Chapter 59 Anorectum
Structure and Function
The structures that make up the anorectum are responsible for the distal storage and voluntary evacuation of feces, and maintenance of fecal continence. These functions are controlled by a complex interaction of the intrinsic and extrinsic nervous system, and include coordination of the muscles, nerves, and supporting tissues that make up the anorectum. Fecal continence is one of the most important functions of the anorectum, and is defined as the ability to retain fecal content, to perceive that the rectum is full, and to determine appropriate conditions for defecation.1 Diseases or disorders of the anorectum may cause fecal incontinence or constipation when there is a loss of coordination of the activities of the smooth and striated muscles of the anorectum. Conversely, disorders of the mucosa lining the anorectum result in signs of inflammatory disease, also known as proctitis, which are observed clinically as tenesmus, hematochezia, or frequent defecation. Because of the important social impact associated with anorectal disorders, and particularly in diseases resulting in fecal incontinence, they are an important cause of euthanasia in pet animals when the problem cannot be corrected or improved.
Anatomy of the Rectum and Perineum
The anorectum consists of the rectum, anal canal, internal and external anal sphincters, muscles of the pelvic canal, and the skin and subcutaneous structures of the perineum (Figure 59-1).1 Although the rectum and anus are confluent, they have separate embryologic origins that account for many differences in blood supply, innervation, and structure.2 The rectum is a short segment of the distal GI tract that begins at the pelvic inlet as a continuation of the distal colon, and extends approximately 5 cm in length to end at the anal canal.3 The majority of the rectum lies within the peritoneal cavity and the serosal surface serves as the visceral peritoneum. The cranial portion of the rectum is suspended by the short mesorectum, a tissue which is continuous with the mesocolon and helps to form the pararectal fossa.3 The rectum is primarily distinguished from the anal canal by the columnar epithelium that lines the mucosal surface.2 The rectum is surrounded by the perineum, which comprises the tissues that make up the boundary of the pelvic outlet. Internally, the perineum is delineated by the ischial arch ventrally, the third coccygeal vertebra dorsally, and laterally (in the dog only) by the sacrotuberous ligament.4 In cats, the lateral margin of the perineum is less-well defined because of the lack of a sacrotuberous ligament.3 Nevertheless, the perineum surrounds the anal and urogenital canals and provides the external structural support for these tissues. The rectum is bounded by the right and left ventral sacrococcygeal muscles dorsally, and the levator ani muscle laterally. The levator ani and coccygeal muscles make up the pelvic diaphragm—the division between the pelvic canal and the ischiorectal fossa. These muscles are crucial in supporting the rectum, and are important not only as a physical partition, but are essential in acting as a counterbalance to the effects of increased intraabdominal pressure. When these muscles fail, as in perineal hernia, abdominal viscera may herniate through the pelvic canal. There are two ischiorectal fossae, each found above the pelvis and lateral to the root of the tail (see Figure 59-1). This fat-filled space contains the arteries, veins, and nerves that supply the distal GI and urogenital tracts—namely the internal pudendal artery and vein, and the pudendal nerve.3 Lymphatic drainage for the perineum also courses through the fossa draining to the medial iliac nodes.
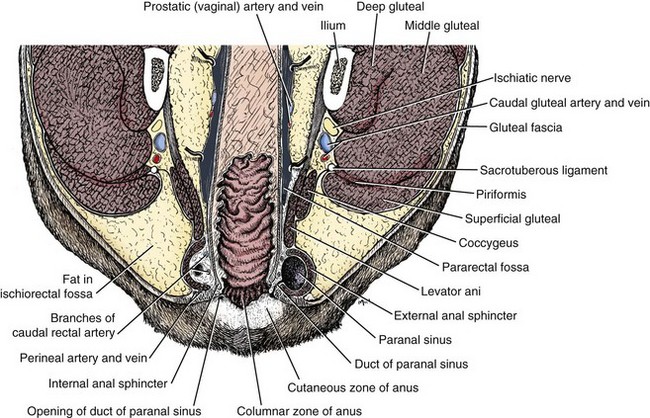
Figure 59-1 Cross-sectional anatomy of the anorectum.
(Reprinted and modified with permission from Evans HE, de Lahunta A. Guide to the Dissection of the Dog, ed 7, St. Louis, 2010, Saunders, p 696.)
As an extension of the colon, the rectum contains the same tunics (layers) that exist in the colon: the mucosa, submucosa, muscularis, and serosa. The submucosa and serosal layers are essentially the same as in other regions of the alimentary tract. However, there are two main differences in the mucosa of the rectum compared to the colonic mucosa: the presence of a large number of solitary lymph nodules and the presence of non-effacing folds in the anal canal, which may be longitudinal or circular, depending on their location. The rectal mucosa also contains columnar epithelium, as in the distal colon, which is rich in goblet cells that secrete mucous. The lymph nodules can be visualized endoscopically in the normal rectal mucosa as either raised or punctate depressions in the rectal mucosa, the so-called rectal pits. Secretion of mucous from the goblet cells is regulated by a submucosal neural plexus in the rectal mucosa. Inflammation of the mucosa results in stimulation of this neural plexus, and this is responsible for the clinical signs often observed in anorectal disease: increased mucus on feces, hematochezia, or tenesmus. At the junction of the rectum and anus, the mucosal epithelium is replaced by a squamous epithelium. This transition point defines the junction of the rectum and internal anal sphincter (or beginning of the anal canal). Embryologically, this transition point marks the site of the cloacal membrane that separates the endoderm from the ectoderm in the embryo.2 Abnormal development of these structures leads to the development of atresia ani and other congenital disorders of anorectal structure such as fistula and clefts.5 The serosal surface of the rectum is continuous with the serosa of the colon; however, the serosa of the anal canal and most caudal portion of the rectum become retroperitoneal as part of the pararectal fossa.3 The muscularis layer of the rectum includes the longitudinal muscles, which are continuous with the longitudinal muscle layer of the colon. The longitudinal and inner circular muscle layers provide tone and structural support to the mucosa and submucosa. In the distal rectum, the fibers of the longitudinal layer sweep dorsocaudally from the sides of the rectum to form the rectococcygeus muscle.3 This muscle passes dorsal to the external anal sphincter and attaches to the bodies of the fifth and sixth coccygeal vertebrae. Anchoring the rectococcygeus muscle on the tail serves not only a means of supporting the anal canal, but also permits movement of the tail during defecation. In the distal rectum the circular muscle layer eventually forms the internal anal sphincter. The internal anal sphincter is composed of smooth (involuntary) muscle, is much smaller than the external anal sphincter, and has questionable significance in maintaining fecal continence.2 Innervation of the rectum is via autonomic nerves from the pelvic plexus, and its blood supply is provided by the caudal rectal arteries.3
Anatomy of the Anal Canal
The terminal portion of the alimentary tract is the anal canal: a short (e.g., 1 cm), highly specialized segment that extends from the rectum to the anal opening. The involuntary smooth muscle of the internal anal sphincter, and voluntary striated muscle of the external anal sphincter are the most important muscles controlling the anal canal. Of these, the external anal sphincter is the most important structural determinant of fecal continence. The external anal sphincter is largely a circular band (approximately 1 to 1.5 cm in width in the dog) of striated muscle that serves as the chief guardian of anal control. The muscle attaches dorsally to the coccygeal fascia and ventrally it blends into the muscles of the external genitalia. Laterally, the sphincter is united by fascia to the levator ani muscles. The mucosa of the anal canal is divided into three zones: columnar, intermediate, and cutaneous.3 The columnar zone connects the rectum to the anus, and is composed of longitudinal ridges of columnar mucosa. The columns formed in this zone encircle the anal canal as an anorectal line. Caudally, these columns loop around to unite as the anal valves, each loop enclosing a tiny pocket called an anal sinus. The ring of anal valves and sinuses forms the dentate line that marks the caudal limit of the deepest part of the anus.2 The intermediate zone (also called the anorectal line) is the transition area between the columnar mucosa and the stratified squamous epithelium that makes up the cutaneous zone. Most caudally, the cutaneous zone is subdivided into an internal and external region. The inner most region contains the anal sacs and the termination of the anal sac ducts. The outer most aspect of the cutaneous zone is keratinized and hairless, and peripheral to the anus itself. The anal opening (or anus) is formed by the plane that separates these two regions of the cutaneous zone. The arterial blood supply to the anus is via the caudal rectal artery, a branch of the internal pudendal artery, which is a large vessel that courses through the ischiorectal fossa. However, venous drainage is via cranial rectal and caudal mesenteric veins to the portal system, and via the caudal rectal and perineal veins to the systemic circulation via the caudal vena cava.3 Lymphatic drainage from the anal canal is also to the medial iliac lymph nodes. Innervation of the anus is supplied by the pudendal nerve.
Anatomy of the Anal Sacs and Glands
In dogs and cats, there are paired anal sacs, which lie ventrolateral (i.e., 4 and 8 o’clock) to the anus in the internal cutaneous zone, lying between the internal and external anal sphincters.3 Anal sacs were first described as structures lined with keratinized epithelium and wrapped in glandular tissue and a connective tissue stroma that is diffusely infused with lymphoid tissue.6 These sacs are sometimes termed paranal sinuses and are now known to contain coiled, apocrine, sudoriparous glands, as well as a few sebaceous glands.3 In cats, there are very few apocrine glands in the anal sacs, with sebaceous glands being found in both the fundus and the ducts of the glands.7 The ducts emptying the canine anal sac open into the lateral margin of the anus at the intermediate zone; however, the anal sac duct in cats opens on a pyramidal prominence 2 mm lateral to the anus.5 It has been suggested that either the increased lipid secretion from the increased sebaceous glands in cats, the location of the duct opening lateral to the anus, or possibly both in combination are responsible for the reduced occurrence of anal sac impaction and abscessation in the cat. The composition of secretions from the anal glands in dogs varies from serous to pasty, and accumulates in the anal sacs, along with desquamated epithelium, bacteria, and yeasts.8,9 The normal canine anal sac secretion is highly variable in its color, consistency, and presence or absence of solid material, even in the anal sacs from the same dog (e.g., right vs. left sacs).8,9 The typical cytologic composition of canine anal sac material contains mostly corneocytes, Gram-positive cocci, and a large amount of amorphous basophilic debris.8 It is also not unusual to find a small number of neutrophils, yeasts, or a few rod-shaped bacteria in normal dogs.9 However, intraepithelial bacteria, undifferentiated epithelial cells, or erythrocytes are not normal components of anal sac secretions and typically indicate the presence of a disease state.8 Under normal circumstances, the anal sacs are emptied when the animal defecates voluntarily or involuntarily (e.g., fear) contracts the external anal sphincter muscle; however, their primary function in the wild is for territorial scent marking.3,3a These sacs are of clinical importance because they may become impacted as a result of anatomic disruption of the ducts caused by increased perineal obesity, anatomic disruption from hernia or neoplasia, or if the secretions become too thick or hardened to allow proper emptying. Impaction of the anal sacs often causes discomfort that is evidenced by scooting or increased licking of the area, and can lead to infection or abscessation. In dogs, anal sac impaction was the third most commonly diagnosed condition in a study of 559 dogs presented for dermatologic assessment.10 In that study, otitis externa and pyoderma were more commonly reported, but these three conditions were clearly the most common reasons dogs were presented for evaluation of dermatologic conditions in private clinical practice, representing 20% of overall admissions in that study.10 In addition to the anal sacs, there are two other types of glands present in the anal area. Circumanal, or hepatoid, glands are nonsecretory, subcutaneous sebaceous glands located in the anal subcutaneous zone. These perianal glands may become clinically important in intact male dogs, as they continue to grow throughout life because of the presence of androgens and may grow to form perianal adenomas late in life.2 In one study, 85% of intact male dogs developed this tumor, making it the most common anal tumor of the dog.4 It is rarely reported in the cat, likely a consequence of the fact that most pet cats are neutered and have fewer numbers of these glands. The “true” anal glands are tubuloalveolar sweat glands located craniolateral to the circumanal glands. These glands produce a fatty secretion that drains into the intermediate zone of the anus and whose function is unknown.
Physiology of Defecation and Fecal Continence
The innervation of the distal alimentary tract is complex and vital to the normal functions of storage, continence, and defecation (Figure 59-2).1 Innervation of the rectum is similar to the colon, in that there is a well-defined enteric nervous system consisting of a myenteric and a submucosal plexus.3 These segments of the autonomic nervous system control the many sensory, integrative and motor neurons that characterize the functions of the rectum, and more specifically control and integrate movements that ensure appropriate storage and transport of feces. The secretions of the goblet cells are also controlled by these systems. The innervation of the anus is more complex (Figure 59-2). The pelvic plexus, specifically the sacral nerve branches of the pelvic nerve, provides parasympathetic fibers which are excitatory to the rectum and inhibitory to the internal anal sphincter. Conversely, sympathetic fibers arise from the hypogastric nerves of the caudal mesenteric ganglion are inhibitory to the rectum (e.g., causing relaxation) and excitatory to the internal anal sphincter (e.g., causing contraction), thus allowing appropriate storage of feces. Relaxation of the internal and external anal sphincters in conjunction with rectal contraction permits defecation. The external anal sphincter is a striated muscle with several muscle bundles that surround the internal sphincter. These muscles are reflexively able to increase anal sphincter pressure, mediated via somatic nerve fibers in the anal branch of the pudendal nerve, while still allowing accommodation of feces within the rectum. The function of the external anal sphincter allows maximal distention (e.g., storage of feces in the rectum) while maintaining anal control. When the pudendal nerve is damaged, the external anal sphincter is incompetent and results in the development of fecal incontinence.
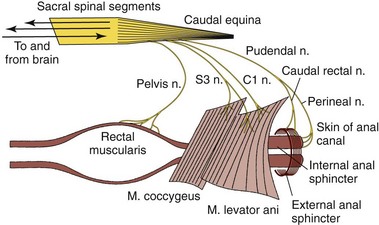
Figure 59-2 Nervous control of defecation.
(Reprinted with permission from Guilford WG: Strombeck’s Small Animal Gastroenterology, ed 3, Philadelphia, Saunders, 1996, p. 507.)
Normal anorectal function and the act of defecation are primarily pressure-based functions that rely heavily on complex interactions between peripheral pressure receptors, myenteric neurons, somatic and autonomic afferent nerve fibers, spinal cord, brainstem, somatic and autonomic efferent nerve fibers, and smooth and striated muscle of the rectum and anal sphincters (see Figure 59-2).5 When the rectum is empty, intraluminal rectal pressure is low. Increased intraluminal pressure is generated by contraction of the internal anal sphincter, either in response to a sudden increase in intraabdominal pressure (e.g., coughing or sneezing) or an increase in rectal filling, and is the primary component of maintenance of fecal continence.11 The main stimulus for defecation is increased pressure in the rectum because of distention. The rectoanal inhibition reflex relaxes the internal anal sphincter along with contractions of the external anal sphincter to allow slow, but continuous filling of the rectum.11,12 As the fecal volume increases in the rectum, conscious awareness of the rectal filling is perceived by sensory signaling of information transmitted via sacral afferent fibers to the cerebral cortex. The rectoanal inhibition reflex allows continued filling of the rectum until defecation is appropriate. If the volume of feces remains small (i.e., stretch is minimal) or defecation is inappropriate, the internal anal sphincter returns to a state of contraction, resulting in propulsion of feces back into the colon.13,14 This activity is also stimulated by descending inputs from motoneurons in the sacral spinal cord and pudendal nerve, which mediate voluntary contractions of the external anal sphincter and levator ani. This back-and-forth process is repeated until the volume of feces is sufficient to initiate rectal distention as well as come into contact with the anal mucosa, resulting in a stronger urge to defecate. When defecation is initiated, distention of the rectum activates parasympathetic efferents which control contraction of colonic smooth muscle (resulting in mass movement of the distal colon), and inhibition of the external anal sphincter and pelvic muscles (resulting in relaxation of the sphincters.)12 Because the rectum itself produces only small contractions that are not propulsive, the main propulsive force to dispel feces out of the body is the contraction of colonic smooth muscles.12 Thus, the rectum serves primarily as a conduit during the process of defecation and as a storage depot in the periods between this process. Defecation is facilitated by proper posture and generation of increased abdominal pressure (closure of the glottis, fixation of the diaphragm, and contraction of the abdominal wall muscles). Conscious suppression of defecation is facilitated by descending impulses to sacral nerves and the pudendal nerve, which mediate contraction of the levator ani and external anal sphincter, resulting in maintenance of fecal continence.
Diagnostic Evaluation
Special Examination
Radiographic examination is not especially useful in the evaluation of inflammatory lesions of the anorectum. However, survey and contrast radiographs may be useful in the evaluation of rectal tumors and rectal stricture. Ultrasonographic studies have assumed increasing importance in the evaluation of lesions of the anorectal canal. Intrarectal ultrasonography, for example, was accurate in assessing depth of rectal tumor penetration as well as the involvement of pararectal lymph nodes in experimentally induced rectal tumors in the dogs.1
Manometric evaluation of anorectal function is useful in selected cases of anorectal disease, for example, external anal sphincter incompetence (see Chapter 14) and congenital aganglionic megacolon (see Chapter 58). Animals with external anal sphincter incompetence may be more objectively evaluated for anal sphincter tone.2
Inflammation
Perianal Fistula
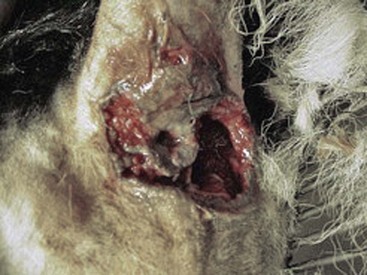
Perianal fistula is a chronic, progressive, often debilitating, disease characterized by one or more ulcerated fistulas or draining tracts affecting the perianal skin and associated tissues surrounding the anus (Figure 59-3). Anal furunculosis is an alternate term for this condition, and may be more appropriate, as true fistulous tracts from the anal canal to the perianal skin are rare. The disease has not been described in cats. The ulcerative lesions are very painful and malodorous because of associated tissue destruction and infection. In most severe and chronic cases, the entire anus is surrounded with open draining tracts that can lead to secondary infection, infestation with ectoparasites, and ultimately to fecal incontinence or rectal stricture if left untreated.
Etiology
Perianal fistula (PF) most commonly affects dogs of the German Shepherd breed or German Shepherd mixed breeds, but Irish Setters, Labrador Retrievers, Old English Sheepdogs, Bulldogs, Spaniels, and Collies are also notably affected.1–5 In one study, German Shepherds accounted for 84% of the reported cases.2 PF primarily affects middle-aged to older dogs of either sex, but the disease has been described in dogs of ages ranging from 1 to 14 years.2,3,6 At this time, the effect of sex hormones on the development or maintenance of PF in dogs is still debated, but it appears that both intact and neutered animals have equal risk of developing PF.5 The cause of PF remains unknown and only partially defined, but anatomic, bacterial, endocrinologic, and immunologic etiologies have all been proposed. Similarities in clinical appearance between canine PF and human Crohn’s disease have caused speculation that these diseases may share a common immunopathogenesis.2,7–10 This notion has been further supported by clinical improvements reported in dogs treated with cyclosporine and other immunomodulating or immunosuppressive drugs (e.g., tacrolimus, azathioprine, prednisone).3,4,9–13 Evidence of immune dysregulation has been reported in some studies, including increased plasma cells, CD 3+ T lymphocytes, immunoglobulin (Ig) A- and IgG-secreting B lymphocytes, and macrophages in affected tissue.14,15 However, immunohistochemical analysis of the number and distribution of B and T lymphocytes has not revealed any simple immunologic defect.15 An alternative hypothesis has been proposed that IgA deficiency in German Shepherds predisposes this particular breed to anal furunculosis.15 This hypothesis is further supported by the findings of House et al. who showed that dogs with PF have increased expression of messenger RNA for interleukin (IL)-1β, IL-6, tumor necrosis factor-α, IL8, IL-10, and transforming growth factor-β.15 Matrix metalloproteinases (MMPs) may contribute to this tissue pathology. MMPs 2, 9, and 13 were found to be significantly increased in tissue biopsies of dogs with PF.16 MMP-9 and MMP-13 are primarily produced by macrophages, and tissue ulceration may occur as a result of the aberrant activation of macrophages in affected dogs.16 It has been suggested that pathologic macrophage activity could result from T-cell secretion of interferon-γ, which might explain why lesions resolve following cyclosporine therapy.16
Pathophysiology
The pathologic changes that occur in dogs with PF are well described and are typical of chronic inflammation.2 In particular, anal furunculosis is characterized by mononuclear infiltration of the fistulous or sinus tracts. Dense aggregates of lymphocytes trigger granulation tissue formation in areas adjacent to the sinus tracts. The inflammatory process can affect the anal sac and its associated duct, the circumanal glands, and the muscles of the external anal sphincter. Sinus tracts form in areas of degenerative squamous epithelium and produce obliteration and ulceration of perineal tissue.2 Erosions and ulcerations typical of PF are believed to result from cell-mediated inflammatory responses and MMP enzyme expression.16 MMPs are enzymes in the family of zinc-dependent endopeptidases, are produced by macrophages, and are involved in the degradation of extracellular matrix. There are several different families of MMPs: collagenases, gelatinases, stromelysins, and membrane-type MMPs. These enzymes play important physiologic roles in the detachment and migration of cells as well as in tissue remodeling for repair and angiogenesis. MMPs can also play a pathologic role in ulcerogenic diseases such as Crohn’s disease, rheumatoid arthritis, periodontitis, and tumor cell invasion and metastasis.17–19 In a pathologic role, MMPs cause tissue destruction instead of extracellular matrix remodeling.16 In ulcerative diseases such as inflammatory bowel disease and Crohn’s disease, significant elevations in MMP-9 and MMP-13 have been documented,20,21 and increased MMP-9 expression is specifically associated with fistulous lesions in Crohn’s disease.22 The finding of MMPs in PF tissues adds further credence to the suggestion that tissue destruction, ulceration, and fistulation are a result of an aberrant cell-mediated inflammatory response.
Clinical Examination
Physical examination may be difficult, if not impossible, without sedation because of the pain associated with the condition. It may be necessary to clip hair and cleanse the area to get a full appreciation of the full extent of the disease. In severe cases, visual inspection will reveal multiple, ulcerated, draining tracts extending into the tissues surrounding the anus (see Figure 59-3). Fistulous lesions are often malodorous and may contain ectoparasites, particularly if the patient resides in warmer climates. Rectal strictures, abnormal anal tone, and granulomatous rectal mucosa may be found during digital rectal examination. In some dogs, the anal sacs are involved in the fistulation resulting in anal sac obstruction and abscessation. In dogs with early disease, the lesions will be more subtle and may require more careful inspection or digital palpation to differentiate other causes of perianal swelling, pain or redness (Figure 59-4). Nevertheless, whether there is one small draining tract or multiple, ulcerated areas with deep fistulas, the diagnosis is the same.
Diagnosis
A diagnosis of PF is made on the basis of signalment, history and clinical signs, and relevant physical examination findings. There are several important differentials for this condition that must be considered, including chronic anal sac abscessation with secondary fistulas, aggressive perianal tumors (e.g., adenocarcinoma), caustic injury, and untreated bite wounds. Although history and physical examination findings are usually sufficient to rule out these other potential causes of perianal disease, a digital rectal examination and probing the extent of the lesions under general anesthesia will confirm the diagnosis. In a recent study of dogs with anal furunculosis, colonoscopy was performed in each case—whether or not the dog had concurrent signs of colitis or colonic disease—and results showed that 50% of the dogs had concurrent colitis.23 The relationship between colitis in dogs and PF is unknown; also unknown is the effect of therapy of colitis on the outcome of dogs with PF. Nevertheless, the study authors recommended that flexible colonoscopy should be performed to obtain colonic biopsies in all dogs with perianal fistulas.23
Treatment
Because of the uncertain etiology of perianal fistulas, a number of medical and surgical treatments have been proposed. Previously, medical therapy included antibiotics, perineal cleansing, antiinflammatory drug therapy (e.g., prednisone), and analgesic drug therapy, all of which were palliative at best.2–424 Surgical excision was frequently cited as the only treatment option in early studies24–26; however, remission rates were highly variable (48% to 97%), complication rates were equally variable (3% to 100%), often involving fecal incontinence, rectal strictures, and delayed wound healing, and recurrence rates were generally near 50%.3,6,24–26 Cryotherapy, Nd-YAG (neodymium:yttrium-aluminum-garnet) laser therapy, and chemical cauterization have been reported with variable success, remission, and recurrence rates.27,28 Following recent accounts of successful use of cyclosporine therapy with remission rates of 72% to 100%,3,29 current therapeutic approaches have been aimed at blunting the immune response that appears to be at the core of disease development.
Immunomodulating drug therapies have been used in PF dogs, including high-dose glucocorticoids,8 cyclosporine,3,4,9,10,29 tacrolimus,12 and azathioprine (alone or in combination with antibiotics or prednisone).11,13,26,29 Low-dose prednisone therapy has been recommended for the initial treatment of the inflammatory component of PF, but as with other earlier medical therapies, low doses of prednisone were found to have mostly a palliative effect.2 Immunosuppressive doses of prednisone may be more effective, but only 33% of dogs in one study achieved resolution of disease.8 Topical tacrolimus, a potent immunosuppressive medication, achieves a resolution of lesions in 50% of dogs and improvement in as many as 90% of dogs.12 Although this approach may provide a viable alternative to other therapies, it is likely to be effective only in dogs with mild to moderate PF, and therefore is not recommended for use in dogs with more severe forms of the disease. Cyclosporine therapy may be the best therapeutic option for this disease. Early studies of cyclosporine showed that upward of 85% of dogs had complete healing with several weeks of therapy.9 Several other studies of cyclosporine, either alone or in combination with ketoconazole or other therapy (including surgery), confirmed the efficacy of cyclosporine in the treatment of canine PF.3,4,10,26,30,31 Success rates of 60% to 98% with cyclosporine have been reported, but most studies also revealed that when the drug was discontinued, recurrence rates were very high, confirming the presence of ongoing immune dysfunction. One of the persistent problems with use of cyclosporine in the treatment of this condition in dogs is the very high cost of the drug—both for the medication, and for the drug-level monitoring that is recommended to assure proper dosing. One approach to reduce the cost of cyclosporine therapy is to combine cyclosporine with ketoconazole. Because cyclosporine is transformed by the hepatic cytochrome P450 3, a mixed-function oxidase, addition of ketoconazole to the therapy acts to competitively inhibit this pathway and effectively increase the half-life of cyclosporine in dog.30 As a result, using this combination of drugs results in an equivalent therapeutic responses, but at a reduction in cost of 50% to 60%.3,30 In another study, investigators showed that cyclosporine at a dose rate of 5 mg/kg q24h is effective in reducing the surface area and severity of lesions in dogs with PF.10 However, an important finding in that study is that doses less than 5 mg/kg q24h are ineffective in resolving PF lesions. Thus, in dogs that are intolerant of ketoconazole combined with cyclosporine, single-dose therapy can be used at a slightly lower dose to make the therapy more cost-effective. Finally, because of the high recurrence rate and, in some dogs, incomplete resolution of lesions, studies have investigated using immunosuppressive therapy with concurrent surgical excision of affected tissues as a combination approach.5 In a recent study, cyclosporine therapy was administered for up to 12 weeks followed by surgical excision of any remaining draining tracts, along with cryptectomy and anal sacculectomy.26 In that study, all 18 dogs were reported to have resolution of the disease, and only one of the 18 had recurrence of the disease 9 months after completion of the therapy and surgery.26 This result suggests that there may be significant merit to using the combination of immune suppression and removal of diseased tissue to achieve the best long-term resolution.
Infection
There are several common diseases affecting the anal sacs, including anal sac impaction, anal sacculitis, and abscessation of the anal sacs. Although each of these may present as different diseases, they likely are variations of the same disease process. These diseases are more common in dogs than in cats, and affect up to 12% of the canine population.1,2
Etiology
The initiating events and exact cause of anal sac disease are unknown in many instances. Nevertheless, a variety of contributing factors likely play contributing or causative roles, including fecal consistency, inactivity, diet, body weight, pudendal nerve dysfunction, generalized seborrheic disorders causing increased anal sac secretions, perianal fistulas or intestinal inflammatory disease, and previous perineal surgery resulting in scar tissue that disrupts normal anal sac function.3–5 In some breeds, such as German Shepherd dogs, the anal sacs lie deeper in the perianal tissues near the rectum, and as a result, may predispose them to an increased risk of impaction or infection within the sac.5 Small-breed dogs, on the other hand, may have an increased risk of anal sac disease because of anatomically small ducts emptying the sacs that are more likely to become obstructed if anal sac secretions become thicker than normal.5,6 Finally, any inflammatory disease occurring in the region (proctitis, perianal fistulas, or dermatitis) can result in secondary inflammation of the anal sacs, leading to anal sacculitis or subsequently abscess formation.
Pathogenesis
The most common cause of inflammation or infection of the anal sac is ductal obstruction, which prevents normal secretion and permits proliferation of bacteria and secondary infection. Bacterial species commonly found in the normal canine anal sac include Streptococcus faecalis, Streptococcus faecium, Escherichia coli, Bacillus spp., Clostridium perfringens, Staphylococcus intermedius, and Proteus spp.1,7 In dogs with pyoderma, a significant increase (from 10% to 30%) in the carriage of S. intermedius in the anal sacs has been reported.7 In addition to these bacteria, yeast (e.g., Malassezia pachydermatis) are often found in anal sacs, often as opportunistic pathogens.7 Dogs with atopic dermatitis have also been found to have higher numbers of bacteria and yeasts in their anal sacs, which could predispose them to an increased incidence of infection and anal sacculitis. Anatomic conformation may play an important role in the tendency to develop anal sacculitis or abscessation, such as small ductal anatomy or aberrant duct placement on the anus that decreases effective emptying. Other contributing factors may include secretions that are thicker than usual, development of abnormal anal tone, production of soft feces, and recent estrus in females.5 Anal sac infection or anal sacculitis can culminate in anal sac abscessation if the secretions and bacteria are retained with ductal obstruction. In dogs with anal sac abscesses, the most common bacteria isolated are E. coli and Proteus spp.8 If the duct is completely obstructed, infection will eventually extend beyond the wall of the sac (anal sacculitis) into the surrounding perineal tissues to induce cellulitis and abscess that will eventually rupture through the skin and perineal tissues via draining tracts. The purulent, malodorous, and often bloody discharge will drain once the abscess ruptures, but the surrounding cellulitis may cause significant pain and swelling of the area. In dogs with recurring episodes of anal sacculitis or impaction that are poorly responsive to medical management, surgical correction with anal sacculectomy should be considered.
Clinical Examination
The most commonly reported clinical signs of anal sac disease are associated with anal pruritus or pain: licking or biting at the tail base or anal region, “tail chasing” behavior, scooting or rubbing the anus on the ground, reluctance to sit, and discomfort when sitting.3,5,8 In more severely affected cases, dyschezia, tenesmus, or reluctance to defecate may result from severe pain. Dogs with long-standing anal sac disease may develop large swellings in the area over the anal sac, and if the abscessed sac ruptures, a draining fistula will be present extending from the affected anal sac.
Diagnosis
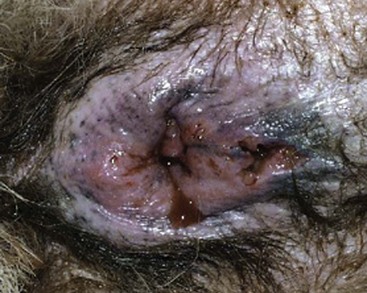
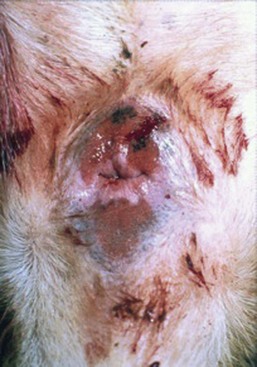
In most dogs, anal sacculitis is suggested by the history of anal pruritus along with either palpable or visual inspection of a perianal swelling at either the 4 o’clock or 8 o’clock positions lateral to the anus. In the absence of significant external evidence of anal sac enlargement, a digital rectal examination is sufficient to confirm the presence of an enlarged, often painful, anal sac(s). Some dogs, and all cats, will require sedation to safely perform a rectal examination because of the accompanying discomfort. Impaction of the anal sacs is confirmed by expression of thick, pasty anal sac material, or inability to easily empty the sacs with otherwise appropriate technique. If the anal sacs are painful when gently palpated, or the material expressed is bloody or purulent, anal sacculitis should be suspected, but not confirmed until cytologic examination finds the presence of a large number of red blood cells and intracellular bacteria in neutrophils.7,9 If the anal sac is abscessed or the surrounding tissues severely infected, fever may be present. An unruptured anal sac abscess presents in affected dogs as a painful, perianal swelling in the region of the anal sac that cannot be expressed using appropriate technique. In dogs with long-standing anal sac abscessation, the skin overlying the sac will be thin, edematous, erythematous, and painful to the touch (Figure 59-5). If the abscess is untreated, the infection will erode through the skin leaving a draining tract surrounding the infected tissues (Figure 59-6). The major differentials to consider in dogs with these clinical signs include perianal fistulas, perianal or anal tumors, bite wounds or other trauma (especially in cats), and in the female dog, the possibility of vaginal infection.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree