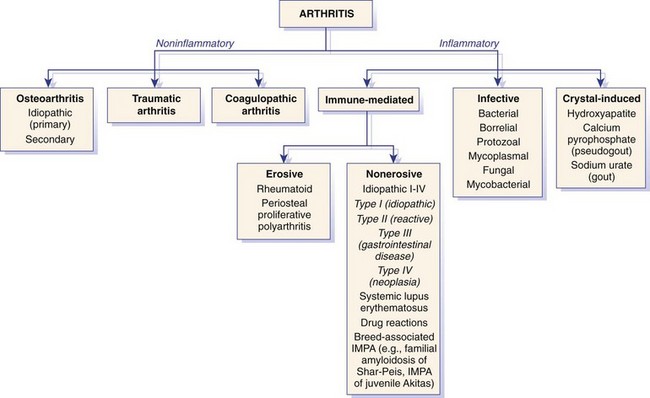
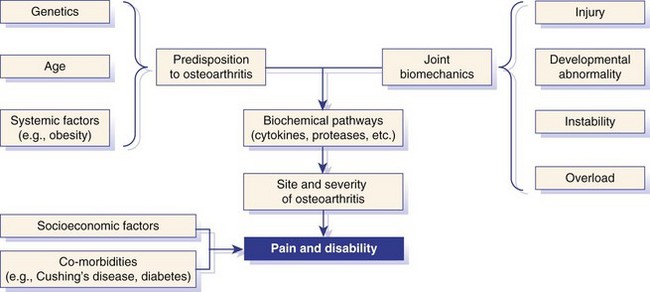
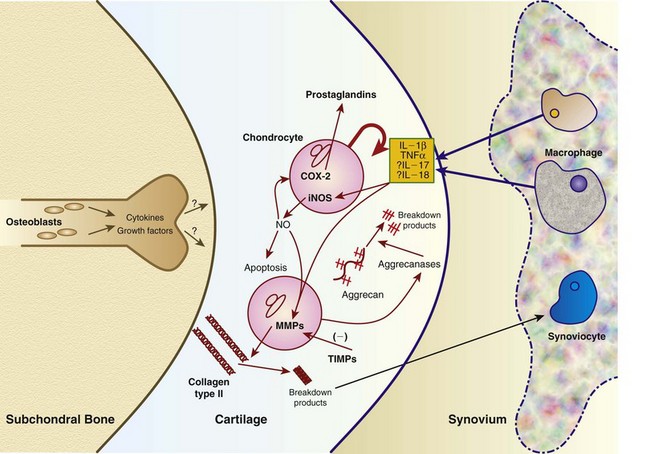
Chapter 68 Arthritis is a broad term that encompasses inflammatory disease processes within the synovial joint. The degree of inflammation may vary considerably between different types of arthritis such that some forms are traditionally described as “noninflammatory” and others as “inflammatory”; in truth, all types of arthritis display some degree of inflammation, albeit in some cases, low grade. Arthritis can be classified (Figure 68-1) to help the clinician determine the type of disease process that is driving the joint pathology. Such a classification is useful to provide a diagnostic and therapeutic framework, although the clinician should realize that the various forms of arthritis share many pathogenic mechanisms. The relative importance of these different processes and the rate at which they progress may differ. Broad categories of arthritis include degenerative, immune mediated, and infective. This chapter will review these categories in turn. Osteoarthritis is the most common form of arthritis in dogs and cats. Although robust epidemiologic data are lacking, it has been estimated that approximately 20% of adult dogs are affected,169 and it was reported that more than 60% of adult cats showed radiographic evidence of the condition in the appendicular skeleton.141 Osteoarthritis should be thought of as a disease process rather than a disease entity because it appears to be a common final pathway for the failing joint. In the dog, it is almost always a secondary phenomenon following an initiating abnormality (e.g., joint laxity or instability, osteochondrosis, trauma). Articular cartilage is considered the key tissue in osteoarthritis, but it must be remembered that the synovial joint is an organ with cross-talk between the various tissues (cartilage, synovium, bone, ligament, synovial fluid, fat). The relative importance of cross-talk between different joint tissues (e.g., between synovium and cartilage) is still unknown. There is no doubt that a morphologic marker of progression of osteoarthritis is the gradual loss of articular cartilage. An understanding of osteoarthritis requires some knowledge of the metabolism of tissues of the synovial joint. The reader is referred to Chapter 40. Osteoarthritis can be classified as idiopathic (syn: primary) or secondary (see Figure 68-1). In dogs, the disorder most frequently occurs secondary to an identifiable abnormality of the joint such as a developmental disorder, joint instability, or trauma (e.g., osteochondritis dissecans, hip dysplasia, cruciate ligament rupture). In cats, this may also be the case (e.g., hip dysplasia187), but often in this species, no initiating cause can be identified. The etiology of osteoarthritis in a particular joint may be obscure; in the case of idiopathic osteoarthritis, or obviously in the case of secondary osteoarthritis, the complexity of the condition extends well beyond identification of its cause. Current models of osteoarthritis indicate that each individual has an inherent susceptibility to osteoarthritis, and superimposed on this are local factors at the joint level. This type of model can be summarized as shown in Figure 68-2. Within this model, the individual’s susceptibility is summarized by genetics, age, and systemic factors such as obesity. The genes that control this susceptibility to osteoarthritis have not yet been identified in dogs, although progress has been made in enhancing our understanding of the genetic basis of diseases that cause secondary osteoarthritis, such as cruciate disease and hip dysplasia.218,345,354,355 Genetics.: In human beings, idiopathic and generalized osteoarthritis is common, and a genetic disposition to osteoarthritis has been clear since it was first reported that generalized nodal osteoarthritis was twice as likely to occur in first-degree relatives as in control individuals.175 However, because of the prevalence of osteoarthritis in the human population and the marked clinical heterogeneity of the disease, the precise genetic contribution to the etiology of osteoarthritis has been difficult to define. Moreover, it is clear that multiple genetic factors can contribute to the incidence and