Fig.1.
Schematic representation of 5-HT receptor subtypes present in brain regions involved in feeding behavior.
Anorexia nervosa and bulimia nervosa in humans are extremely complex compared to the “simplicity” of animal models. However, animal models represent the first required step to propose molecular therapeutic targets and alternatives. Results obtained in animal models may further ensure the “anecdotal evidence that anorexia sufferers might be hooked on the self-starvation and self-control involved in the disorder” (26).
Using animal models, we postulated that anorexia might involve altered signaling events within the NAc (6). To address this possibility, we examined the effects on food intake of directly stimulating or inactivating 5-HTR4 in the NAc (Fig. 2, microsurgery in freely moving animals). The 5-HTR4 have been selected because we have previously observed that mice lacking these receptors displayed attenuated feeding responses to the restraint stress compared to wild-type mice (Fig. 2) (22). We also investigated whether these receptors are further involved in the anorectic effect of MDMA by using a combination of pharmacological, biochemical (Homogeneous Time-Resolved Fluorescence-based: HTRF), immunocytochemical, and molecular biology techniques (Quantitative Real-Time PCR: QR-PCR) that include intracerebral injection of siRNA-mediated 5-HTR4 (si5-HTR4) knockdown into the NAc (siRNA-mediated knockdown in adult mouse). Using 5-HTR4 knockout (KO) mice, we further determined that 5-HTR4 contributes to the appetite-suppressant effect of MDMA. In addition, the generation of 5-HT receptor knockout mouse required the use of different and specific complex methods previously and extensively reviewed (27–29). Additional experiments revealed that the activation of accumbal 5-HTR4 increases the mRNA level of the satiety factor cocaine- and amphetamine-regulated transcript (CART) via a cAMP/PKA signaling pathway. Finally, we provide evidence that increased CART mRNA expression mediates the appetite-suppressant effects of both accumbal 5-HTR4 stimulation and MDMA, the psychogenic compound of ecstasy. Using this set of combined methods, the 5-HTR4 was observed to mediate upregulation of CART in the NAc, which triggers anorexia-like behavior and the appetite-suppressant effects of ecstasy.
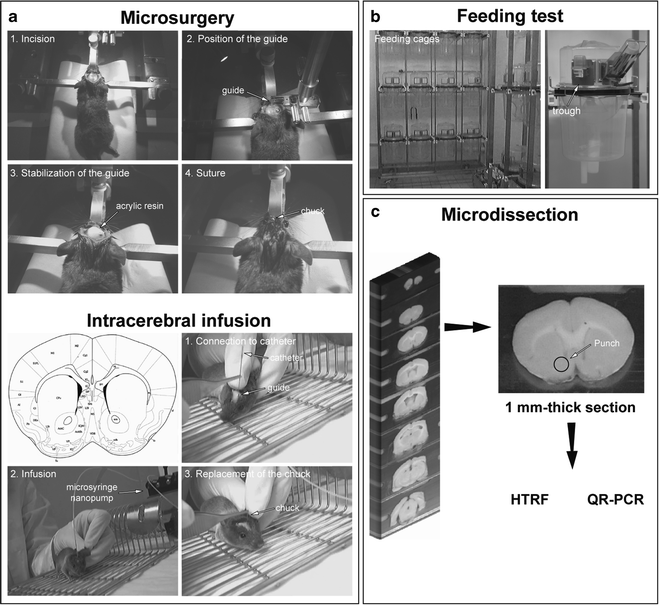

Fig. 2.
Overview of the experimental strategy: from molecule to behavior analyses. (a) microsurgery for intracerebral infusion of compound (agonist, antagonist, inverse agonist, siRNA, transformed virus) in freely moving animals. Five days before any infusion of compounds into a specific brain area, a steel guide is unilaterally implanted at coordinates from the bregma according to the brain atlas (described in detail in Sect. 2.1). (b) Following classic measurement of the intake of food (excluding the spillage), animals are sacrificed at a determined and precise time period following the treatment, and are infused at 7 min intervals between each mouse. The brains are quickly frozen in isopentane cooled with liquid nitrogen (or dry ice) and stored at −80°C. (c) Brains are sliced into 1 mm, and tissue samples are microdissected at −20°C. Tissue samples are then treated using the HTRF technique (described in detail in Sect. 2.2) or QR-PCR.
2 Materials and Procedures
2.1 Microsurgery Materials, Equipment, and Setup
Intracerebral infusion of the compound into the NAc or any other brain area in freely moving mice requires the implantation of a permanent sterile stainless steel guide (internal diameter: 0.160 mm, external diameter: 0.405 mm). A chuck is required to temporarily fill the guide until injection. The lengths of the guide and chuck are strictly equal and have to be determined according to the coordinates of the brain atlas (30). The coordinates are adapted for mice weighing 30 g. In any case, the precise location of the site of injection has to be assessed using a classic cresyl violet staining.
The guide is stabilized and maintained on the bone surface with a nontoxic acrylic resin (liquid/powder mixture). These products have to be stored in a cool place away from direct sunlight. The liquid is highly flammable and has to be stored to avoid sources of ignition. The liquid/powder mixture reaches a dough-like state 15 s after mixing. The manipulation should be finished before 2 min after mixing when setting starts.
Mice are deeply anesthetized by intraperitoneal (i.p.) injection with ketamine (60 mg/kg) and xylazine (15 mg/kg). Ketamine is an anesthetic and xylazine has a double advantage of being sedative and analgesic. Lachrymal frost is systematically used in order to protect the eyes of each animal from light. This mixture of both ketamine and xylazine is recommended in the Guide for Care and Use of Laboratory Animals established by the Centre National de la Recherche Scientifique (CNRS, France). Each compound used is stored and maintained systematically at 4°C until use and is dissolved in NaCl (9%) on the surgical day. The dissolved solution is maintained at 4°C for the entire surgical session. On the treatment day, each compound is infused at a precise rate (1 μL/min) into the target brain area using a microsyringe nanopump. The guide is connected to a catheter on one side while the other extremity is linked to a syringe placed on the microsyringe nanopump.
2.2 Restraint Stress or Forced Immobilization Materials, Equipment, and Setup
Animals are handled and cared for in accordance with the Guide for the Care and Use of Laboratory Animals and the European Communities Council Directive of 24 November 1986 (86/609/EEC). Mice are housed 3–6 per cage and allowed to acclimatize for at least 5 days after their arrival at the facility. All animals are then housed in individual cages and handled daily for 5–7 days before the experiment to minimize handling-associated stress; this period is thought to correspond to the complete adaptive process (31). Some mice were subjected to acute immobilization stress or restraint stress according to a well-established protocol (32, 33), as described in detail below. The animals are randomly assigned to stress procedure or control groups. Two methods can be used for the execution of immobilization stress: immobilization-induced stress with a restrainer device or immobilization-induced stress without a restrainer.
2.3 Micropunch Technique Materials, Equipment, and Setup
Animals are sacrificed after the various treatments and the brain area (e.g., NAc: 1.2 mm3; hypothalamus: 2 × 3.9 mm3) is microdissected from 1-mm-thick sections at −20°C using a micropunch following the landmarks of the stereotaxic atlas (NAc: A +1.6 mm, hypothalamus: A +0.58 and −1.58 mm, from bregma) (30). Figure 3 illustrates the micropunch technique (6, 34). The 1-mm-thick brain sections are obtained using a brain slicer matrice, allowing the precise sectioning of the brain. From these tissue samples, total mRNA can be then isolated, for example, and the mRNA of interest can be analyzed (34, 35).
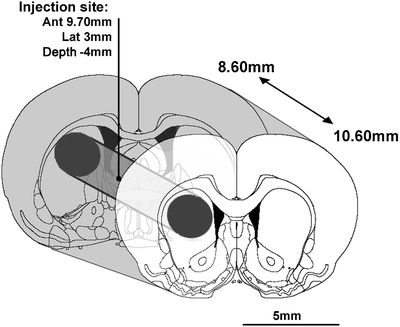

Fig. 3.
Illustration of the punch technique. 2 mm striatal sections are obtained at −20°C using a specific slicer designed for the present investigation. Inside sections, a cylinder (2 mm diameter) was dissected with a micropunch allowing the experimenter to obtain cylindric striatal samples of 6.28 mm3 according to the coordinates of the stereotaxic atlas (73): anterior: 8.60–10.60 mm from interaural line. Punched samples included the treatment injection site located at anterior: 9.70 mm from the interaural line.
2.4 Microsurgery Procedures
One night before the surgery, mice are placed in the surgery room in order to adapt and avoid any stress related to the novelty of the surgery room compared to their usual room. Mice have to be operated on in a manner that allows them recover sufficiently before the active (dark) period.
Mice are anesthetized by i.p. injection of ketamine (60 mg/kg) and xylasine (15 mg/kg) and placed in a stereotaxic frame. The skin is precisely cut in only one movement from the top to the bottom of the skull in sterile conditions. Only the bone is treated with a diluted solution of H2O2 (1/10). Do not touch the skin with the H2O2 solution. Small incisions are made at the surface of the bone in order to fix the resin in a later step. The bone is drilled in order to implant the guide. A saline solution is used to remove all bone dust. The guide is placed slowly according to the coordinates. The resin is then applied using a small spatula such that the mixture surrounds, but does not touch, the guide. The experimenter should wait 3 min for the resin to solidify and the guide to be permanently implanted. Using sterile surgical thread, the skin is stitched. The localizations of the injection sites are then systematically assessed for each mouse.
2.5 Restraint Stress and Forced Immobilization Procedures
Immobilization stress (IS) differs from the restraint stress. Restraint stress is widely and commonly used as an appropriate stress paradigm model for the induction of acute stress. Immobilization stress is believed to be the most severe type of stress in rodent models and has comparative effects in human. IS, which prevents any body movement because of the taping of the legs and trunk to a wooden apparatus outside the home cage of animal, is considered a stronger stress than other paradigms (36) such as restraint stress, in which the mouse is restrained in its home cage within a cylindrical wire mesh net that prevents locomotion but is flexible and allows some body motility. For this, immobilization is considered as a complex stressor and includes physical as well as psychological dimensions.
In the restraint stress paradigm, the animal has to be immobilized individually in an adjustable, semicylindrical, acrylic restrainer with air holes (Plexiglas restrainers) for 110 min. Other designs with device variations have been proposed. For example, to restrain the animal, a cylindrical wire mesh restrainer may be clamped at both openings and placed inside the animal’s home cage during the restraint stress session. In another alternative, mice subjected to acute restraint stress are placed individually in a well-ventilated polypropylene tube (40 mm in diameter and 90 mm in length). This method ensures minimum movement, including that of the tail, and involves no pain. Control mice are left undisturbed in their home cages. The body weight of each animal is measured daily, beginning 1 week before stress and lasting until sacrifice.
In the immobilization paradigm, mice are placed on wooden boards in prone position by taping their limbs and shoulders to metal mounts at 0930 hours for 60 min, after which they are returned to their home cages. Head movements can be restricted with an appropriate loop around the neck. For the chronic stress, the experimental group of mice is subjected to immobilization daily for 1–10 days; control mice are handled each day for the same time period. All stress procedures begin at the same time of the day, between 0930 hours and 1030 hours, 3–4 hours after the start of the light cycle, to reduce the disruption to the circadian rhythm and associated variations on stress hormone levels as much as possible. The body weight and 24-hour-cumulative food intake of each animal are measured daily (at 0730 hours and 1500 hours) from 1 week before the chronic stress period, until sacrifice. Depending on the assay used, mice are sacrificed by decapitation after stress; controls are sacrificed at the same time.
2.6 Micropunch Technique Procedures
Following sacrifice, brains should be frozen in powdered dry ice to keep their original form. Blocks of dry ice would alter the form of the brain, and the tissue samples may not contain the target area. We commonly use the micropunch technique in mice, but it also can be used in rats. The brain matrice, micropunch, slides, pincer, and tubes for collecting tissue samples should be put into the cryostat and maintained at −20°C; otherwise, tissue samples could melt.
3 Anticipated Results and Notes
3.1 Anticipated Results
Using the restraint stress model in mice, to mimic anorexia-like behavior, we found that the 5-HTR4 knockout mice displayed attenuated feeding responses to acute restraint stress (22). We also explored the molecular mechanisms behind anorexia-like behavior and discovered similarities to ecstasy’s effects. Ecstasy mimics the appetite loss characteristic of anorexia. We surmised that these effects might be centered in the NAc. Stimulating these receptors in mice reduced their drive to eat and increased production of the same transcripts stimulated in response to cocaine and amphetamines (CART: Fig. 4). Blocking the receptor with RNA interference increased food intake. Mice lacking the 5-HTR4 displayed attenuated feeding responses to the appetite-suppressant effects of ecstasy (Fig. 4).
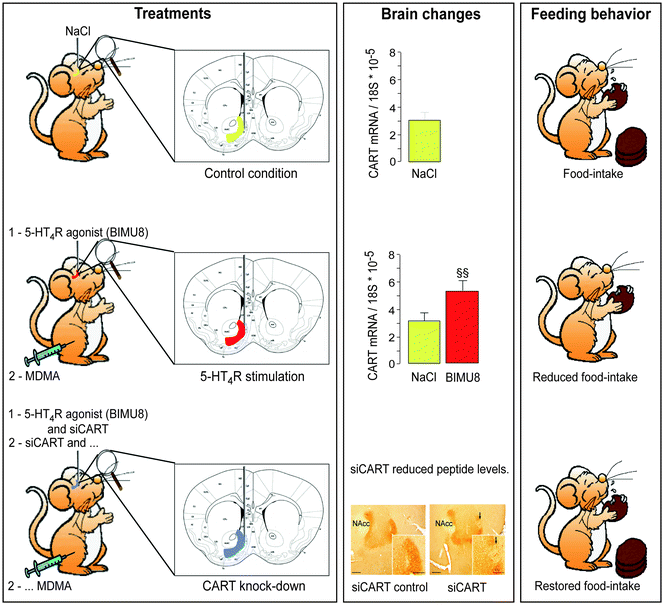

Fig. 4.
Schematic representation of stimulating the 5-HT4 receptors in mice reducing their drive to eat and increasing production of the same transcripts (CART) stimulated in response to MDMA (psychogenic compound of ecstasy).
3.2 Notes
1.
Experimenters have to be extremely precise. For each mouse, the site of injection has to be precisely assessed. If the guide is not placed in the expected location, experimental value cannot be taken into account. It then requires precisely analyzing the location of the site of injection on frontal brain sections using cresyl violet staining. When the aim is to evaluate the intake of food, it requires assessment before any period of treatments that feeding behavior is similar compared with a nonoperated group of mice.
2.
Following surgery, we recommend placing the mice on a warm-water plastic pocket in order to preserve the life of the animals.
3.
At the end of the surgery session, we recommend hydrating the mice with one i.p. injection of NaCl (9%; 800 μL/30 g). Food is directly placed inside the cage for each isolated mouse.
4.
The micropunch technique requires being extremely systematic to dissect the tissue samples at the same location. The samples should be maintained at −20°C. The cryostat chamber is then appropriate to perform this technique.
5.
Prior to the restraint stress or immobilization and particularly before beginning handling, ensure that there are no animals presenting weight loss, abnormal locomotor activity, or wounds.
6.
If several mice display abnormal weight loss during handling procedures, they should be excluded. Be sure that strain(s) used are not sensitive to handling.
7.
It is important to minimize the impact of environmental conditions by keeping constant the dark/light cycle, temperature, humidity, water, and standard mouse chow (which should be available ad libitum), and the same experimenter for at least 1 week before and during the entire experimental period.
8.
Make sure that mice are stressed in an isolated room (avoiding noise influences).
9.
Since the procedure used in immobilization implies taping limbs, it may be necessary to remove the tape gently in order to avoid nociception.
4 Conclusion
There are clear benefits in combining different methodological approaches (stress, microsurgery, and molecular techniques) to analyze mechanisms underlying eating disorders, such as anorexia. Adaptive techniques allowing the visualization of a molecular cascade of events in the human brain are not yet available. The use of the stress paradigms proposed is appropriate and mimics the psychological stress and associated behavioral changes seen in anorexia.
The goal of detecting therapeutic targets using animal models can be realized (melanocortin 4 receptors and 5-HT2C receptors for obesity). Our animal models are highly predictive in human clinical applications because, in agreement with our previous studies (6), recent findings of the G. Knudsen’s team have observed that the 5-HTR4 binding site density is modified in the NAc in humans suffering from eating-related disorders (personal communication), but also from hyperphagia (37), which further encourages investigation of the role of 5-HTR4 in eating disorders.
References
1.
Reda M, Sacco G (2001) Anorexia and the holiness of Catherine of Siena (translated from the Italian by Newman G.). J Crim Justice Pop Cult 8:37–47
2.
Vandereycken W, Van Deth R (1989) Who was the first to describe anorexia nervosa: Gull or Lasègue? Psychol Med 19:837–845PubMedCrossRef
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


