hosts’ cells are often damaged by cytolysis. Although domesticated animals are usually incidental hosts for these infections, in some cases, they serve as reservoirs. As unnatural hosts, domesticated animals may be subclinically affected or show signs of disease (usually nonsuppurative, neurotropic encephalitis, abortion, or teratology). The clinical susceptibility of humans, dogs, and cats for each disease also varies. Results of serologic testing indicate that a large number of dogs and cats may be exposed to these viruses; however, clinical illness is uncommon. Because serologic cross-reactivity between certain viruses can occur, and because dogs and cats can be subclinically infected, the following discussion emphasizes instances in which virus isolation and consistent pathologic findings have been present or experimental inoculation of dogs or cats has been performed. TABLE 24-1 Arthropod-Borne Viral Infections Affecting Dogs and Cats C, Cat; D, dog; H, human; ?, uncertain. bAlso includes Kunjin, Alfuy, Kokobera, Koutango (West Nile virus subtypes), and Murray Valley encephalitis viruses in Australia. cSixteen serogroups are in this family. Members of the California serogroup include Jamestown Canyon, La Crosse, and Tahyna viruses. Craig E. Greene and Richard A. Bowen Alphaviruses in this family are grouped into seven antigenic complexes, and three of these (see Table 24-1) occur in the New World, causing sporadic equine, human, canine, and feline infections. Natural and experimental susceptibility of dogs to Venezuelan equine encephalitis (VEE) virus has been well described.66 In both natural and experimental infections involving mosquitoes, viremia and seroconversion occur without clinical illness. For this reason, dogs have been considered good sentinel hosts for human VEE infection. Dogs also have been used to monitor the spread of VEE infection into geographic areas. Parenteral inoculation of VEE in dogs has produced fever, leukopenia, and neurologic deficits at the peak of the febrile response, and high mortality.45 Cerebrovascular hemorrhage and infarction were detected. Naturally occurring VEE was also suspected as causing encephalitis in a puppy.66 Everglades virus is one variant in the VEE complex circulating among rodent hosts and vector mosquitoes in Florida. It causes febrile illness in humans with rare neurologic manifestations. Only subclinical infections have been noted in dogs, in which seropositive results have been used to monitor its distribution.42 Eastern equine encephalitis (EEE) has been diagnosed in late spring or summer in naturally infected pups (age range of 10 days to 6 months) from south-central Georgia.12,53 All of the pups with EEE were younger than 6 weeks, with fever (above 40° C [104° F]) and signs of diffuse encephalitis exhibited by mental depression, ataxia, nystagmus, tremor, excessive salivation, paralysis, and seizures. Petechial hemorrhages have been grossly evident on the surface of the brain at necropsy. Histologic lesions in the brain were inflammation with macrophage infiltration and perivascular cuffing, edema, gliosis, hemorrhage, and multifocal necrosis (Fig. 24-3). Mononuclear and neutrophilic infiltrates have been present in the meninges. Lesions were randomly distributed; however, they were most prevalent in gray matter of the cerebrum and midbrain. Lesions were milder in the cerebellum with mild perivascular cuffing and microglial nodule formation. Foci of myocardial degeneration and necrosis with mononuclear infiltration have been found in the heart. The viral association has been confirmed by specific neutralization of EEE virus by available serum from one pup, by use of reverse transcriptase (RT)-PCR on brain tissues from two pups, and viral culture isolation with specific immunofluorescent and PCR detection of EEE viral antigen and nucleic acid, respectively. EEE has also been isolated from the central nervous system (CNS) of a litter of dying 6-week-old puppies in which no microscopic lesions could be demonstrated.13 The infection may have been coincidental. Experimentally, dogs have developed diffuse encephalitis after intracerebral or parenteral inoculation of western equine encephalitis virus.144 Ross River and Barmah Forest viruses are endemic in Australia, primarily affecting kangaroos and wallabies (family Macropodidae) as major mammalian reservoirs. Serum neutralizing antibodies are widespread in human and mammalian populations, and some dogs and cats have neutralizing antibodies to these viruses. Dogs and cats experimentally infected by feeding mosquitoes infected with these viruses were relatively resistant to both viral infections.26 Only a few animals seroconverted, and none developed viremia or clinical signs. Although these animals are naturally exposed, they are not likely to be important reservoirs of these viruses. Tenshaw virus, a Bunyavirus present in the Southeastern United States, was inoculated into dogs and cats and produced asymptomatic viremia, and mosquito-mediated transmission from infected dogs was demonstrated.153 Rift Valley fever virus (RVFV) has produced viremia, severe hepatic necrosis, myocarditis, splenic congestion, meningitis, diffuse petechiae, and death in puppies and kittens younger than 3 weeks.84,116,170,171 Virus can also be transmitted from puppies to their mother and to other puppies. Older puppies do not succumb to infection but do develop viremia. The virus can cause abortions and stillbirths in pregnant bitches, which is its hallmark in ruminants. Inhalation or ingestion of the virus from infected carcasses may occur under natural circumstances. (For a complete discussion of RVFV infection, see the next section.) La Crosse virus infection is remarkable in adaptation to at least one of its vectors, Aedes triseriatus. Vector infection is lifelong, and the virus is transmitted transovarially to subsequent generations of mosquitoes. It is the primary cause of pediatric arboviral encephalitis in the United States. Two litters of puppies younger than 3 weeks of age developed a sudden onset of seizures and neurologic dysfunction after infection with La Crosse virus.20 An adult dog from Florida developed CNS signs of forebrain disease that responded to simultaneous treatment with antibacterials and glucocorticoids; however, it then developed seizures.157 Severe diffuse necrotizing to granulomatous meningoencephalitis affecting primarily the cerebral cortex was noted in the affected dogs described previously (Figs. 24-4 and 24-5). Immunohistochemical testing on the CNS demonstrated La Crosse virus within the affected brain tissue. Whether La Crosse virus is a cause of idiopathic granulomatous meningoencephalitis in dogs is uncertain (see Chapter 82, Neurologic Diseases of Suspected Infectious Origin and Prion Disease). RVFV, a Phlebovirus infection of Africa and the Middle East, is capable of being transmitted by many species of mosquitoes. Among domestic species, RVFV affects predominantly sheep, cattle, and goats, and occasionally human beings. In humans, the disease is characterized by hepatocellular failure, acute renal failure, and hemorrhagic manifestations.2 Sera from wild and domestic dogs and cats in endemic regions have had positive test results for antibodies to RVFV. However, in a study using the more specific plaque reduction neutralization test, only lions from areas of three prior outbreaks had positive test results.75 The mammalian reservoir for infection may exist in exotic carnivores such as the lion; however, domestic dogs and cats can be experimentally infected and maintain a viremia sufficient to infect mosquitoes. Clinical illness in inoculated dogs is seen only in the first few weeks of life.170 Some pups became hyperthermic during infection, although all died in a terminal hypothermic state. Some of the animals showed signs of CNS dysfunction near death. In older animals, 50% develop viremia and a corresponding increase in serum neutralization titer. Clinical signs in older animals were inapparent; however, transmission of infection from pup to mother and from pup to pup was demonstrated. High mortality has been observed when kittens less than 3 weeks of age were inoculated with RVFV.171 A transient febrile response was followed by terminal hypothermia. Neurologic signs were ataxia followed by recumbency, and paddling movements were observed within 24 hours before death. As with pups, evidence indicated that the virus spread to other kittens and adults. On gross necropsy of dead pups and kittens, the liver and spleen were enlarged and soft in consistency.116 In the liver, faint gray to yellow foci, 1 mm in diameter, were surrounded by thin hyperemic borders. Petechial and ecchymotic hemorrhages were found on the serosal surfaces of the heart, abdominal lymph nodes, and mucosal surfaces of the gastrointestinal tract. Histologically, hepatocellular necrosis has been the most consistent finding. In less severe areas, foci of necrosis are evident, whereas in the most severe cases, the entire liver is necrotic with little normal parenchyma. Multifocal inflammation and necrosis have been evident in the myocardium. Perivascular nonsuppurative meningoencephalitis, characteristic of other arboviral infections, was found in the CNS. Lesions in experimentally infected dogs and cats did not show characteristic intrahepatic eosinophilic cytoplasmic inclusions or Councilmanlike bodies observed in other hosts.116 Orbiviruses are one of nine genera in the family Reoviridae. Dogs, which can be infected subclinically with African horse sickness virus, develop a serologic response and a viremia enabling them to transmit the infection.44,141 They are thought to acquire infection naturally and suffer illness and death from eating infected horse meat or from bites of infected Culicoides midges.76 Abortion in pregnant bitches and their subsequent death have occurred 7 to 9 days after vaccination with a blue-tongue virus-contaminated, modified live virus combination canine vaccine.1,52,52 Lesions in the dam consisted of interstitial pneumonia, myocardial degeneration, hepatic vasculitis, and renal glomerulitis.52 Natural disease with this virus in dogs is unlikely based on the observed low seropositivity of dogs tested in endemic areas.77 Flaviviruses are small, icosahedral, enveloped virions (45 to 60 nm diameter; 45 nm for West Nile virus (WNV) with a single-stranded, positive-sense RNA genome.122 The genus type species is the yellow fever virus (flavi, Latin for “yellow”); the genus also includes the St. Louis encephalitis virus, which serologically cross-reacts with WNV. Saint Louis encephalitis, Murray Valley encephalitis, and Kunjin viruses, along with other closely related viruses, belong to the Japanese encephalitis serocomplex. Infections caused by these viruses are maintained in nature by cycles involving birds as vertebrate hosts and predominantly Culex mosquitoes as vectors. Although dogs appear resistant to Saint Louis encephalitis virus, serosurveys have demonstrated that they develop antibody during human epidemics, suggesting subclinical infection. Parenteral inoculation of Japanese encephalitis virus resulted in subclinical infection, but intracranial inoculation resulted in encephalitis with corresponding neurologic deficits.79 Dogs have developed fever, viremia, and serologic response to Powassan virus.56 Wesselsbron virus was isolated from the CNS of a dog with encephalitis.149 Inoculation of brain tissue from this animal into naïve dogs produced seroconversion, viremia, and transient paralysis in one dog. Yellow fever virus has produced a transient viremia in cats after inoculation; however, puppies could not be productively infected, even when splenectomized. European tick-borne encephalitis and louping-ill—two tick-transmitted flavivirus infections that can infect dogs—are discussed later in this chapter. Cats have been reported to be refractory to Saint Louis encephalitis virus because they do not develop a humoral immune response after experimental infection and have not shown measurable antibody levels in field serosurveys.94 Cats do develop antibodies to Powassan virus.94 Richard A. Bowen and Craig E. Greene WNV is a mosquito-borne virus in the family Flaviviridae, genus Flavivirus, and a member of the Japanese encephalitis serocomplex, which includes such notable pathogens as Japanese encephalitis, Murray Valley encephalitis, and St. Louis encephalitis viruses. WNV was originally isolated in 1937 from a febrile woman in the West Nile area of Uganda. Two major genetic lineages of WNV are recognized. The virus now endemic in North America is a member of lineage 1 and is most closely related to an isolate from an outbreak in Israeli geese in 1998; it has caused massive outbreaks of disease in birds, horses, and humans.5,15,19,100 Lineage 2 WNV has been recognized as a cause of human and equine neurologic disease, and avian mortality, in South Africa and central Europe.11,23,51,169 Three additional lineages of WNV have been proposed, based on viruses isolated from Europe, Russia, and India.10,22 WNV is one of the most broadly distributed arboviruses, occurring in Africa, the Middle East, Asia, Australia (the Kunjin variant), and North, Central, and South America; it also is seen with some frequency in parts of Europe. WNV is maintained in a bird-mosquito-bird sylvatic cycle and spills over into populations of mammals, which are generally dead-end hosts.* In the 1990s, several outbreaks with neurologic disease in humans and horses occurred in Europe and the Middle East, and a 1998 outbreak in Israel was the first reported outbreak with substantial avian mortality.9,32,91,121 WNV was not recognized in the Western Hemisphere before its emergence in New York City in 1999. Subsequently, WNV spread with astonishing speed across the United States, and into Canada, Mexico, and Central and South America.69,162 Before the emergence of WNV in the United States, clinical disease due to WNV infection had been reported in a single dog and no cats. The affected dog died with neurologic disease over 35 years ago in Botswana; the agent isolated from this case was originally identified as Wesselsbron virus, but was subsequently confirmed by genomic sequencing to be WNV.29,149 Serologic surveys have indicated that dogs in WNV-endemic areas frequently become subclinically infected with WNV: 37% in Africa in 1988; 24% in India in early 1990s; 5% in New York City in 1999; 2.4% in Missouri in 2002; 26% in Louisiana in 2002; 38% in Turkey in 2005; and from 55% in 2003 to 87% in 2006 in Harris County, Texas.* The study conducted in a transmission focus in Louisiana in 2002 demonstrated that 9% of cats were seropositive for WNV, supporting the concept that a large majority of WNV infections in both dogs and cats are subclinical.86 The epizootic of WNV infection in the United States has been the largest arbovirus epidemic ever recorded. It deviated from historic outbreaks of WNV by its marked avian mortality and increased diversity of affected host species. Mortality in corvids (crows and blue jays) was especially high. Deaths associated with WNV have been reported in more than 150 avian species.† Birds were a direct source of virus spread, because virus is shed in saliva and cloacal excretions, and carnivores may ingest carcasses.8,14,14 In the U.S. outbreak, in addition to widespread disease in birds, humans, and horses, published reports document WNV disease in squirrels, dogs, wolves, sheep, an alpaca, and several hundred farmed alligators.‡ Serologic surveillance studies have identified a number of other animals that can become infected with WNV, including bats, skunks, raccoons, opossums, and cats.17,86,95,99 Mosquitoes are the major transmission vectors of WNV, although other ectoparasites such as ticks can be viable vectors, and birds are the major vertebrate reservoir host. Although most of the natural transmission in the United States has been associated with three Culex subspecies (pipiens, quinquefasciatus, and tarsalis), WNV has been isolated from at least 59 mosquito species.70,117 Most WNV disease is associated with the mosquito season, and disease usually peaks in late summer. However, the means by which the virus overwinters has not been clearly established, and disease can occur in midwinter.14,57 In general, mammals are considered incidental or dead-end hosts. The viremia is thought to be too low for transmission to mosquitoes; at most, rare inefficient transmission might occur.8,28,71,91 In initial experimental infections of cats and dogs, cats developed higher virus serum levels (103 to 104 plaque-forming units [PFU] per milliliter, 0.5 to 8 days postinoculation) than did dogs (101.6 to 102.2 PFU/mL between 0.5 and 3.5 days postinoculation).8 However, the levels in the cats were still low and transient compared with those found in competent species of birds; therefore, even cats are unlikely to be efficient reservoirs for mosquito infection. The viral level needed for transmission varies with mosquito species and is not precisely defined, but likely requires concentrations of greater than 104 PFU/mL for reasonable efficiency. Additional routes of nonvector transmission have been identified during the North American epizootic of WNV infection, including ingestion (predation, milk consumption by nursing), blood transfusion, organ transplantation, transplacental transfer, and needle stick.8,71 Oral transmission was documented experimentally in cats, alligators, and raptors and is the likely route of a large outbreak in farmed alligators fed tissues from infected horses.8,89,115,126 Summer 2002 was the first time that WNV disease was recognized in canids in North America; details have been reported on several occasions (in adult dogs and juvenile captive wolves).27,31,102,104,135 More than 30 additional dogs and 3 cats have been reported to surveillance databases as possibly being affected. The outcome of WNV infection in birds varies considerably among species, ranging from high-titer viremia and near uniform mortality in corvids to low-titer viremia and very low mortality in chickens. Other birds, such as house sparrows, develop a high-titer viremia but experience relatively low mortality.92 Mammals are readily infected by WNV, but most (horses, dogs, cats, ruminants) develop a viremia of insufficient magnitude to infect feeding mosquitoes. Exceptions to this generalization occur. For example, dogs develop low-titer viremia and are considered incompetent as amplifying hosts, but treatment of dogs with glucocorticoids before infection resulted in levels of viremia roughly 50 times what was seen in untreated dogs.25 The hallmark of WNV-induced disease in mammals is encephalitis or encephalomyelitis. Neuroinvasion appears to be possible through several routes, including hematogenous spread across the endothelium of the blood-brain barrier, transport via infected leukocytes, or retrograde axonal transport from the peripheral nervous system.87 A majority of the work on immunology of WNV infection has used mice, and both B and T cells participate in preventing or clearing infection.46 Humoral antibody can prevent productive infection, as observed after passive immunization or vaccination, and also limits dissemination of virus. Cellular immunity, mediated by CD4+ and CD8+ T cells, is essential to clearing virus from the CNS, but in some cases, cellular immune responses appear to contribute to immunopathogenetic lesions in the CNS.173
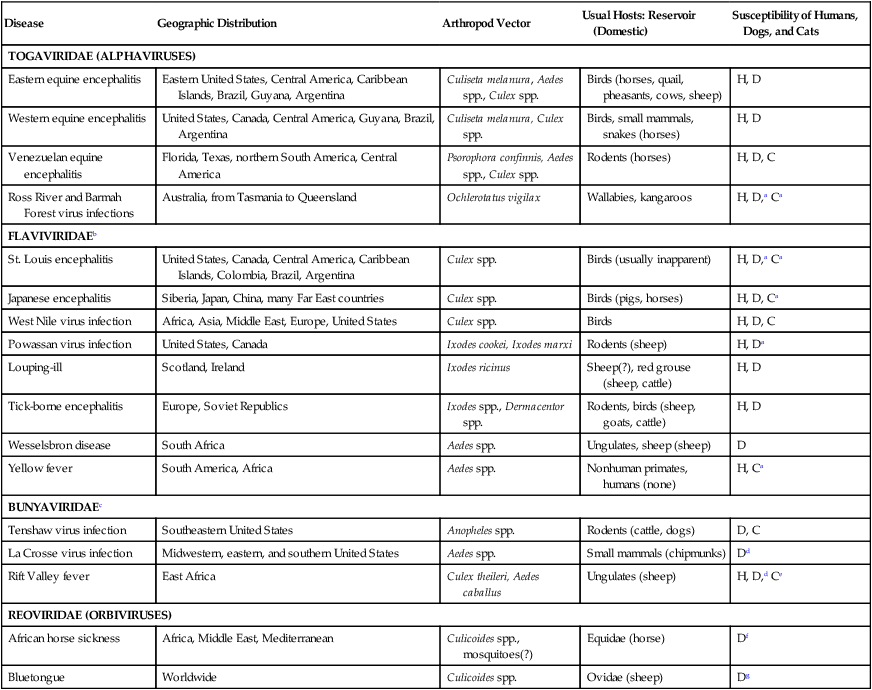
Arthropod-Borne Viral Infections
Disease
Geographic Distribution
Arthropod Vector
Usual Hosts: Reservoir (Domestic)
Susceptibility of Humans, Dogs, and Cats
TOGAVIRIDAE (ALPHAVIRUSES)
Eastern equine encephalitis
Eastern United States, Central America, Caribbean Islands, Brazil, Guyana, Argentina
Culiseta melanura, Aedes spp., Culex spp.
Birds (horses, quail, pheasants, cows, sheep)
H, D
Western equine encephalitis
United States, Canada, Central America, Guyana, Brazil, Argentina
Culiseta melanura, Culex spp.
Birds, small mammals, snakes (horses)
H, D
Venezuelan equine encephalitis
Florida, Texas, northern South America, Central America
Psorophora confinnis, Aedes spp., Culex spp.
Rodents (horses)
H, D, C
Ross River and Barmah Forest virus infections
Australia, from Tasmania to Queensland
Ochlerotatus vigilax
Wallabies, kangaroos
H, D,a Ca
FLAVIVIRIDAEb
St. Louis encephalitis
United States, Canada, Central America, Caribbean Islands, Colombia, Brazil, Argentina
Culex spp.
Birds (usually inapparent)
H, D,a Ca
Japanese encephalitis
Siberia, Japan, China, many Far East countries
Culex spp.
Birds (pigs, horses)
H, D, Ca
West Nile virus infection
Africa, Asia, Middle East, Europe, United States
Culex spp.
Birds
H, D, C
Powassan virus infection
United States, Canada
Ixodes cookei, Ixodes marxi
Rodents (sheep)
H, Da
Louping-ill
Scotland, Ireland
Ixodes ricinus
Sheep(?), red grouse (sheep, cattle)
H, D
Tick-borne encephalitis
Europe, Soviet Republics
Ixodes spp., Dermacentor spp.
Rodents, birds (sheep, goats, cattle)
H, D
Wesselsbron disease
South Africa
Aedes spp.
Ungulates, sheep (sheep)
D
Yellow fever
South America, Africa
Aedes spp.
Nonhuman primates, humans (none)
H, Ca
BUNYAVIRIDAEc
Tenshaw virus infection
Southeastern United States
Anopheles spp.
Rodents (cattle, dogs)
D, C
La Crosse virus infection
Midwestern, eastern, and southern United States
Aedes spp.
Small mammals (chipmunks)
Dd
Rift Valley fever
East Africa
Culex theileri, Aedes caballus
Ungulates (sheep)
H, D,d Ce
REOVIRIDAE (ORBIVIRUSES)
African horse sickness
Africa, Middle East, Mediterranean
Culicoides spp., mosquitoes(?)
Equidae (horse)
Df
Bluetongue
Worldwide
Culicoides spp.
Ovidae (sheep)
Dg
Mosquito-BORNE and Gnat-Borne Infections
Togaviridae
Bunyaviridae
Rift Valley Fever
Reoviridae
Flaviviridae
West Nile Virus Infection
Etiology
Epidemiology
Pathogenesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Arthropod-Borne Viral Infections