Antiinfective Drugs
Learning Objectives
After studying this chapter, you should be able to
1. Identify the classes of antiinfective drugs.
2. Describe the adverse side effects of antiinfective drugs.
3. Explain the clinical uses of antiinfective drugs.
4. Explain the clinical uses of antifungal agents.
Key Terms
Antibacterial
Antibiotic
Antimicrobial
Bacteria
Bactericidal
Bacteriostatic
Beta-lactamase
Detergent
Disinfect
Efficacy
Fungicidal
Fungistatic
In vitro
In vivo
Iodophor
Microorganism
Nephrotoxic
Ototoxic
Sporicidal
Tachypnea
Teratogenic
Thrombophlebitis
Introduction
Microorganisms are ubiquitous in the environment. Some microorganisms have pathogenic potential, but others do not. Animals usually make initial contact with an infectious agent somewhere on the body’s surface (e.g., mucous membranes, skin, respiratory tract, or digestive tract). In the fight against infection, several hundred antimicrobial drugs have been developed since the early 1900s. These drugs have been used to fight disease in both humans and animals.
Not all antimicrobials have the same degree of effectiveness against microorganisms. A determination can be made to distinguish different types of bacteria with the use of a Gram stain. The Gram stain is a laboratory procedure in which dyes are used to stain bacteria (Figure 12-1). Gram-positive bacteria stain dark blue to purple. Gram-negative bacteria stain pink to red. However, some bacteria cannot be identified through the Gram stain technique. For differentiating acid-fast bacilli, carbol fuchsin stain can be used and then decolorized with ethyl alcohol and hydrochloric acid. Other bacteria must be identified by special techniques such as dark-field examination or Gimenez stain. Giemsa and Wright stains may be used to identify parasites and intracellular microorganisms. Bacteria with similar staining properties tend to respond to the same antimicrobial therapy. Still other bacteria are classified by their ability to survive with or without oxygen. Aerobes are bacteria that must have oxygen to live and replicate. Other bacteria are able to live and multiply without oxygen; these are known as anaerobes. Anaerobes may be hardy and difficult to eradicate.
Mechanism of Action
Through analysis of the effects that a drug’s action has on bacteria, antimicrobial drugs can be divided into two categories: bactericidal and bacteriostatic. However, some strains of mutant bacteria have greater resistance to some antimicrobials. Resistant strains of bacteria can make antimicrobial therapy difficult. Therefore, to prevent mutant strains from developing, antimicrobial drugs must not be used indiscriminately. Sometimes, it may be necessary to use two different types of antimicrobial drugs to treat patients with infections caused by two or more different organisms.
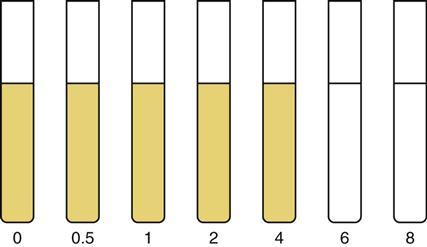
After a laboratory has identified the type of organism that is causing an infection, an antibiotic sensitivity test may be performed. Several tests are available for testing the susceptibility of an organism to a specific antimicrobial drug. Most commonly, the disk susceptibility test is used in small laboratories (Figure 12-2). With this test, an agar plate with a standard amount of cultured organism is used. Using a dispenser, paper disks impregnated with various antimicrobial drugs are placed within the agar plate. Incubation is carried out, along with measurement of the zones of inhibition. These zones show which antimicrobial agents are susceptible or resistant to each particular antimicrobial and how effectively they may perform in vitro. The Kirby-Bauer procedure is commonly used in many laboratories.
The broth dilution susceptibility test is also used in many laboratories (Figure 12-3). An organism is inoculated into a series of tubes or wells in a microculture plate. These tubes or wells contain different concentrations of antimicrobials. The lowest concentration that macroscopically inhibits the growth of an organism is the minimum inhibitory concentration (MIC). The MIC represents the degree of susceptibility of an organism to a specific concentration of a particular antimicrobial drug. The antimicrobials that are effective in vitro may not always be the best choice for use in vivo. A clinician chooses which agent to use by considering the diagnosis and assessing each agent’s pharmacodynamics and pharmacokinetics. This process allows a clinician to choose the most efficient and efficacious drug to treat a specific condition.
