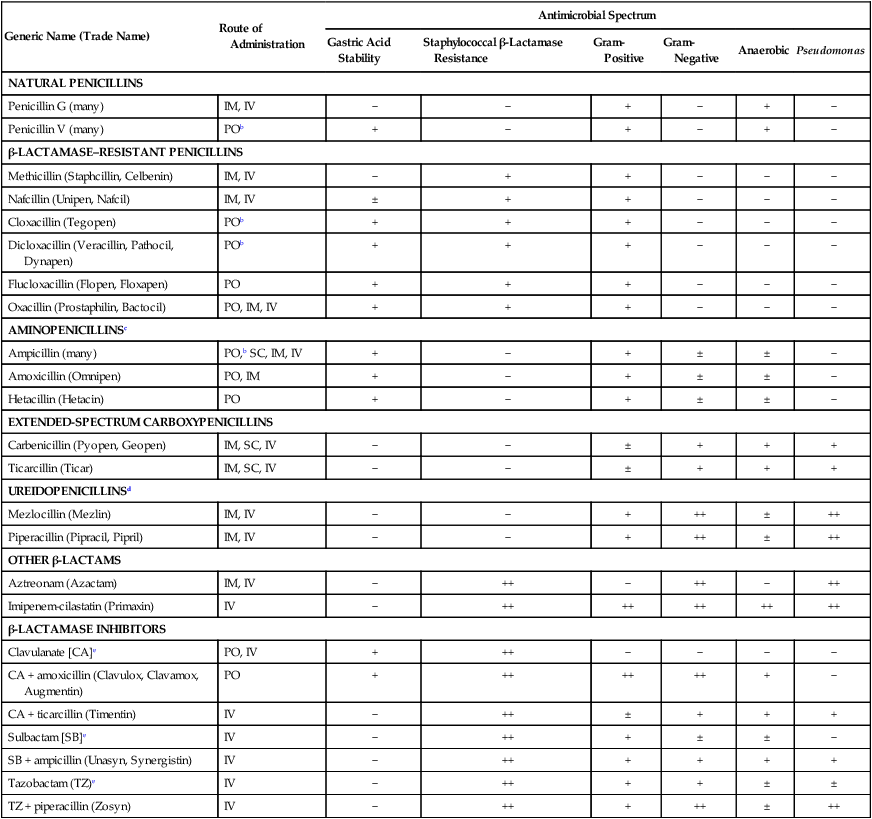
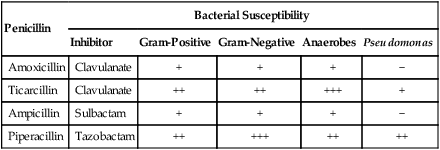
The terms antibiotic, antibacterial, and antimicrobial are often used interchangeably, despite different meanings. Antibiotics are chemicals (e.g., penicillin) produced by organisms (“natural”) intended to suppress other organisms (generally, but not exclusively, bacteria), whereas antimicrobial refers to any compound, whether natural, synthetic, or between, that suppresses microbial growth. Antibacterials target bacteria; antifungals target fungi, and so forth.61 Antibacterials, among the most widely used drugs in veterinary practice, are sometimes administered without adequate documentation that a bacterial infection exists. Although this practice may not immediately harm the patient, routine and irrational use of antimicrobials may have several undesirable consequences. Unrestricted use encourages the selection of resistant strains of bacteria, which subsequently limits the choice of effective agents.135 Long-term treatment with antibacterials may suppress the animal’s resident microflora, thereby allowing overgrowth of more resistant microorganisms. The worldwide increase in antimicrobial resistance has paralleled the use of drugs to combat microbial infections in humans and animals.406 For example, in a number of studies of antibacterial susceptibility data over time intervals during the past two decades, increases in prevalence of multiresistant bacteria have been observed.29,204,204 In comparing the intervals of 1990 to 1992 and 2002 to 2003, a significant decrease in susceptibility of canine bacterial isolates to commonly used antimicrobials was observed.26 Selection of bacterial subpopulations with greater antimicrobial resistance than the original population is an inevitable consequence of indiscriminant antibacterial treatment. Selection pressures are highest when bacteria are exposed to suboptimal dosage regimens (i.e., concentrations of drug below the minimum inhibitory concentration [MIC]). The therapeutic aim is to treat for an appropriate time interval at a therapeutically active dose that achieves bactericidal concentrations at the site of infection. Once resistance emerges, selection pressure forces it to remain if antimicrobial use is continued. Therefore, veterinary hospitals must develop protocols that minimize inappropriate and indiscriminate antibacterial use.442,555 Judicious use is essential because there are very few new antibacterial drugs on the horizon with novel mechanisms of action.110 Antimicrobial overuse and development of microbial resistance to effective drugs have become a major concern of the veterinary scientific community. The American College of Veterinary Internal Medicine,379 the American Association of Feline Practitioners (AAFP),12,163 the American Animal Hospital Association,11 and the American Veterinary Medical Association13 have all published consensus statements and guidelines addressing these concerns. One veterinary teaching hospital has published their experiences in implementing antimicrobial oversight.607 Selection of antimicrobials can be empirical or based on susceptibility data for the involved microorganisms. Empirical choices are based on the organ system of involvement, the suspected organisms involved, and a knowledge of the available drugs and their antimicrobial activity. Knowledge of the specific organ system infections can be obtained in Chapters 84 to 92. Unfortunately, the widespread and increasing use of antimicrobials in dogs and cats has caused a parallel increase in the resistance patterns of infectious organisms. Therefore, performance of susceptibility testing and proper interpretation of test data are needed. See Chapters 84 to 92 for the various collection methods, for specimens to be collected of body tissues and fluids, and Chapter 29 and Boothe61 for a discussion of methods of susceptibility testing and interpretation of results. The current chapter and the Drug Formulary in the Appendix, can be used to aid in selecting the proper drug and an appropriate dosage regimen. Good antimicrobial stewardship begins with critical evaluation as to the need to use antimicrobials in a particular clinical situation. Determining the need includes both identifying whether infection is causing clinical illness and confirming that antimicrobials are indicated for treatment of those clinical signs. Once the need for antimicrobial therapy is confirmed, the next consideration is to match the drug to the implicated organism, such that the most narrow-spectrum antimicrobial might be selected. The final, and equally important, consideration is to design a dosing regimen that is appropriate for the patient. This decision must take into account a multitude of factors presented by the chemotherapeutic triangle: host (e.g., target tissue, impact of inflammation, immunocompetence), microbial (e.g., presence of virulence factors, biofilm, resistance), and drug (e.g., lipophilicity, concentration versus time dependency, adverse events) factors.60 Paramount to successful antimicrobial therapy is resolution of clinical signs. Paramount to judicious use is optimizing the dosing regimen to avoid development of resistance. The two goals are not mutually inclusive. Antibacterials can be divided into two groups based on their patterns of bactericidal activity: concentration-dependent drugs and time-dependent drugs.62,127 For the concentration-dependent drugs, optimal activity is achieved with high peak-drug concentration compared to the MIC of the infecting microbe. This group comprises the aminoglycosides and quinolones (fluoroquinolones) and, under select circumstances, other drugs. Efficacy of quinolones also is associated with area under the curve (AUC). The highest safe, achievable drug concentration is the most important factor for aminoglycosides and quinolones. Pulse infusion or once-daily dosing is likely to maximize the efficacy of these agents and induce the least amount of bacterial resistance. Most of the remaining antimicrobials are the time-dependent drugs, which show optimal activity when plasma concentrations of approximately four times the MIC are maintained for 50% to 80% of the dosage interval.562 Drugs of this type are the β-lactams (penicillins, cephalosporins, monobactams, carbapenems), vancomycin, tetracyclines, phenicols, clindamycin, and most macrolides. For tetracyclines, macrolides, and vancomycin, efficacy is related to AUC, which is an index relative to maintaining necessary blood or tissue concentrations. Administration of these drugs by continuous infusion or multiple daily doses would be most efficacious for drugs of these groups with short half-lives. Antimicrobial combinations are used widely in veterinary practice, but few instances have occurred in which a synergistic outcome is documented; in many cases, combined use might reduce efficacy or enhance toxicity. Combination therapy may be most indicated for treatment of infections for which the risk of resistance is high. However, several potentially effective combinations exist. β-Lactams act on bacterial cell walls and increase the uptake of aminoglycosides. Cell wall–active agents such as penicillins, cephalosporins, imipenem, and vancomycin have been synergistic with the aminoglycosides streptomycin, gentamicin, and amikacin.2 Agents acting sequentially in a pathway, for example, sulfonamides and diaminopyrimidines (see the later discussion of diaminopyrimidines-sulfonamides), have also been effective and more bactericidal than either drug by itself. Combinations of agents acting on the cell wall, such as aminopenicillin (ampicillin, amoxicillin) together with a cephalosporin (cefotaxime, ceftriaxone) have been more effective against bacteria in vitro and in vivo, presumably because they attach to different binding proteins on the bacterial cell wall. The streptogramins (see the later discussion of streptogramins) consist of two components that act synergistically to inhibit protein synthesis in bacteria. β-Lactamase inhibitors (see the later discussion of other β-lactamase inhibitors) increase the activity and spectrum of β-lactams against various bacteria. Other synergistic antimicrobial combinations include quinolones, macrolides, or tetracyclines with rifampin, especially against persistent intracellular microorganisms in difficult-to-reach areas such as pyogranulomatous lesions or meninges. Presumably rifampin facilitates intracellular penetration of the other drugs. Combinations of quinolones with β-lactams or aminoglycosides may also be beneficial.487 Clinical applications that justify combination therapy include bacterial endocarditis, resistant Pseudomonas infections, meningitis, febrile neutropenia, and Brucella, Helicobacter, and Mycobacterium infections. The prophylactic use of antimicrobials to prevent anticipated infections is controversial because of the increased risk of selecting for resistant microorganisms.157 Indiscriminant use of antibacterials may alter the patient’s microbial flora and allow colonization by resistant bacteria. For example, this can occur when concurrent antibacterials and indwelling urinary catheters are used in intensive care patients.404 However, in other instances, prophylactic administration has been beneficial, such as when infection is anticipated from contamination of otherwise sterile tissues. These cases include contaminated wounds after trauma, surgical procedures when contamination is expected, surgery in immunosuppressed patients, and during surgical procedures involving prolonged exposure of healthy tissue to air. Antibacterials may also be indicated prophylactically before major dental procedures and high-risk medical conditions, including diabetes mellitus, hyperadrenocorticism, immunosuppressive or cancer chemotherapy, and chronic bronchopulmonary disease. In humans undergoing cancer chemotherapy, prophylactic quinolone administration for neutropenia decreased the rate of development of infections; however, when infections occurred, they were caused by highly resistant microoganisms.37 For further discussion of prophylactic antimicrobials in traumatic and surgical wounds, see Surgical and Traumatic Wound Infections, Chapter 53. This chapter presents a review of the general properties of antibacterial drugs by pharmacologic groups. Information on specific antibacterial drugs used in dogs and cats can be found in the Drug Formulary in the Appendix at the end of this book. Because of space limitations, a discussion of general principles of antibacterial chemotherapy has been omitted here. Further coverage of antibacterial drug resistance and prophylaxis is in Chapter 93. Dosage charts for the administration of antibacterial drugs in treating bacterial infections of various organ systems are provided in Chapters 84 through 92. This group of drugs, which includes penicillins, cephalosporins, monobactams, and carbapenems, acts by disrupting bacterial cell wall synthesis. These drugs are most effective when organisms are reproducing rapidly because of the high rate of cell wall formation. The most common mechanism of bacterial resistance to β-lactams is production of the enzyme β-lactamase, which damages the β-lactam ring of these compounds. More than 300 uniquely different enzymes have been described. Combination of penicillins with a β-lactamase protector (e.g., clavulanate or sulbactam) improves efficacy and thus broadens the spectrum of the penicillin toward susceptible organisms that have acquired resistance through β-lactamase production. Cephalosporins are generally more resistant to β-lactamases produced by Staphylococcus species, but are more susceptible to those produced by anaerobic organisms.620 More problematic are the emerged extended-spectrum β-lactamases, which target most third-generation drugs, but not the second-generation drug cefoxitin, or clavulanate or carbapenems.104 Extended-spectrum β-lactamases are not generally detected by routine susceptibility methods.23 Genes for β-lactamase, encoded on bacterial chromosomes or plasmids, may spread within and among these populations of bacteria. An increasing number of staphylococci have a modified penicillin-binding protein in their cell walls with a reduced affinity for all β-lactam drugs. These organisms have been classified as methicillin-resistant types and make up a majority of nosocomial isolates from patients in human hospitals. Methicillin resistance in Staphylococcus aureus (MRSA) and Staphylococcus pseudintermedius (MRSP) is indicated by the presence of the mecA gene, which encodes a mutation in PBP2a, thus reducing its affinity for the β-lactam ring, rendering the organism resistant to all β-lactams.69,320 Detection of MRSA or MRSP on susceptibility generally is based on resistance to oxacillin, which is more stable than methicillin in disks used for testing. Although it is community-acquired MRSA strain USA300 that appears to be most commonly associated with increased colonization in clinically healthy dogs and cats, it is USA100, most frequently associated with hospital-acquired MRSA infections in humans, that most commonly causes clinical infections in dogs and cats (see Staphylococcal Infections, Chapter 34).171 Penicillin G (benzyl penicillin) is a naturally occurring bactericidal antibiotic produced by certain molds of the genus Penicillium. It primarily inhibits synthesis of the gram-positive bacterial cell wall, causing osmotic fragility of susceptible bacteria. Penicillin G has three significant therapeutic limitations: it is degraded by gastric acid, which reduces systemic availability after oral administration; it is inactivated by β-lactamases produced by certain staphylococci and many gram-negative organisms; and at usual therapeutic dosages, its spectrum is limited mainly to gram-positive aerobic and facultative anaerobic organisms (Table 30-1). Newer semisynthetic penicillins have been produced to overcome these disadvantages. In general, penicillins are believed to work synergistically with aminoglycosides in vivo. TABLE 30-1 Properties of Penicillins and Associated β-Lactam Derivativesa IM, Intramuscular; IV, intravenous; PO, by mouth; SC, subcutaneous; −, none; ±, variable; +, good; ++, excellent. aSee the Drug Formulary in the Appendix for dosages. bFor best results, administer on empty stomach. cAlso includes bacampicillin (Spectrobid), cyclacillin (Cyclapen), epicillin, pivampicillin, and talampicillin. dAlso includes azlocillin (Azlin). eSusceptibility data indicate efficacy of inhibitor alone, although the drug is not available by itself. Despite the production of newer derivatives, penicillin G remains the most active drug against many gram-positive aerobic bacteria. Most streptococci, except for enterococci, are susceptible (see Chapter 33). Many gram-positive bacilli and anaerobic bacteria, with the exception of β-lactamase-producing Bacteroides fragilis, are susceptible. Staphylococci are frequently resistant because of β-lactamase production. Procaine penicillin and benzathine penicillin are poorly soluble compounds that slowly dissolve at the site of injection, liberating penicillin G. Carbenicillin and ticarcillin have increased activity against gram-negative aerobes, including Acinetobacter, Proteus, Enterobacter, some Klebsiella, and some anaerobes. Ticarcillin is especially active against Pseudomonas. Carboxypenicillins are destroyed by β-lactamase and are less effective against gram-positive organisms. These drugs are generally not well absorbed orally and must be given parenterally for systemic activity, but carindacillin and carfecillin are two esters of carbenicillin that are available for oral administration. The MICs for susceptible organisms are relatively high; therefore, large doses must be administered and the drugs should be given at shorter intervals. Coadministration with an aminoglycoside improves efficacy against Pseudomonas and reduces development of drug resistance. Ticarcillin and ticarcillin-clavulanate have been used successfully in managing canine otitis externa caused by Pseudomonas aeruginosa (see the Drug Formulary in the Appendix and Chapter 84, Otitis Externa).396 Expense generally limits systemic use of carboxypenicillins. Dosing schedules for β-lactams have been based on maintenance of blood levels above MIC for all or most of the dosing interval.574 β-Lactams exhibit time-dependent killing. For optimal effect, plasma drug concentrations should exceed the MIC for 50% to, at, or above 80% of the treatment interval so that bacterial cell walls can be disrupted as they form. A bactericidal effect is usually achieved at between one and four times the MIC. Higher doses of β-lactams have often been used for more severe infections, but reaching the MIC may be all that is necessary, and impairment of excretory mechanisms in septic animals may permit use of lower doses.343 Because of their low toxicity, β-lactam doses generally can be increased in order to maintain effective concentrations with minimal risk of adverse reactions. Additional studies on bioactivity and pharmacokinetics in ill and organ-impaired animals will be needed to resolve the dosing schedules for these drugs. Unlike aminoglycosides and quinolones, penicillins and cephalosporins have no PAE on gram-negative bacteria at clinically achievable concentrations. A PAE for certain gram-positive organisms, such as some staphylococci, has occurred. It is the PAE that allows antibacterial concentrations to fall below the MIC for a portion of the treatment interval without impairing efficacy. Nevertheless, for most infections, β-lactams should be administered at a dose and interval sufficient to maintain plasma concentrations high enough to affect the target organism in the tissues of concern. In severe infections with resistant organisms, this action may necessitate constant intravenous infusions. Bleeding has been an important side effect in human patients treated with antipseudomonal and extended-spectrum penicillins. This effect has been attributed to various factors, including delayed fibrin polymerization, suppression of vitamin K-dependent procoagulants, and platelet dysfunction. Acute postoperative (within 5 days) azotemia has been ascribed to administration of nafcillin to dogs during surgery.423 Diarrhea has been a side effect of oral or parenteral therapy with penicillins and is caused by inducing selective alterations in intestinal microflora.217 In experimental studies in dogs, oral administration of recombinant β-lactamase eliminated the intestinal fraction of parenterally administered ampicillin, preserved serum levels of the drug, but prevented alterations of the fecal microflora associated with diarrhea.231 This group includes imipenem, panipenem, biapenem, meropenem, and ertapenem. Imipenem is active against most gram-positive and gram-negative aerobes and anaerobes. It is primarily indicated for treatment of infections caused by cephalosporin-resistant members of the family Enterobacteriaceae and some anaerobes. Resistant Pseudomonas isolates have emerged during therapy. Breakdown of imipenem by dehydropeptidase-1 in the kidney and other tissues produces nephrotoxic metabolites and decreases urine concentrations of active drug. Coadministration of cilastatin, a metabolic inhibitor of dehydropeptidase-1, increases drug concentrations in urine and decreases potential nephrotoxicity. Parenteral administration of imipenem-cilastatin is necessary because neither drug is absorbed orally. The dose should be reduced in renal failure. Panipenem is combined with betamipron for the same reason. Biapenem and meropenem are more stable and do not require that any inhibitor be given concurrently. Meropenem pharmacokinetics have been studied in dogs.48 The protein binding of meropenem is such that plasma concentrations parallel those in interstitial fluid.49 Ertapenem has a narrower spectrum of activity than others in this class. The dose should be reduced in renal failure. Adverse effects include nausea, vomiting, diarrhea, phlebitis at the infusion site, fever, and seizures. See the Drug Formulary in the Appendix for further information on each of these drugs. Some naturally occurring β-lactam drugs have low antibacterial activity by themselves but bind irreversibly to and inactivate bacterial β-lactamase. Concurrent administration of these agents increases the activity of penicillins and decreases the in vitro MIC required to inactivate many β-lactamase-producing organisms, such as staphylococci, Escherichia, Capnocytophaga canimorsus, some Proteus and Klebsiella, B. fragilis, Salmonella, and Campylobacter (Table 30-2). Organisms such as Enterobacter, Serratia, Citrobacter, and Pseudomonas remain resistant. TABLE 30-2 Comparison of Antibacterial Activity of Selected β-Lactam and β-Lactamase Inhibitor Combinationsa +++, Excellent; ++, very good; +, good; −, poor. aSee the Drug Formulary in the Appendix for further drug information and dosages. An orally administered product combining amoxicillin with potassium clavulanate is licensed for small animal use. This combination is best suited to treat resistant infections caused by members of the family Enterobacteriaceae (except some Pseudomonas spp.) and anaerobes. It is given commonly to treat infections of the skin, lower respiratory tract, soft tissue, middle ear, and sinuses. The primary side effect has been diarrhea. Studies over a 9-year period on bacteria isolated from dogs and cats showed little decrease in relative susceptibility to this drug combination.582 However, evidence indicates increasing resistance at least by Escherichia coli.500 A parenteral formulation of ticarcillin with potassium clavulanate is licensed for human and equine use. Cephaosporins have been separated into four generations or classes, based on the chronology of discovery, chemical structure, and therapeutic activity. Generally, increasing generations are associated with decreased susceptibility to β-lactamase destruction, although ESBL has affected this distinction. The characteristics of the classes are compared in Table 30-3, and important features of common cephalosporins are presented in Table 30-4. For further information on specific drugs and dosage, see the Drug Formulary in the Appendix. TABLE 30-3 Comparison of Antimicrobial Activity of Cephalosporins
Antibacterial Chemotherapy
β-Lactam Drugs
Penicillins
Natural Penicillins
Generic Name (Trade Name)
Route of Administration
Antimicrobial Spectrum
Gastric Acid Stability
Staphylococcal β-Lactamase Resistance
Gram- Positive
Gram- Negative
Anaerobic
Pseudomonas
NATURAL PENICILLINS
Penicillin G (many)
IM, IV
−
−
+
−
+
−
Penicillin V (many)
POb
+
−
+
−
+
−
β-LACTAMASE–RESISTANT PENICILLINS
Methicillin (Staphcillin, Celbenin)
IM, IV
−
+
+
−
−
−
Nafcillin (Unipen, Nafcil)
IM, IV
±
+
+
−
−
−
Cloxacillin (Tegopen)
POb
+
+
+
−
−
−
Dicloxacillin (Veracillin, Pathocil, Dynapen)
POb
+
+
+
−
−
−
Flucloxacillin (Flopen, Floxapen)
PO
+
+
+
−
−
−
Oxacillin (Prostaphilin, Bactocil)
PO, IM, IV
+
+
+
−
−
−
AMINOPENICILLINSc
Ampicillin (many)
PO,b SC, IM, IV
+
−
+
±
±
−
Amoxicillin (Omnipen)
PO, IM
+
−
+
±
±
−
Hetacillin (Hetacin)
PO
+
−
+
±
±
−
EXTENDED-SPECTRUM CARBOXYPENICILLINS
Carbenicillin (Pyopen, Geopen)
IM, SC, IV
−
−
±
+
+
+
Ticarcillin (Ticar)
IM, SC, IV
−
−
±
+
+
+
UREIDOPENICILLINSd
Mezlocillin (Mezlin)
IM, IV
−
−
+
++
±
++
Piperacillin (Pipracil, Pipril)
IM, IV
−
−
+
++
±
++
OTHER β-LACTAMS
Aztreonam (Azactam)
IM, IV
−
++
−
++
−
++
Imipenem-cilastatin (Primaxin)
IV
−
++
++
++
++
++
β-LACTAMASE INHIBITORS
Clavulanate [CA]e
PO, IV
+
++
−
−
−
−
CA + amoxicillin (Clavulox, Clavamox, Augmentin)
PO
+
++
++
++
+
−
CA + ticarcillin (Timentin)
IV
−
++
±
+
+
+
Sulbactam [SB]e
IV
−
++
+
±
±
−
SB + ampicillin (Unasyn, Synergistin)
IV
−
++
+
+
+
+
Tazobactam (TZ)e
IV
−
++
+
+
±
±
TZ + piperacillin (Zosyn)
IV
−
++
+
++
±
++
Carboxypenicillins
Pharmacodynamics
Toxicity
Other β-Lactam Drugs
Monobactams
Carbapenems
β-Lactamase Inhibitors
Penicillin
Bacterial Susceptibility
Inhibitor
Gram-Positive
Gram-Negative
Anaerobes
Pseudomonas
Amoxicillin
Clavulanate
+
+
+
−
Ticarcillin
Clavulanate
++
++
+++
+
Ampicillin
Sulbactam
+
+
+
−
Piperacillin
Tazobactam
++
+++
++
++
Clavulanate
Cephalosporins
Generation
Bacterial Susceptibility
Gram-Positive
Gram-Negative
Anaerobes
β-Lactamase Resistant
Selected Susceptible Organisms
Firsta
+++
+
++
+
Staphylococcus
Secondb
++
++
++
+
Proteus
Thirdc
+
+++
+
++
Pseudomonas
Fourthd
+
+++
+
+++
Enterobacteriaceae ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antibacterial Chemotherapy