severity of osteoarthritis, and that these may differ according to specific joint, gender, and race. Given the variety of candidate genes that predispose to osteoarthritis, evidence indicates that there may be an additive effect of individual genes in the development of disease.322 Several candidate genes encoding proteins of the extracellular matrix of the articular cartilage have been associated with early-onset osteoarthritis.150 In addition to point mutations in type II collagen, inherited forms of human osteoarthritis may be caused by mutations in several other genes that are expressed in cartilage, including those encoding types IV, V, and VI collagen, as well as cartilage oligomeric matrix protein (COMP). In addition to structural proteins, genes encoding nonstructural proteins have been identified as associated with risk of osteoarthritis. For example, growth differentiation factor (GDF)-5 has been identified in several studies in different populations.101,321,324 A recent genome-wide association scan in affected and unaffected women with knee osteoarthritis suggested the existence of cis-acting regulatory polymorphisms that are in, or near to, the prostaglandin endoperoxidase synthase-2 (PTGS2) gene, which encodes the cyclooxygenase (COX)-2 enzyme.323 Age.: Age also appears to influence susceptibility to osteoarthritis. Aging influences the structure of joint tissues, including articular cartilage. As chondrocytes age, they synthesize smaller, less uniform aggrecan molecules and less functional link proteins, their mitotic and synthetic activities decline, and their responsiveness to anabolic mechanical stimuli and growth factors decreases.209,216,217 Accumulation of advanced glycation endproducts within the type II collagen network is another notable feature of aging.87–89,333 These age-related cross-links of collagen appear to reduce turnover of the collagen network. In the canine cranial cruciate ligament transection model, advanced glycation endproducts were associated with increased severity of osteoarthritis.90 With aging, the length and uniformity of aggrecan molecules are also diminished.56 C-terminal truncation of aggrecan occurs through the activity of matrix metalloproteinases and aggrecanases.188 Such shorter aggrecan molecules contain fewer chondroitin sulfate side-chains but greater quantities of keratan sulfate160 and thus have less ability to imbibe water into the tissue, thus reducing compressive stiffness. Overall, aging is associated with multiple changes in articular cartilage such that cellular activity and responsiveness, repair mechanisms, and extracellular matrix features favor the loss of tissue. Numerous laboratory-based studies are supported by data from clinical studies of canine patients, which suggest that long-term outcome for dogs with cruciate disease and secondary osteoarthritis is not as good in older dogs.161 Bodyweight.: Data on the effects of bodyweight on the development or progression of osteoarthritis in dogs are not clear. Bodyweight is not a surrogate for “overweight” or “obese,” but in many studies it is used as a variable because information on body condition score or body fat content may not be available. Bodyweight appears to increase the risk of some initiating causes of osteoarthritis, such as hip dysplasia. In a birth cohort of Boxers,327 it was found that high birthweight was associated with increased risk of the development of clinical signs of hip dysplasia. In addition, the risk of cranial cruciate ligament rupture is increased in dogs with higher bodyweight.340 Overweight and Obesity.: Obesity, another key risk factor for osteoarthritis,194 is defined as a disease in which excess body fat has accumulated, such that health may be adversely affected. Inbred canine populations have proved useful in defining the contribution of dietary restriction in ameliorating the appearance and progression of osteoarthritis. In studies of colonies of dogs at high risk for canine hip dysplasia and obesity, Labrador Retrievers (n = 48) were paired at age 6 weeks by gender and bodyweight within each of seven litters, and then were assigned randomly within the pair to a “control fed” (ad libitum) group or a “25% diet restriction” group.195 Beginning at age 8 weeks, each control fed dog was given the dry, extruded diet ad libitum, and each diet restriction pair-mate was given 75% of the amount of food that its control fed pair-mate had consumed the previous day. Each feeding group was given the same diet; only the quantity offered differed between the control fed and diet restriction feeding groups. When the dogs were 3.25 years old, the control fed group’s rations were held constant at a daily dietary energy level consistent with an ideal bodyweight for that breed. Among control fed dogs at age 2 years, 42% had radiographic evidence of hip osteoarthritis, compared with 4% hip osteoarthritis among diet restriction dogs.174 By 5 years of age, 52% of control fed dogs had radiographic evidence of hip osteoarthritis, compared with 13% of diet restriction dogs. Bodyweight at 5 years of age correlated moderately with severity of hip osteoarthritis, suggesting that body weight alone might not be the primary driving force for development of hip osteoarthritis in the dog. Radiographic hip osteoarthritis in the whole group of 48 dogs had increased in linear fashion over the 14.5-year period of feeding and data collection, from a prevalence of 15% at age 2 years to 67% by age 14 years. By the end of the study, 83% of control fed dogs had developed radiographic hip osteoarthritis, compared with 50% of the diet restriction group, which had a longer median life span.298 Diet restriction also resulted in lower prevalence and severity of osteoarthritis in the shoulder273 and elbow joints151; at 8 years of age, the prevalence of osteoarthritis in two or more joint types was 77% among control fed dogs and 10% among diet restriction dogs. Thus, the evidence is compelling that optimal body condition score limits the appearance (and progression) of osteoarthritis in dogs. Overweight and obesity may be an etiologic factor for osteoarthritis by causing increased load on the joint, as has been demonstrated in human subjects.53 However, evidence in human patients also suggests that obesity may alter joint alignment,102,293 causing focal overload of joint tissues. Furthermore, canine obesity is associated with a systemic subclinical proinflammatory state with increased concentrations of circulating adipokines such as tumor necrosis factor (TNF), interleukin (IL)-6, and leptin117; clearly, such changes also raise the hypothesis that obesity may cause osteoarthritis through the action of such adipokines. Evidence is ample to implicate TNF and IL-6 in the degradative processes of articular cartilage, but other evidence supports a role for leptin in the metabolism of joint tissues.184,276 Gender Status.: The association between gender and osteoarthritis in dogs and cats has not been extensively researched. However, gender is a well-recognized risk factor for osteoarthritis in human beings. From epidemiologic studies, it is known that before the age of 50, the prevalence of osteoarthritis is higher in men,346 but after age 50, the prevalence is higher in women,240 with postmenopausal women being at significantly greater risk for knee and hand osteoarthritis in particular.84 The effect of hormonal status on the development of osteoarthritis in women remains unclear; epidemiologic studies suggest a link between osteoarthritis and ovarian function, along with a protective effect of estrogen. Estrogen receptors alpha and beta, through which estrogen signals, have been found in several tissues of the joint, including cartilage, bone, ligaments, and synovium. Although this indicates that these tissues have the machinery to be responsive to estrogens, estrogen replacement therapy has showed contradictory effects on osteoarthritis.128,136 Animal models have therefore been used to elucidate the role of estrogen depletion (through ovariectomy) and estrogen treatment. A recent systematic review302 of ovariectomy animal models of osteoarthritis reported that in 11 animal models, ovariectomy caused biomechanical, compositional, or histologic changes in the articular cartilage; four studies reported no effect, and only one study observed less osteoarthritis after ovariectomy. Thus the data are inconclusive at the current time. The effect of estrogen treatment on osteoarthritis was also inconclusive, with 11 of 22 studies reporting a beneficial effect, and 6 studies showing a detrimental effect. Data on the effect of gender status on canine osteoarthritis are sparse. Some data address the association between gender and the risk of initiating factors such as hip dysplasia, elbow dysplasia, and cranial cruciate ligament rupture.186,239,295,340 Of note, a Swedish study was undertaken to address the association between grading of hip status as assessed by radiographic examination (hip screening) and subsequent incidence of veterinary care and mortality related to hip dysplasia in five breeds of insured dogs.214 This study provides information on the likelihood that these dogs will develop clinical signs of hip pain, which in many cases will have been caused by osteoarthritis secondary to hip dysplasia. In this study, gender did not show any significant effect on hip dysplasia-related veterinary care or mortality. However, in a study of hindlimb lameness caused by hip dysplasia in Boxers and the effects of gender,327 neutering at least 6 months before making a diagnosis of clinical hip dysplasia influenced the risk that clinical hip dysplasia would develop over time; neutered dogs were 1.5 times more likely to develop clinical hip dysplasia, compared with sexually intact dogs (mean age at the time of neutering was 3 years). Studies on gender and the risk of cranial cruciate ligament rupture have shown that neutering in both males and females is associated with increased risk of ligament rupture.95,295 As for elbow osteoarthritis, males were found to be at 1.8 times greater risk of developing osteoarthritis than females,186 but the effect of neutering on elbow osteoarthritis is not clear, probably because studies of elbow dysplasia and osteoarthritis tend to rely on data from radiographic screening programs for breeding animals, in which neutered animals are not included. Exercise, Diet, and Housing.: Candidate environmental factors that may contribute to the risk or progression of canine osteoarthritis include variables such as nutrition, exercise, and housing conditions. However, very few studies have explored the effects of environmental factors on the development or progression of canine osteoarthritis. One study of a birth cohort of Boxers found that the risk of clinical signs of hip dysplasia or hip osteoarthritis was increased if the floor upon which puppies were housed (up to the first 49 days of life) was categorized as “slippery.” The category of “slippery” was used when it was covered with tarpaulin or newspapers, and “nonslippery” was used when covered with carpet, rubber, blankets, sawdust, or straw.327 These data suggest that breeders of “at risk” breeds should house their puppies on nonslippery surfaces. The effects of exercise on canine osteoarthritis have received little attention in the literature to date. One recent study investigated ten dogs with chronic and stable hindlimb lameness associated with osteoarthritis, along with ten healthy control dogs. Dogs were subjected to force platform gait analysis to determine baseline data; they were thereafter trotted for a distance of 1.2 km on a short leash, after which gait analysis was immediately repeated to determine postexercise values. In the control group, differences between baseline and postexercise data were not significant. Conversely, postexercise peak and impulse values of the vertical force, as well as the peak of the propulsion force values, were significantly lower than baseline in the osteoarthritis group.36 A Swedish case control study investigated 325 dogs from a year 2000 birth cohort of Labrador Retrievers with known status on both hip dysplasia and elbow dysplasia at the age of 12 to 24 months.274 Each case of hip dysplasia was matched with a control dog of the same sex, born at maximum 15 days apart. Owners of case subjects and controls were sent a validated questionnaire regarding diet, bodyweight and condition score, and exercise. Type, frequency, and amount consumed of commercial food, table food, and homemade diets were recorded. Owners were also questioned about treats and the use of vitamin and mineral supplements. Another section of the questionnaire, concerning exercise and the way the dogs perform and lead their lives, recorded time spent on, and seasonal variations in, different training activities. The final section identified the weight and size of the dog compared with averages for the breed, as well as owner-perceived body condition score. Results showed no significant difference between cases and controls for hip or elbow dysplasia among the proportions fed homemade diets, table foods, commercial diets, or treats. High dietary intake of fat and high proportions of energy derived from fat were shown to be significant risk factors for elbow osteoarthritis. Identified risk factors for hip and elbow dysplasia included being exercised by running after balls and sticks thrown by the owner. The pathogenesis of osteoarthritis involves changes in all tissues of the synovial joint. Central to this are alterations in metabolism and morphology of articular cartilage, dramatic changes in subchondral bone metabolism and architecture, osteophyte and enthesophyte formation, and synovial inflammation and fibrosis. Current evidence increasingly implicates cross-talk between various tissues of the joint, particularly synovium and cartilage. In addition, it should be remembered that osteoarthritis is associated with changes in other tissues such as surrounding muscles, ligaments, and tendons as part of the process of disuse and inhibition of ipsilateral musculature. Furthermore, changes in the central nervous system caused by this chronic condition can lead to the phenomenon of pain sensitization (see Chapter 22). Articular Cartilage.: Much of our knowledge of events in canine osteoarthritis comes from experimentally induced models such as the Pond-Nuki cranial cruciate ligament transaction model.258 Typical features of osteoarthritis include degeneration or progressive loss of structure and functionality of articular cartilage. Grossly, the tissue loses compressive stiffness and tensile strength, and the surface of the tissue begins to fibrillate. As the disease process progresses, cartilage tissue is lost and erosion and ulceration ensue. Overall, the pathophysiologic process of osteoarthritis can be divided into three overlapping stages: in the beginning, the extracellular matrix degrades on a molecular level, the water content increases, the size of aggrecan molecules within the tissue decreases,188 and the structure of the collagen network is damaged, all of which leads to reduced stiffness of the cartilage. Second, chondrocytes try to compensate for the damage through enhanced proliferation and metabolic activity—cell clusters, formed by cloning, appear surrounded by newly synthesized matrix molecules. This condition can remain for several months to years. In stage three, the chondrocytes are not able to keep up their repair activity, and complete loss of cartilage tissue is the consequence. Within osteoarthritic cartilage, an imbalance is evident between anabolic and catabolic processes in the tissue, with both degradation and synthesis upregulated. Many cytokines and growth factors are produced by synovial lining cells and chondrocytes.210,211 In the short to medium term (1 to 3 years), an increase in cartilage thickness occurs,166 which is associated with tissue swelling and an anabolic response that produces more cells and more extracellular matrix.2 However, as disease progresses, cartilage tissue is lost and end-stage disease involves ulceration of cartilage and eburnation of subchondral bone.49 Degradation of the components of the extracellular matrix of articular cartilage and cell death are key processes in osteoarthritis (Figure 68-3). Inflammatory cytokines such as IL-1, IL-17, IL-18, and TNF-α upregulate the synthesis of certain matrix metalloproteinases and other proteolytic enzymes112; concomitantly, synthesis of their inhibitors (tissue inhibitors of metalloproteinases [TIMPs]) is decreased. Given the potential role of inflammatory prostaglandins and the use of nonsteroidal antiinflammatory drugs (NSAIDs) for the treatment of osteoarthritis, there has been considerable interest in the role of COX in osteoarthritis. Chondrocytes from human osteoarthritic cartilage explants express COX-2 and spontaneously produce prostaglandin E2 (PGE2).294 In addition, it was recently reported that PGE2 produced by osteoarthritic cartilage explants decreased proteoglycan synthesis and enhanced the degradation of both aggrecan and type II collagen. These effects are associated with downregulation of MMP-1, together with upregulation of MMP-13 and disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5).13 In addition, some evidence suggests that COX inhibition may provide beneficial effects in cartilage.83 Among other inflammatory mediators of interest in the pathogenesis of osteoarthritis are both oxygen- and nitrogen-derived free radicals. Reactive oxygen species such as superoxide anion, hydrogen peroxide, and hydroxyl radicals directly promote chondrocyte apoptosis, most probably via mitochondrial dysfunction.5 Nitric oxide (NO), produced by the inducible isoform of nitric oxide synthase (iNOS), appears to be another major catabolic factor produced by chondrocytes in response to cytokines such as IL-1β and TNF-α. Considerable evidence indicates that overproduction of NO by chondrocytes plays a role in the progression of cartilage loss in osteoarthritis. Although normal cartilage does not express iNOS nor produce NO without stimulation by inflammatory cytokines, osteoarthritic cartilage explants spontaneously produce considerable quantities of NO. Degradation of aggrecan appears to be a very early event in canine osteoarthritis334 and is followed by disruption of the collagen network. Aggrecan can be degraded by matrix metalloproteinases such as MMP-13, but the “aggrecanase” enzymes appear to be particularly important. The aggrecanases,318 also known as ADAMTS-4 and ADAMTS-5, cleave the aggrecan protein core in the interglobular domain between G1 and G2142; this action releases most of the molecule, including the negatively charged sugar side-chains, from the matrix.165 It is currently thought that ADAMTS-5 is upregulated in murine models of osteoarthritis, and recent studies indicate that this enzyme may be critical for disease progression.107,125,308 However, which of these enzymes is most important in canine and feline osteoarthritis remains unknown. The intact triple helix of type II collagen can be degraded only by MMP-1 and MMP-13, and possibly MMP-8 and MMP-14.43,81,236 The enzymes are secreted as pro-forms that are activated by partial proteolysis. In addition, the action of these enzymes is controlled by natural inhibitors, tissue inhibitors of metalloproteinases, which are produced such that the balance between proteolytic activity and inhibitors is critical.236 Cartilage oligomeric matrix protein is the most abundant noncollagenous protein of articular cartilage. It putative role is seen in the assembly of collagen fibrils,135,259 and it has been extensively studied in human osteoarthritis as a biomarker of human disease progression.60,281,292 Data are limited on the role of cartilage oligomeric matrix protein in canine osteoarthritis, but they suggest increased cartilage oligomeric matrix protein catabolism in early osteoarthritis.123,231 Anabolic changes in osteoarthritic cartilage probably represent a repair response. Although an anabolic response occurs, disturbances to the anabolic mediators are not fully understood at the current time. Synthesis of cartilage matrix molecules can be stimulated by various growth factors such as insulin-like growth factor (IGF-1 and IGF-2) and transforming growth factor-β (TGF-β). Both IGF and TGF-β can stimulate aggrecan and collagen synthesis. The availability of IGF is controlled by circulating and locally produced binding proteins (IGFBPs), which act to extend the circulating half-life of IGF, but also act to control the local availability of IGF to bind to its receptor. Disturbance to the IGF-IGFBP “system” in canine osteoarthritis104 reveals that the availability of IGF may be decreased. In addition, expression of TGF-β is reduced in osteoarthritis.80 Synovium.: The synovium is the joint capsule lining layer; although it is often called the synovial membrane, it is not in fact a membrane but a discontinuous layer of fibroblast-like and macrophage-like cells. Osteoarthritis involves variable synovitis and capsular fibrosis, and indeed interest in this aspect of the disease process is increasing.47,48 As a species, the dog seems particularly prone to development of synovitis during the process of osteoarthritis, particularly during the early stages. Most available information has been gathered by evaluating change in the stifle joint associated with, or following, cranial cruciate ligament transection. Synovial histologic changes include synovial hypertrophy and often hyperplasia with an increased number of lining cells, often accompanied by marked infiltration of the sublining tissue with foci of lymphocytes.50,147,198 Cartilage breakdown products, derived from the articular surface as a result of mechanical or enzymatic destruction of the cartilage, can provoke the release of collagenase and other hydrolytic enzymes from synovial cells and macrophages.81 Indeed the macrophage is likely to be a key cell in driving synovial control of cartilage metabolism through the release of catabolic cytokines such as IL-1β and TNF-α, which are probable contributors to the degradative cascade (see Figure 68-3). Experiments in the collagenase-induced mouse model of osteoarthritis investigated the role of macrophages by depleting the synovium of macrophages using the bisphosphonate, clodronate, encapsulated in liposomes and delivered intra-articularly.46 These experiments showed a reduction in synovial matrix metalloproteinases associated with a reduction in articular cartilage aggrecanolysis. Such data implicate the synovium in the progression of chondropathy in osteoarthritis. Furthermore, the synovium is likely to be a key tissue in the origin of pain in osteoarthritis.77 Subchondral Bone.: Osteophyte formation and subchondral bone sclerosis are key features of osteoarthritis. The genuine osteophyte, or osteochondrophyte, arises in the periosteum overlying the bone at the junction between cartilage and bone.326 Osteophytes can contribute both to the functional properties of affected joints and to clinically relevant symptoms. Osteophyte formation is highly associated with cartilage damage, but osteophytes can develop without explicit cartilage damage. Mesenchymal stem cells present in the periosteum or synovial lining are thought to be the precursors of osteophytes. In murine experimental osteoarthritis, osteophytes originate primarily from the periosteum covering the bone at the cartilage/bone junction. However, cell populations from the synovium can be triggered to form cartilage in vitro, and synovium-derived mesenchymal stem cells have been shown to be even more efficient in cartilage formation than bone marrow–derived mesenchymal stem cells. Growth factors of the TGF-β superfamily appear to play a crucial role in the induction of osteophytosis. TGF-β, when introduced into the joint in experimental animals, induces osteophyte formation, and TGF-β expression is observed in osteophytes in human patients and experimental animals with osteoarthritis.326,328 Several studies have evaluated the subchondral bone changes associated with canine osteoarthritis. In the canine cranial cruciate transaction model of osteoarthritis, thinning and increased porosity of the subchondral bone plate301 is followed by sclerosis.85 Recent data suggest that subchondral bone plate thinning is associated with cartilage damage,167 but the colocalization of pathologies in these adjacent tissues does not elucidate cause and effect. Data from gene knockout mouse models of osteoarthritis, induced by destabilization of the medial meniscus, suggest that pathology in articular cartilage and that in subchondral bone are progressive over time but are likely independent of each other.206 Pain in Osteoarthritis.: Our current understanding of joint pain is poor, and osteoarthritis provides additional challenges with respect to understanding the relationship between joint pathology and pain, which apparently is not linear and is not predictable. Much information on nociception in the joint comes from human studies or animal models. Pain in the joint is often dull and aching and is poorly localized in contrast to cutaneous pain. To date, the neuronal organization of joint pain has not been fully elucidated, but most available information on this topic describes the innervation of joints. Joint nerves contain Aβ-, Aδ-, and C-fibers. Corpuscular endings of Aβ-fibers are found in ligaments and in the fibrous joint capsule, and free nerve endings are present in all structures of the joint except normal articular cartilage. From all joint structures including ligaments, fibrous capsule, adipose tissue, meniscus, periosteum, and synovial layer, but not cartilage, conscious sensations can be evoked. In human beings who are awake, direct stimulation of fibrous structures with innocuous mechanical stimuli evokes pressure sensations. However, pain is elicited when noxious mechanical, thermal, and chemical stimuli are applied to fibrous structures such as ligaments and fibrous capsule.99 No pain is elicited by stimulation of cartilage, and stimulation of normal synovial tissue rarely evokes pain.176 In daily life, pain in a normal joint is most commonly elicited by twisting or contusing the joint. Movements in the working range of a normal joint usually are not painful, and palpation of a normal joint does not hurt. A large group of mainly C-fibers are so-called silent nociceptors because they do not respond even to noxious mechanical stimuli of the normal joint. They begin to respond to mechanical stimulation during inflammation of the joint. During inflammation, numerous silent nociceptors develop sensitivity for mechanical stimulation of the inflamed joint. This recruitment of fibers significantly increases input into the spinal cord,131a,285a,285b and neuronal changes provide a plausible explanation for the occurrence of mechanical hyperalgesia or pain in the inflamed joint. Whether they also evoke other sensations such as pressure or stiffness is unknown. In summary, the joint is equipped with a large number of nerve fibers that are suitable to encode painful mechanical stimuli.284,285 It is thought that sensitization of primary afferent fibers for mechanical stimuli is produced by inflammatory mediators, including TNF-α, IL-6, bradykinin, PGE2, PGI2, serotonin, substance P, galanin, neuropeptide Y, and nociception.283 For example, in a study using collagen-induced arthritis in mice, the neutralization of TNF-α reduced both mechanical hyperalgesia (as judged from the testing of withdrawal responses in behavioral experiments) and the inflammatory process.157 Thus it appears that inflammatory mediators play a significant role in adapting the responses of nociceptors within the articular tissues. Evidence also indicates that joint pain results in the development of central sensitization,228,237 which is one of the mechanisms leading to increased pain. Further, it has been demonstrated that COX enzymes play a role in central sensitization,275,332 and that COX inhibitors can prevent the establishment of central sensitization.332 Growing evidence suggests that central sensitization can actually drive the progression of osteoarthritis pathology, and that downward modulation of central sensitization can result in decreased joint pathology.105,296 In addition, a direct effect of NSAIDs at the level of the joint may result in a reduction in disease progression.253,254 One such mechanism is seen in the prevention of NO-induced cell death. Several studies evaluating animal models or human beings have shown that osteoarthritis cartilage has a higher number of apoptotic chondrocytes compared with normal cartilage.7,143,182,325 The production of NO may represent an important component in the pathogenesis of osteoarthritis. NO is produced in large amounts by chondrocytes upon proinflammatory cytokine stimulation. Selective inhibition of COX-2 significantly inhibits NO-induced cell death.171 Osteoarthritis often is not a diagnosis of sufficient accuracy in canine orthopedics because the disease is usually secondary and is the result of some other primary joint abnormality (e.g., instability, laxity, fracture). Other chapters in this textbook discuss methods used in reaching such diagnoses, and a seamless transition is often seen between the primary joint disease and the process of osteoarthritis. Idiopathic osteoarthritis is diagnosed when all attempts to identify an initiating cause have failed. Examples include osteoarthritis of the small joints of the manus and pes, as seen in older dogs (Figure 68-4), and osteoarthritis of the elbows, as seen in older cats (Figure 68-5).
Arthritis
Introduction and Classification of Arthritis
“Noninflammatory” Types of Arthritis
Classification
Etiology
Pathogenesis of Osteoarthritis
Diagnosis and Staging of Osteoarthritis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Arthritis
Only gold members can continue reading. Log In or Register to continue