 Aminocyclitols
Aminocyclitols
Pharmacodynamics/Pharmacokinetics
Aminocyclitols belong to a class of drugs that are sugar-derived and that demonstrate important biologic value. Aminocyclitols are components that make up aminoglycoside-type antibiotics. Aminoglycoside antibiotics act on susceptible bacteria presumably by irreversibly binding to the 30S ribosomal subunit, thus inhibiting protein synthesis. Antimicrobial activity of aminoglycosides is enhanced in an alkaline environment. Aminoglycoside antibiotics are inactive against fungi, viruses, and most anaerobic bacteria (Plumb, 2011).
Aminoglycosides are not absorbed well after oral or intrauterine administration. They can be absorbed from topical administration when used in irrigations during surgical procedures. After intramuscular (IM) administration to dogs and cats, levels peak from 30 minutes to 1 hour later. After absorption, aminoglycosides are distributed primarily in the extracellular fluid. They do not readily cross the blood–brain barrier nor do they penetrate ocular tissue. Aminoglycosides tend to accumulate in the inner ear and kidneys, which explains their toxicity to those organs. Elimination of aminoglycosides after parenteral administration occurs almost entirely by glomerular filtration (Plumb, 2011). See Table 12-1.
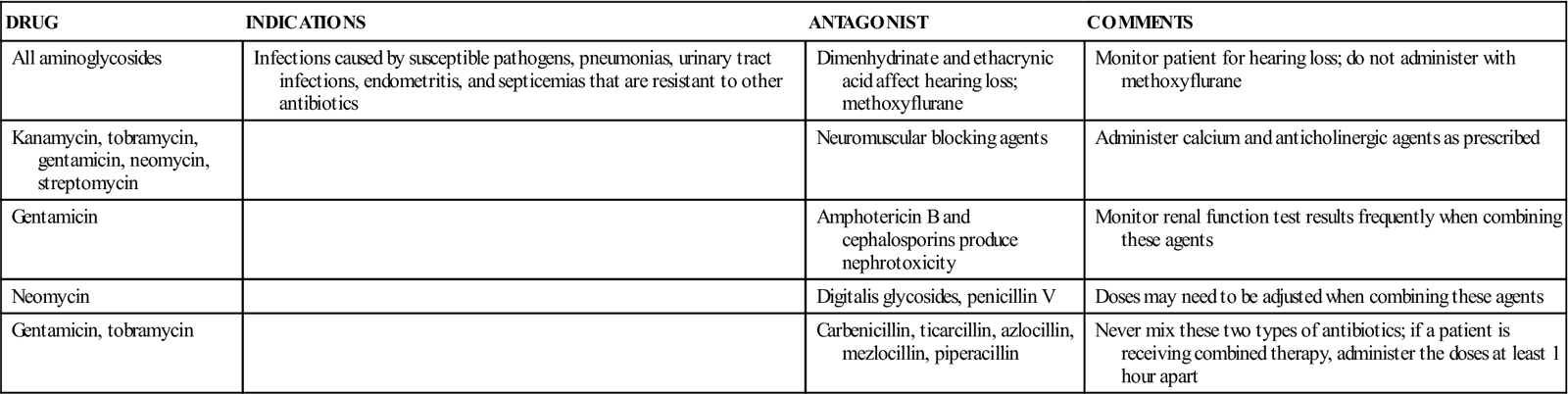
TABLE 12-1
Aminoglycoside Preparations, Indications, and Antagonistic Drugs
| DRUG | INDICATIONS | ANTAGONIST | COMMENTS |
| All aminoglycosides | Infections caused by susceptible pathogens, pneumonias, urinary tract infections, endometritis, and septicemias that are resistant to other antibiotics | Dimenhydrinate and ethacrynic acid affect hearing loss; methoxyflurane | Monitor patient for hearing loss; do not administer with methoxyflurane |
| Kanamycin, tobramycin, gentamicin, neomycin, streptomycin | Neuromuscular blocking agents | Administer calcium and anticholinergic agents as prescribed | |
| Gentamicin | Amphotericin B and cephalosporins produce nephrotoxicity | Monitor renal function test results frequently when combining these agents | |
| Neomycin | Digitalis glycosides, penicillin V | Doses may need to be adjusted when combining these agents | |
| Gentamicin, tobramycin | Carbenicillin, ticarcillin, azlocillin, mezlocillin, piperacillin | Never mix these two types of antibiotics; if a patient is receiving combined therapy, administer the doses at least 1 hour apart |
Amikacin
Clinical Uses
Parenteral use is only Food and Drug Administration (FDA)-approved in dogs and is used to treat serious gram-negative infections. Amikacin is FDA-approved for intrauterine infusion in mares and for intraarticular injection in foals to treat septic arthritis.
Dosage Forms
All of the following are veterinary-label products.
Adverse Side Effects
This drug is ototoxic and nephrotoxic. Cats are very susceptible to the vestibular effects of amikacin. In the United States, amikacin is not FDA-approved for use in cattle or other food animals.
Storage of the Drug
Amikacin can be stored at room temperature and is stable for up to 2 years. Solutions over time may become pale yellow, but this does not decrease the drug’s efficacy.
Apramycin
Clinical Uses
Some countries use this drug to treat bacterial enteritis, colibacillosis, and salmonellosis in pigs, calves, and poultry (Plumb, 2011).
Dosage Forms
Adverse Side Effects
Do not use this drug in cats. Do not use in patients with myasthenia gravis.
Storage of the Drug
Apramycin powder should be stored in a cool dry place, in a tightly closed container. It should be protected from moisture. It should be stored at less than 25°C (or 77°F).
Gentamicin
Clinical Uses
This drug is used in the treatment of serious gram-negative infections.
Dosage Forms
All of the following are veterinary-label products.
Adverse Side Effects
Gentamicin should be used with extreme caution in patients with chronic renal failure (CRF). Do not use in patients that are debilitated, who have a fever, sepsis, or dehydration. Use this drug with caution in working dog breeds such as seeing-eye dogs, herding dogs, or dogs for the hearing impaired because it can cause ototoxicity. Do not use in animals with neuromuscular disorders or in patients with botulism. IM injections in horses may cause muscle irritation, so intravenous (IV) injections are preferred. Do not use gentamicin in rabbits.
Storage of the Drug
Gentamicin sulfate for injection and the oral solution should be stored at room temperature (15°C–30°C [59°F–86°F]). Do not store the drug in rusty containers, nor offer medicated-drinking water in rusty containers because the drug will be destroyed.
Neomycin
Clinical Uses
Neomycin is more nephrotoxic than the other aminoglycosides. It is also less effective against gram-negative organisms. Its use topically is mainly for the skin, eyes, and ears; orally, it is used to treat enteric infections, reduce microbe numbers in the colon before surgery, and reduce ammonia-producing bacteria in the treatment of hepatic encephalopathy.
Dosage Forms
All of the following are veterinary-label products.
Adverse Side Effects
Rarely, neomycin has caused ototoxicity, nephrotoxicity, severe diarrhea, and malabsorption of the intestines.
Storage of the Drug
Neomycin should be stored at room temperature, in tightly sealed, light-resistant containers. Neomycin (in the dry state) is stable for at least 2 years at room temperature.
Spectinomycin
Clinical Uses
This is sometimes used in dogs, cats, and horses. Spectinomycin only has FDA approval for cattle, chickens, turkeys, and swine.
Dosage Forms
All of the following are veterinary-label products.
Adverse Side Effects
When spectinomycin is used correctly, adverse effects are uncommon. It probably has less ototoxicity and nephrotoxic activity than other aminoglycosides. Cattle that are injected subcutaneously (SC) have developed swelling at the site of injection.
Storage of the Drug
Store at room temperature. Avoid freezing.
Tobramycin
Clinical Uses
This drug is used for the prevention of cystine urolithiasis in patients that show no improvement after the use of dietary therapy combined with urinary alkalinization.
All of the following are veterinary-label products.
Adverse Side Effects
According to Plumb (2011), increased liver enzymes, lethargy, dermatologic effects, aggressiveness, sulfur odor to the urine, or myopathy may be noted with this drug.
Storage of the Drug
Store tablets at room temperature in tightly sealed containers.
 Carbapenems
Carbapenems
Pharmacodynamics/Pharmacokinetics
Carbapenems are a class of beta-lactam antibiotics with a wide range of antibacterial activity. Carbapenems inhibit bacterial cell wall synthesis, and they are usually bactericidal.
There are currently no pharmacokinetic data available for dogs and cats. In humans, these drugs are excreted in the urine or feces.
Ertapenem
Clinical Uses
Ertapenem may be useful in treating gram-negative bacterial infections and works well in the place of aminoglycosides when they should not be used.
Dosage Form
There are no veterinary label products available.
Adverse Side Effects
The adverse effects in dogs and cats are unknown.
Storage of Drug
Ertapenem should be stored at room temperature.
Imipenem–Cilastatin
Clinical Uses
This drug combination is useful in equine or small animal medicine to treat serious infections when less expensive antibiotics perform poorly.
Dosage Forms
Only human-label products are available.
Adverse Side Effects
Gastrointestinal (GI) problems such as vomiting, anorexia, and diarrhea may be seen along with central nervous system (CNS) toxicity, pruritus, and anaphylaxis. It may cause seizures if the IV dose is given too rapidly.
Storage of Drug
Store at room temperature. After reconstitution, the solution is stable for 4 hours at room temperature or 10 hours if refrigerated. Do not freeze solutions.
Meropenem
Clinical Uses
Meropenem is useful in treating resistant gram-negative bacterial infections, especially when aminoglycosides may pose a risk.
Dosage Form
Only human-label products are available.
Adverse Effects
When given SC, meropenem may cause animals to have hair loss at injection sites.
 Cephalosporins
Cephalosporins
Pharmacodynamics/Pharmacokinetics
Cephalosporins are a group of broad-spectrum, semisynthetic antibiotics derived from Cephalosporium acremonium, a species of soil-inhabiting fungi, which share the nucleus 7-aminocephalosporanic acid. Cephalosporins that were named before 1975 are spelled with ph, and those named after 1975 are spelled with f. First-generation cephalosporins are active mainly against gram-positive bacteria. Second-generation cephalosporins have a broader spectrum of activity, and third-generation cephalosporins are mainly used against gram-negative organisms. Fourth-generation cephalosporins have an extended spectrum and have increased resistance to hydrolysis and B-lactamases (Plumb, 2011). See Table 12-2.
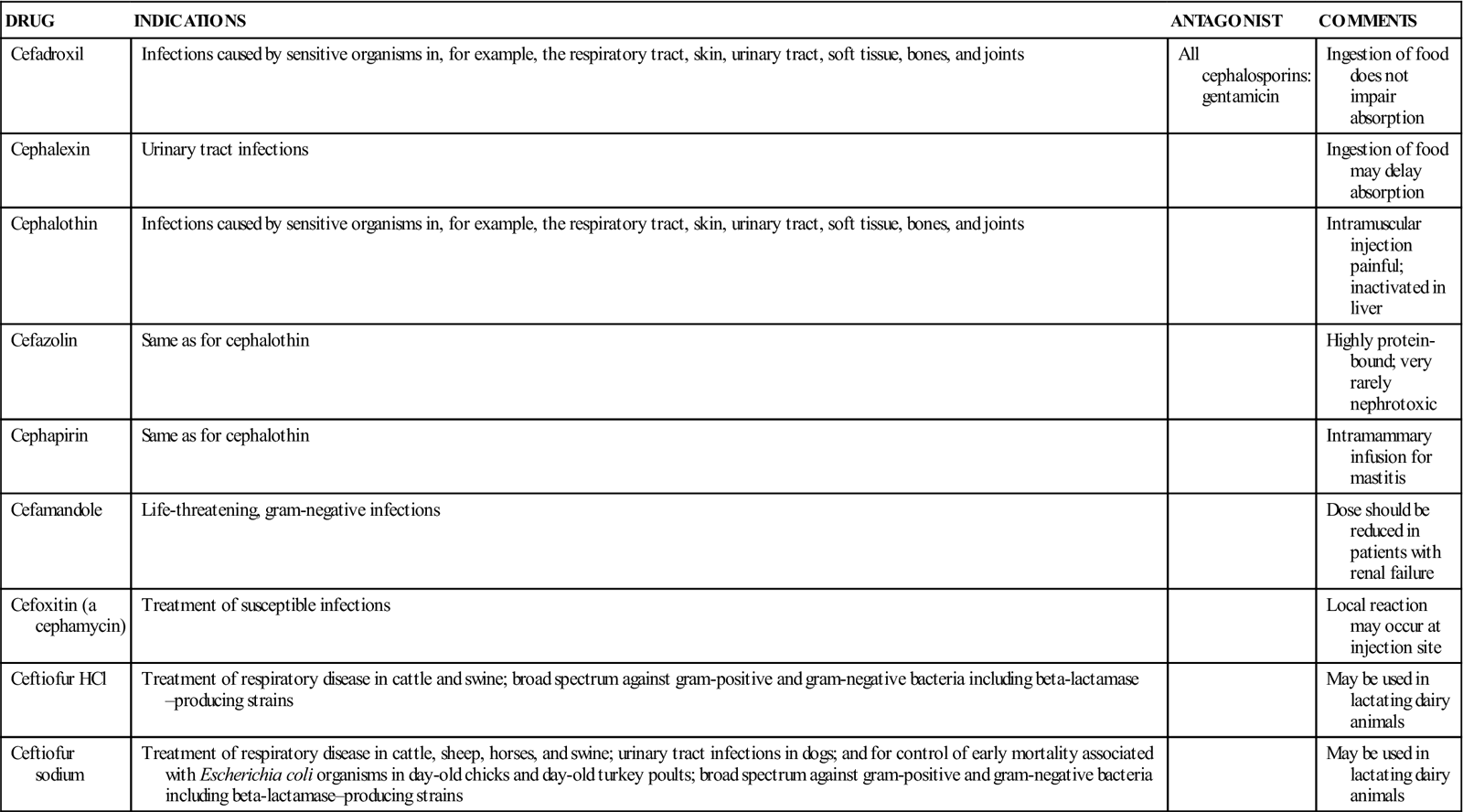
TABLE 12-2
Cephalosporin Preparations, Indications, and Antagonistic Drugs
| DRUG | INDICATIONS | ANTAGONIST | COMMENTS |
| Cefadroxil | Infections caused by sensitive organisms in, for example, the respiratory tract, skin, urinary tract, soft tissue, bones, and joints | All cephalosporins: gentamicin | Ingestion of food does not impair absorption |
| Cephalexin | Urinary tract infections | Ingestion of food may delay absorption | |
| Cephalothin | Infections caused by sensitive organisms in, for example, the respiratory tract, skin, urinary tract, soft tissue, bones, and joints | Intramuscular injection painful; inactivated in liver | |
| Cefazolin | Same as for cephalothin | Highly protein-bound; very rarely nephrotoxic | |
| Cephapirin | Same as for cephalothin | Intramammary infusion for mastitis | |
| Cefamandole | Life-threatening, gram-negative infections | Dose should be reduced in patients with renal failure | |
| Cefoxitin (a cephamycin) | Treatment of susceptible infections | Local reaction may occur at injection site | |
| Ceftiofur HCl | Treatment of respiratory disease in cattle and swine; broad spectrum against gram-positive and gram-negative bacteria including beta-lactamase–producing strains | May be used in lactating dairy animals | |
| Ceftiofur sodium | Treatment of respiratory disease in cattle, sheep, horses, and swine; urinary tract infections in dogs; and for control of early mortality associated with Escherichia coli organisms in day-old chicks and day-old turkey poults; broad spectrum against gram-positive and gram-negative bacteria including beta-lactamase–producing strains | May be used in lactating dairy animals |
Orally Active
Parenterally Active
Cephalosporins that are active by parenteral administration are placed into four groups.
Group I
This group has fair activity against gram-positive bacteria and moderate activity against gram-negative bacteria; the drugs show poor activity against Pseudomonas.
Group II
This group has high activity against Enterobacteriaceae.
Group III
This group has high activity against Pseudomonas and other gram-negative bacteria.
Group IV
This group is resistant to β-lactamase.
Cefaclor
Clinical Uses
Cefaclor is used to treat infections that are resistant to first-generation cephalosporins.
Dosage Forms
Only human-label products are available.
Adverse Side Effects
In humans, this drug can cause GI upset and hypersensitivity reactions. It may cause increases on liver function tests, as well as intermittent increases in blood urea nitrogen (BUN) and serum creatinine levels.
Storage of the Drug
Capsules, tablets, and powder for suspension should be kept at room temperature. After the oral suspension has been reconstituted, it should be refrigerated and discarded after 14 days.
Cefadroxil
Clinical Uses
Cefadroxil is useful in treating infections of the skin, soft tissue, and genitourinary tract in dogs and cats.
Dosage Forms
Products are human-label unless otherwise specified.
Adverse Side Effects
These are usually not serious and occur rarely. They include hypersensitivity, GI effects, the potential for nephrotoxicity, and tachypnea, rarely.
Storage of the Drug
Cefadroxil should be stored at room temperature. After reconstitution, the suspension should be refrigerated and discarded after 14 days.
Cefazolin
Clinical Uses
In the United States, there are no FDA-approved cefazolin products for veterinary use. It may be used for surgical prophylaxis in humans.
Dosage Forms
Only human-label products are available.
Adverse Side Effects
These are usually not serious and do not often happen. Some signs that may be seen include hypersensitivity, fever, eosinophilia, and lymphadenopathy. Pain at the injection site may occur when given IM, and it may have the potential for nephrotoxicity.
Storage of the Drug
Cefazolin sodium powder and solutions for injection should be protected from light. The powder should be stored at room temperature. After reconstitution, the solution is stable for 24 hours when kept at room temperature or 96 hours if refrigerated. After reconstitution, if freezing is desired, the preparation is stable for at least 12 weeks.
Cefepime
Clinical Uses
Cefepime is useful in treating serious infections in dogs or foals when aminoglycosides are contraindicated.
Dosage Form
Only human-label products are available.
• Maxipime
Adverse Side Effects
Clinical signs that may be seen include loose stools in dogs. IM injections may be painful.
Storage of the Drug
The powder for injection should be stored between 35.6°F and 77°F.
Cefixime
Clinical Uses
Uses for cefixime in veterinary medicine are limited. It should be used when other cephalosporins or fluoroquinolones are contraindicated.
Dosage Form
Only human-label products are available.
• Suprax
Adverse Side Effects
GI upset, hypersensitivity, and possibly fever may occur.
Storage of the Drug
Cefixime powder for suspension should be stored at room temperature in tightly sealed containers. After reconstitution of oral suspension, refrigeration is not required. The solution should be discarded after 14 days regardless of whether it has been refrigerated.
Cefotaxime
Clinical Uses
Uses for this drug in veterinary medicine are limited. Its use should be reserved for cases in which other drugs are ineffective.
Dosage Form
Only human-label products are available.
• Claforan
Adverse Side Effects
GI distress, hypersensitivity, and possibly fever may occur.
Storage of the Drug
Cefotaxime’s storage is the same as that used for Cefixime.
Cefotetan Disodium
Clinical Uses
Cefotetan is a good choice for treating serious infections.
Dosage Form
Only human-label products are available.
Adverse Side Effects
This drug appears to be well tolerated. The following may be seen: hypersensitivity, pain at the injection site, and gut flora alteration. Cefotetan may cause nephrotoxicity.
Storage of the Drug
The sterile powder for injection should be stored below 22°C (71.6°F). Darkening of the powder over time does not harm its efficacy. After reconstituting with sterile water, the solution is stable for 24 hours if stored at room temperature or 96 hours if refrigerated.
Cefovecin
Clinical Uses
Cefovecin is FDA-approved to treat skin infections in dogs and in cats to treat wounds and abscesses.
Dosage Form
Adverse Side Effects
This drug is well-tolerated in dogs and cats. In dogs, it may cause some changes in laboratory results such as increases in gamma glutamyl transpeptidase (GGT) and alanine aminotransferase (ALT). In cats, changes in laboratory results include mild increases in ALT, increases in BUN, and moderately increased creatinine levels. In dogs, clinical signs including depression, lethargy, and vomiting may be seen.
Storage of the Drug
Store the powder and reconstituted product in the original container and keep refrigerated at 36°F to 46°F. Use the entire contents within 28 days after reconstitution. Protect from light. Solution that darkens over time does not adversely affect the drug’s potency.
Cefoxitin
Clinical Uses
Cefoxitin can be used clinically when injectable cephalosporins are indicated.
Dosage Form
Only human-label products are available.
Adverse Side Effects
These are usually not serious and rarely occur. Hypersensitivity and pain at the injection site may be seen, it may alter gut flora, and it has potential for nephrotoxicity.
Storage of Drug
Cefoxitin should be stored at temperatures less than 30°C.
Cefpodoxime Proxetil
Clinical Uses
This drug is used in the treatment of skin infections.
Dosage Forms
Adverse Side Effects
Cefpodoxime proxetil may cause diarrhea, vomiting, and allergic reactions.
Storage of the Drug
Store at 68°F to 77°F.
Ceftazidime
Clinical Uses
Ceftazidime is useful in treating gram-negative bacterial infections. It may be used in the treatment of gram-negative infections in reptiles.
Dosage Forms
Only human-label products are available.
Adverse Side Effects
Ceftazidime may cause GI disturbances, pain when administered by IM injection, and allergic reactions.
Storage of the Drug
The drug should be stored at 59°F to 86°F.
Ceftiofur Crystalline Free Acid
Clinical Uses
This drug is used in swine for the treatment of respiratory disease. It is used in cattle for the treatment of bovine respiratory disease (BRD), shipping fever, and pneumonia. In dairy cattle, it is used for the treatment of subclinical mastitis.
Dosage Forms
Only veterinary-label products are available.
Adverse Side Effects
Hypersensitivity reactions may occur.
Storage of the Drug
The ready-to-use injectable product should be stored at 68°F to 77°F.
Ceftiofur Sodium
Clinical Uses
Ceftiofur sodium is used in cattle for the treatment of respiratory disease and acute interdigital necrobacillosis. It is used in swine for the treatment of bacterial pneumonia. In sheep and goats, it is used for the treatment of pneumonia. In horses, it is used for the treatment of respiratory infections. It is used in dogs for the treatment of urinary tract infections and is used in chicks and poults for the control of early mortality. Withdrawal time in cattle is 4 days before slaughter. No milk discard time is needed. In sheep and goats, no withdrawal time for slaughter or withholding milk is needed. Do not use in horses intended for human consumption.
Dosage Form
• Naxcel
Adverse Side Effects
These are usually rare. Pain may follow injection. Administration to horses under stress may be associated with acute diarrhea that could be fatal. Hypersensitivity reactions may occur.
Storage of the Drug
Unreconstituted powder should be stored at room temperature. Protect from light. Color change will not affect efficacy. After reconstitution, the solution is stable for up to 7 days when refrigerated.
Ceftriaxone
Clinical Uses
Ceftriaxone is used to treat serious infections. It has activity against Borrelia burgdorferi, which makes it an excellent choice for treating Lyme disease.
Dosage Form
Only human-label products are available.
• Rocephin
Adverse Side Effects
These include hematologic effects, “sludge” in bile, hypersensitivity reactions, increased liver enzyme, BUN, and creatinine levels, and urine casts. Pain on IM injection may occur.
Storage of the Drug
The powder for reconstitution should be stored at or below 77°F.
Cefuroxime
Clinical Uses
Cefuroxime may be a good choice in small animal medicine for treating bacterial infections that are not responsive to first-generation cephalosporins.
Dosage Forms
Only human-label products are available.
Adverse Side Effects
GI effects may occur.
Storage of the Drug
Store tablets in tightly sealed containers at room temperature. Store powder for suspension at 35.6°F to 46.4°F.
Cephalexin
Clinical Uses
Cephalexin is not FDA-approved for veterinary use in the United States. It has been used to treat dogs, cats, horses, rabbits, ferrets, and birds that are infected with Staphylococcus.
Dosage Forms
Only human-label products are available.
Adverse Side Effects
There is a low occurrence of problems associated with cephalexin. Cephalexin has been known to cause salivation, tachypnea, and excitability in dogs and emesis and fever in cats.
Cephapirin
Clinical Uses
An intramammary cephapirin sodium product is FDA-approved in the United States for the treatment of mastitis.
Dosage Forms
All of the following are veterinary-label products.
Adverse Side Effects
These rarely occur. Allergic reactions, rashes, fever, and lymphadenopathy may be seen.
Storage of the Drug
Cephapirin intramammary syringes should be stored at controlled room temperature.
 Macrolides
Macrolides
Pharmacodynamics/Pharmacokinetics
Many of the macrolides inhibit protein synthesis by penetrating the cell wall and binding to the 50S ribosomes subunits in susceptible bacteria. Many of them are considered bacteriostatic antibiotics. They have a broad spectrum of activity. The majority of these drugs are excreted from the body in the bile.
Azithromycin
Clinical Uses
This is a good broad-spectrum agent. It may be used to treat Bordetella in canines.
Dosage Form
Only human-label products are available.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree