Chapter 12
Anesthesia
Comprehensive reviews of reptile anesthesia and analgesia, including cardiopulmonary anatomy and physiology and selection of effective drugs and dosages for various species, have been published previously.1–8 An update on analgesic techniques is provided in Chapter 18, whereas this chapter reviews and highlights the current knowledge of reptile anesthesia, the effects of anesthetic agents on cardiopulmonary performance, and the application and limitation of monitoring techniques in reptile anesthesia.
Preanesthetic Evaluation
Diseases of the respiratory tract are commonly seen in reptiles and may affect both the upper and lower respiratory tract.9 Most commonly, a viral or bacterial cause is identified, and the patient may require effective antimicrobial therapy before induction of general anesthesia. Determination of respiratory rate and effort will provide limited information on the presence of respiratory tract disease. Imaging modalities such as radiography or computed tomography and collection of diagnostic samples will assist in identifying the severity and etiology of respiratory tract disease.
Assessment of cardiovascular performance should be part of the physical examination. Unfortunately, few studies have determined normal values for cardiovascular performance (e.g., heart rate and arterial blood pressures) in reptiles. Although reference values for various cardiac variables need to be established in common reptile species, determination of heart rate and rhythm, radiographic evaluation of cardiac morphologic characteristics, and echocardiography will give valuable information on cardiac performance and abnormalities. Reviews of reptile cardiology, including anatomy, physiology, and diagnostic procedures, have been published previously.10,11
Supportive Measures
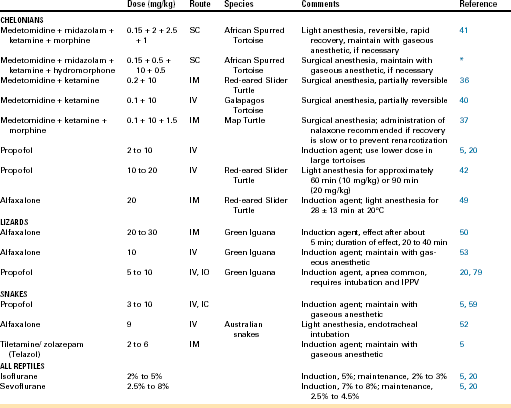
Many reptiles require supportive care before anesthesia because of underlying chronic disease processes and dehydration, which can often be identified during the preanesthetic evaluation. Effective fluid therapy is indicated for correcting fluid and electrolyte imbalances and returning the patient to a normovolemic state. Intravenous (IV) or intraosseous (IO) fluid therapy is most effective and should be administered as a constant rate infusion if vascular or intraosseous access has been established (Figure 12-1). IV catheters can be placed percutaneously in the jugular veins of chelonians (Figure 12-1, A) and in ventral tail veins of lizards. After cut-down techniques, IV catheters can also be placed in the cephalic and ventral abdominal veins of lizards (Figure 12-1, B) or in the jugular veins of snakes. In lizards, the tibia is a common site for IO catheterization (Figure 12-1, C) when IV access is not possible.
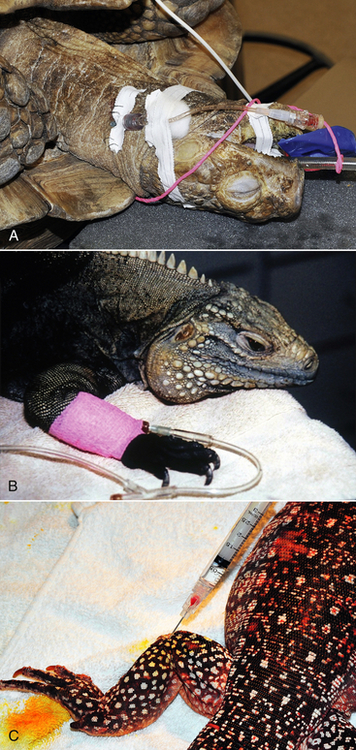
FIGURE 12-1 Intravascular access in reptiles. A, Intravenous catheter in the jugular vein of an African Spurred Tortoise (Centrochelys sulcata). B, Intravenous catheter in the cephalic vein of a Rock Iguana (Cyclura spp.) (Photo courtesy Dr. Douglas R. Mader, Marathon Veterinary Hospital, Marathon, Fla.) C, Intraosseous catheter placement in the left tibia of a Red Tegu (Tupinambis rufescens). Correct intraosseous catheter placement is confirmed by aspiration of hemorrhagic aspirate (needle hub), consistent with bone marrow.
Analgesia
A balanced analgesic regimen takes advantage of distinct analgesic pathways and may include the systemic administration of different classes of analgesics (e.g., nonsteroidal antiinflammatory drugs [NSAIDs] and opioids) and/or the provision of local anesthetics. At present, local anesthetic regimens are not commonly utilized in reptiles. Although little data are available on effective drugs, dosages, and techniques, local anesthesia in combination with systemic analgesic therapy offers the advantages of reduced dosages for systemic analgesic agents and will reduce maintenance requirements during general anesthesia. The same principles established in domestic animals should be followed and should include administration of lidocaine or preferably the longer acting bupivacaine at the surgery site. Advanced techniques such as spinal anesthesia have been investigated and are described further on. It should be part of the anesthetic protocol to provide preoperative, intraoperative, and postoperative analgesia. Opioid agents are most effective in controlling acute pain resulting from trauma and surgery, whereas NSAIDs are most commonly used for the management of chronic pain.1,12–14
Common opioid analgesic agents used in reptiles to manage acute pain include morphine (1 to 2 mg/kg) and hydromorphone (0.5 to 1 mg/kg).1,13 At present, the most common NSAID used in reptiles is meloxicam at 0.2 mg/kg IV, intramuscularly (IM), or per os (PO) every 24 to 48 hours. For further information on effective pain management and protocols see Chapter 18.
Sedation
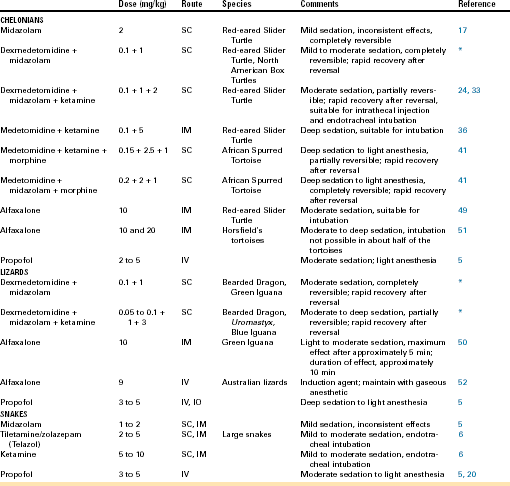
Sedation is commonly used in human and domestic animal veterinary medicine to facilitate diagnostic sample collection, medical treatment, and positioning for diagnostic imaging (Figure 12-2, A-C). Sedation is defined as a state of drug-induced altered consciousness that allows the patient to better tolerate stressful or unpleasant procedures without depressing protective airway reflexes or having a significant cardiopulmonary depressant effect.15 Currently, diagnostic procedures are often performed in conscious reptiles or under general anesthesia. For sedation of reptiles, a variety of injectable anesthetic drugs at lower subanesthetic doses can be used alone or, preferably, in combination.16–19 Drugs used for sedation should be short-acting or reversible, so that prolonged or unpredictable recoveries are avoided. Sedative drugs should be chosen based on the desired level of sedation (mild to deep), the general condition of the patient, and the diagnostic and/or therapeutic procedure to be performed. Commonly used sedative protocols are summarized in Table 12-1. Under sedation, spontaneous respiration should be maintained and reptiles should respond to painful stimulation (e.g., toe pinch). The combination of procedural sedation, with either local or spinal anesthesia, offers the possibility of performing surgical or invasive procedures that would otherwise only be possible under general anesthesia (Figure 12-2, D). When sedation is combined with local or spinal anesthesia, the dose of general anesthetic drugs can be reduced, which will lead to reduced cardiovascular depression, more rapid recoveries, and potentially fewer anesthetic complications compared with general anesthesia.
TABLE 12-1
Sedation Protocols Commonly Used in Reptiles
IM, Intramuscular; IO, intraosseous; IV, intravenous; SC, subcutaneous.
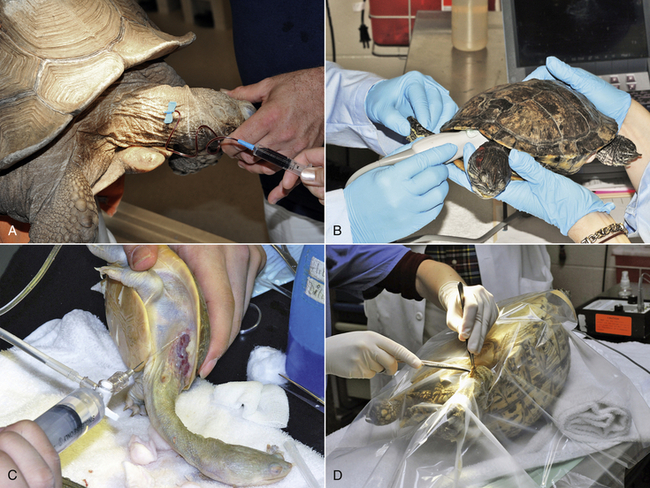
FIGURE 12-2 Procedural sedation in chelonians. In all animals, spontaneous respiration was maintained and a response to painful stimulation could be elicited. A, Blood collection from the right jugular vein of a sedated African Spurred Tortoise (Centrochelys sulcata). B, Echocardiography in a sedated Red-eared Slider (Trachemys scripta elegans). C, Wound debridement and lavage in a sedated Side-necked Turtle (Chelodina spp.). D, Surgical skin abscess removal under local lidocaine anesthesia in a sedated Leopard Tortoise (Stigmochelys pardalis).
Regional Anesthesia and Analgesia
Local Anesthesia
Local anesthesia is currently underutilized in reptile medicine but offers significant benefits, such as the ability to reduce the depth of general anesthesia or sedation for certain surgical procedures. In some cases, local anesthesia can be used solely in manually restrained or sedated animals for minor surgical procedures (see Figure 12-2, D).20 Common indications for the use of local anesthetics as part of an anesthetic protocol in reptiles (see Table 12-2) include distal limb surgery, tracheal or lung washes, or infiltration of incision sites before coeliotomy. Lidocaine and bupivacaine are readily available in most veterinary clinics, and both are effective local anesthetics in reptiles. The toxic dosages for local anesthetics have not been determined in reptiles. In mammals, toxic dosages for lidocaine have been reported to range from 5 to 20 mg/kg.20 Although toxic dosages in reptiles are unknown, care should be taken not to exceed those dosages used in mammals, particularly in small reptile patients, so that systemic side effects can be avoided.
Spinal Anesthesia
In mammals and humans epidural anesthesia and spinal anesthesia (which is also known as intrathecal or subdural), are two estabilished forms of regional anesthesia of the spinal cord and nerves. In particular, epidural anesthesia is common practice. These techniques offer substantial advantages if used as part of a balanced anesthetic protocol: they reduce the amount of general anesthetics needed to perform certain surgical procedures. Consequently, the dose-dependent cardiorespiratory depressant effects of general anesthetics are reduced, and in addition, improved and often longer term analgesia can be provided. Different classes of anesthetics and analgesics can be injected epidurally or intrathecally, which results in either desensitization of motor and sensory fibers (local anesthetics) or a sensory block only (i.e., opioid analgesics) without an effect on motor function. In humans and mammals, possible indications for epidural or spinal anesthesia include surgical procedures involving the tail, perineum, genitals, hindlimbs, and caudal abdomen.21,22
Regional anesthesia and analgesia of the spinal cord is currently in its infancy in reptiles and has only been reported in turtles and tortoises.23–26 In chelonia, epidural anesthesia is not possible due to the lack of a sufficiently developed epidural space. However, a well-developed intrathecal (subdural) space, which directly surrounds the spinal cord and is filled with cerebrospinal fluid (CSF), is present (Figure12-3) and allows for intrathecal administration of various anesthetic and analgesic drugs. Because of the presence of the carapace and the fusion of the vertebral column to the carapace, access to the intrathecal space is limited in chelonia to the cervical and coccygeal vertebrae. Only intrathecal injections in the coccygeal area are of clinical interest.
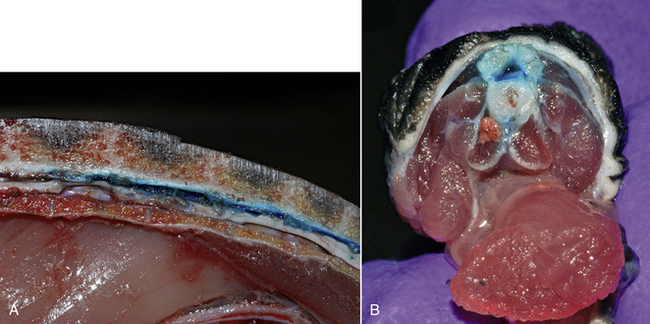
FIGURE 12-3 Methylene blue dye injected in the intrathecal (subdural) space surrounding the spinal cord in Red-eared Slider (Trachemys scripta elegans) carcasses. A, Cross-section of the proximal tail base. Note the close proximity of the skin overlying the neural arch dorsally. B, Sagittal view of the spinal canal at the level of the last dorsal and sacral vertebrae.
In chelonia, the cloaca, genitalia, and hindlimbs are innervated by caudal branches of the sacral plexus and coccygeal nerves.27,28 The sacral plexus arises from spinal nerves XVII through XXI, located at the last dorsal and sacral vertebrae28,29 Within the sacral plexus, the nerves interconnect and subdivide several times before terminating in the inguinal, pelvic, and hind leg muscles.28,29 Therefore the temporary desensitization of these nerves by intrathecal injection of local anesthetics and analgesics can provide regional anesthesia and analgesia sufficient for a multitude of surgical procedures involving the cloaca, urinary bladder, genitals, and hindlimbs in turtles and tortoises. More cranial spreading of intrathecally administered anesthetics will lead to desensitization of spinal nerves located more cranially, which supply the prefemoral fossae and caudal coelomic organs, and potentially allow for surgical procedures via the prefemoral approach.
While anecdotal reports on the use of spinal anesthesia in chelonians exist, the clinical application has only been published in male Galapagos tortoises (Chelonoidis [Geochelone] nigra) with a body weight of 32 to 97 kg.25 In these conscious tortoises 20G, 3-cm hypodermic needles were placed in the mid to distal dorsal coccygeal region after aseptic preparation (Figure 12-4, A). Needle placement was performed through an intervertebral space, which was identified by palpation. Correct intrathecal needle placement was confirmed by aspiration of CSF before intrathecal injection of 2% lidocaine at an average dosage of 0.8 mg/kg (0.04 mL/kg body weight or 1 mL of 2% lidocaine per 20 to 25 kg body weight). In all 15 animals, the level of regional anesthesia was graded as excellent and allowed for successful completion of phallectomy in all tortoises. At the reported dosage, no hindlimb paralysis was induced and no side effects were noticed. Repeated administration of local anesthetics into the intrathecal space after placement of an indwelling intrathecal catheter has also been reported in a Galapagos tortoise (body weight, 105 kg) for management of chronic cloacal prolapse.26
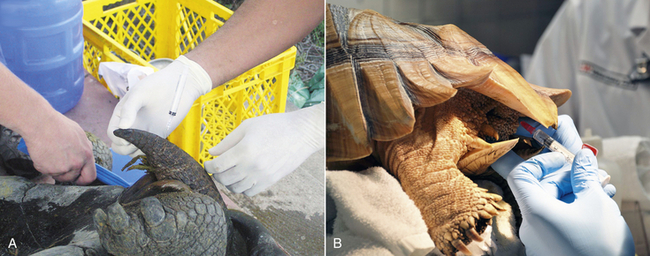
FIGURE 12-4 Spinal anesthesia in tortoises. A, Intrathecal injection of lidocaine in a conscious male Galapagos Tortoise (Chelonoidis [Geochelone] nigra) in dorsal recumbency before phallectomy. The needle is placed in the intervertebral space at the distal third of the tail. (Image courtesy of S. Rivera.) B, Intrathecal injection of lidocaine at the proximal tail in a sedated female African Spurred Tortoise (Centrochelys sulcata) before prefemoral cystostomy.
Research into the feasibility and efficacy of spinal anesthesia in Red-eared Sliders (Trachemys scripta elegans) has been performed.23,24 Spinal anesthesia was successfully induced in turtles of both sexes, with body weights ranging from 0.5 to 1.0 kg. Injections were performed in sedated turtles, which allowed for proper restraint of the tail and correct intrathecal injection at the level of the mid to proximal coccygeal vertebrae. The turtles were positioned in horizontal ventral recumbencey and the tails were aseptically prepared before insertion of 28G needles, attached to 0.5-mL insulin syringes (Figure 12-5).23,24 After penetration of the neural arch, the needle was advanced into the intrathecal space and careful aspiration was performed. If significant amounts of blood are aspirated, then the needle has been inadvertently placed in the prominent intravertebral venous plexus surrounding the intrathecal space. The needle position is readjusted until no aspiration of blood occurs, followed by injection of anesthetic and analgesic drugs over approximately 3 to 5 seconds. Successful intrathecal injection and induction of spinal anesthesia was confirmed by complete motor block (relaxation) of the tail, cloacal sphincter, and hindlimbs. A fast onset period was observed within 1 to 5 minutes. Following the initial injection of either preservative-free lidocaine (4 mg/kg, 2%) or bupivacaine (1 mg/kg, 0.5%) spinal anesthesia was successfully induced in about half of the turtles. In turtles in which no spinal anesthesia was induced after the initial injection, a second intrathecal injection of the same drug and dose was administered 15 minutes later. The repeated intrathecal injection increased the overall success rate to approximately 80% to 90 %.23,24 In male and female Red-eared Sliders, the duration of motor block of the tail, cloacal sphincter, and hindlimbs was approximately 1 hour following lidocaine injection (4 mg/kg, 2%).23, 24 Intrathecal bupivacaine injection (1 mg/kg, 0.5%) in male Red-eared Sliders led to motor block of the tail, cloacal sphincter, and hindlimbs lasting approximately 2 hours.24 A large variation in duration of spinal anesthesia was reported in male and female turtles. A possible explanation for this high degree of variation is the well-developed and prominent intravertebral venous plexus, which is located within the spinal canal directly overlying the intrathecal space predominately dorsally and laterally. Therefore a shorter duration of spinal anesthesia was attributed to a partial intrathecal dose delivery, secondary to an inadvertent IV injection into the intravertebral venous plexus. Consequently, care should be taken with needle placement within the intrathecal space by means of repeated aspiration before and during intrathecal injection.
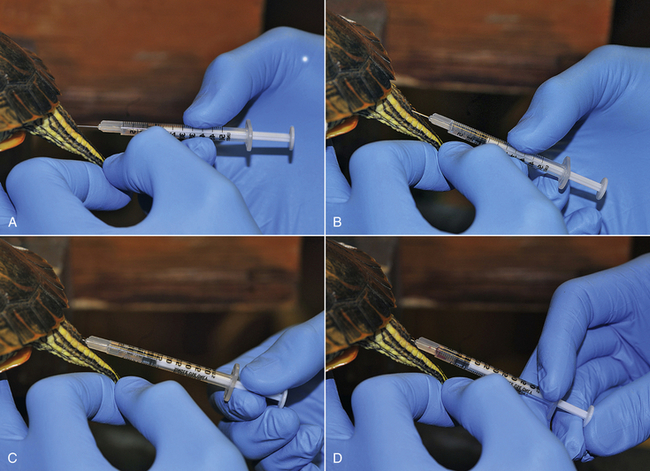
FIGURE 12-5 Intrathecal injection technique for induction of spinal (intrathecal) anesthesia and analgesia in a female Red-eared Slider (Trachemys scripta elegans). A, Penetration of the skin and neural arch with the needle at an approximately 45-degree angle. B, Advancement of the needle into the spinal canal at an approximately 20-degree angle. C, Aspiration is performed to ensure correct placement in the intrathecal place. If an excessive amount of blood is aspirated, the needle is repositioned until no further blood is aspirated. D, Injection of the drug into the intrathecal space over 5 to 10 seconds.
In addition to the injection of anesthetic drugs, drugs with primary analgesic properties can also be injected intrathecally for provision of long-term spinal analgesia. Intrathecally administered morphine relieves somatic and visceral pain by selectively blocking nociceptive impulses without interfering with sensory and motor function or causing sympathetic nervous system blockade leading to hypotension. Morphine is a highly hydrophilic drug that tends to induce long duration of action after intrathecal administration because of delayed systemic absorption and longer maintenance in the spinal cord.30,31 In male Red-eared Slider Turtles, intrathecal administration of morphine (0.1 to 0.2 mg/kg, 4 mg/mL, preservative-free) and lidocaine (4 mg/kg, 2%) resulted in thermal antinociception of the hindlimbs for up to 48 hours.24 No sedation was noted in any of the animals after intrathecal morphine administration. Therefore this combination of drugs should be considered so that short-term spinal anesthesia and concurrently long-lasting spinal analgesia can be achieved; if the analgesic effects last throughout the immediate postsurgical period, then the need for systemic opioid analgesic drugs is potentially reduced. It is currently unknown if intrathecal morphine has a potential respiratory effect in turtles, as has been documented for systemically administered morphine.14
In summary, spinal anesthesia and analgesia are clinically feasible techniques in chelonia. However, significant anatomic differences exist, particularly the presence of a prominent intravertebral venous plexus in Red-eared Slider Turtles. This makes general recommendations regarding technique and doses across chelonian species challenging. Furthermore, the amount of drug injected intrathecally will affect the extent of the sensory or motor block. Therefore different procedures will require different amounts of anesthetics and analgesics to be injected intrathecally. This technique may have to be repeated depending on the outcome. Currently, no studies have systematically evaluated the long-term effects and safety of intrathecal injections in chelonia. Strict aseptic techniques should be used to avoid iatrogenic complications, and only preservative-free drugs should be injected into the intrathecal space so that spinal toxicity and secondary neurologic complications are avoided. In contrast to mammals, in which the spinal cord terminates in the distal lumbar or lumbosacral region, the spinal cord in chelonia extends caudal to the last coccygeal vertebrae, and a cauda equina is not developed in chelonians.32 Therefore intrathecal needle placement in chelonia can potentially lead to iatrogenic mechanical trauma of the spinal cord in the coccygeal area and neurologic deficits. Sedation before intrathecal injection is recommended so that the tail is sufficiently restrained and immobilized and the caudal scratch/kick reflex is reduced; otherwise the reflex makes intrathecal injections in conscious turtles and tortoises challenging. In contrast, in male Galapagos tortoises, intrathecal injections were successfully performed without the use of sedation.25
Anesthetic Agents
Ketamine, a dissociative agent with dose-dependent anesthetic, sedative, and analgesic properties, is frequently used in reptile anesthesia. However, when administered alone, muscle relaxation is considered inadequate and recoveries excessively prolonged, especially when high dosages are used. Administering an alpha-2-adrenergic agonist (e.g., dexmedetomidine) or a benzodiazepine (e.g., midazolam) along with ketamine allows for reduction of the dose of ketamine administered and provides the benefit of partial reversibility of these protocols, leading to more rapid recoveries and increased safety. Even at lower subanesthetic dosages (less than 5 mg/kg), ketamine can provide additional sedation and analgesia if combined with other anesthetic drugs.24,33
Tiletamine/zolazepam (Telazol, Fort Dodge Laboratories, Fort Dodge, Iowa) is a commercially available drug combination composed of a dissociative anesthetic (tiletamine) and a benzodiazepine (zolazepam). Dosages of 2 to 10 mg/kg have been recommended for sedation, induction of general anesthesia, or facilitation of intubation.5,6,20 The anesthetic efficacy of tiletamine/zolazepam can be unpredictable, and recovery may be prolonged. In dehydrated patients or in those with underlying renal or metabolic disorders, the use of this drug combination is contraindicated.20 Tiletamine/zolazepam is also useful in large or dangerous species (e.g., crocodilians and large monitor lizards), and although not recommended for routine use in most reptiles, some have reported consistently good results (Mader DR: Pers. com., Feb, 2013 ).
Alpha-2-adrenergic agonists, such as medetomidine and dexmedetomidine, provide sedation, muscle relaxation, and possibly, analgesia in reptiles. Dose-dependent cardiovascular depression has been documented in reptiles after medetomidine administration.19,34,35 Alpha-2-adrenergic agonists are commonly used in combination with ketamine, especially in chelonian species, for safe, reliable, and reversible sedation but can also be combined with benzodiazepines for procedural sedation.36–41 Combining ketamine with medetomidine or dexmedetomidine allows reduction of both drug dosages, and reversibility of the alpha-2-adrenergic agonists with atipamezole will lead to faster and more predictable recoveries. Morphine or hydromorphone can be added to alpha-2 adrenergic-agonist-plus-ketamine protocols in cases in which analgesia is needed.37 If morphine or hydromorphone are used, reversal with naloxone (0.04 to 0.2 mg/kg, subcutaneously [SC]) should be considered after termination of the anesthetic procedure, particularly in freshwater turtles, if respiratory depression is profound or if prolonged recoveries or renarcotization are to be avoided.37
Benzodiazepines, such as midazolam and diazepam, have sedative and muscle relaxant properties. Midazolam is water soluble, can be administered SC, IM, and IV, and is considered a more appropriate choice than diazepam, which is not recommended for IM or SC injection.4 Midazolam, used alone, provides mild but highly variable sedation, which may be sufficient for minor clinical procedures.16,17 Midazolam, combined with ketamine or an alpha-2-adrenergic agonist, reduces all drug dosages and attenuates the dose-dependent cardiovascular depressant effects and prolonged recoveries commonly observed with high dosages of ketamine. The effects of benzodiazepines can be antagonized with the use of flumazenil (0.05 mg/kg, SC), which shortens recovery from sedation or anesthesia.41
Propofol, a short-acting nonbarbiturate anesthetic agent, can be administered IV or IO to provide short-term anesthesia, facilitating procedures such as placement of an esophagostomy tube or abscess debridement, or to induce general anesthesia and allow for endotracheal intubation and maintenance of anesthesia with an inhalational anesthetic agent. Respiratory depression is more profound when propofol is administered rapidly.20,42 Because propofol does not accumulate in tissues and is rapidly metabolized, complete recovery can be expected with assisted ventilation in cases of overdose. Propofol requires intravascular administration, which can be challenging in some species. Recently, published articles have described complications secondary to accidental extravascular injection of propofol intrathecally and into the subcarapacial sinus or the coccygeal vein of chelonian species.43–45 Complications associated with intrathecal propofol injection included forelimb and hindlimb paralysis, coma, and spinal necrosis.43–45 In addition, complications with propofol in sea turtles have been reported and include respiratory arrest and death several hours postanesthesia (Mader DR: Pers. com., Feb 2013).
Alfaxalone is a short-acting steroid anesthetic due for commercial release in the United States in 2013 that has a long history of use in other countries for induction of sedation or anesthesia. Alfaxalone is rapidly cleared and its metabolism is independent of organ function. Similar to propofol administration, alfaxalone administration is associated with smooth and rapid recoveries and dose-dependent cardiovascular and respiratory depression in mammals.46–48 One major advantage of alfaxalone is that it can be administered IM as well as IV.46,49–52 However, dose volumes are often large and may need to be divided between injection sites; thus the cost may be prohibitive in larger patients.
The IV administration of alfaxalone in Green Iguanas (Iguana iguana) (10 mg/kg) resulted in rapid induction of light anesthesia, which allowed endotracheal intubation.52,53 The effects of IM administration of alfaxalone have been investigated in Red-eared Sliders, Horsfield’s tortoises (Agrionemys horsfieldii) and Green Iguanas.49–51, At a dose of 10 mg/kg IM light-to-moderate sedation, suitable for minor nonpainful procedures such as blood collection and physical examination was induced. At 20 mg/kg, light anesthesia was induced and allowed for endotracheal intubation in all Red-eared Sliders and Green Iguanas, but only in about half of the Horsfield’s tortoises, which were moderately to deeply sedated. The administration of 30 mg/kg in Green Iguanas resulted in surgical anesthesia for up to 40 minutes.50 The induction times after IM alfaxalone administration in Green Iguanas were short (approximately 5 to 10 minutes at 24°C to 27°C), and recovery was smooth. The induction, duration, and recovery times after alfaxalone administration were significantly shorter at 35°C compared with 20°C in Red-eared Slider Turtles.49 At 20 mg/kg IM, the induction times were 19 ± 6 (20°C) and 7 ± 5 minutes (35°C), and the plateau phase lasted 28 ± 13 (20°C) or 8 ± 5 (35°C) minutes.49
Inhalational agents such as isoflurane and sevoflurane can be used for both induction and maintenance of anesthesia in many reptiles (but often not chelonians and crocodilians due to breath holding). Both agents offer the advantages of relatively fast induction and recovery times as well as limited organ toxicity, especially in patients with renal or hepatic impairment. Sevoflurane is commonly used in human and domestic animal anesthesia and has a low blood solubility. A rapid increase in alveolar concentration can be seen during induction with this agent. The low blood solubility allows for rapid changes in anesthetic depth. Investigations of the use of sevoflurane in various reptile species have shown variable results in that some species have not achieved a surgical plane of anesthesia. Few studies have investigated comparative cardiopulmonary effects of sevoflurane versus isoflurane in reptiles.14 Administration of inhalational agents follows similar principles as those established for domestic animal anesthesia. After induction of anesthesia, the trachea should be intubated with an appropriately sized endotracheal tube, which should be connected to a non–rebreathing (body weight, less than 10 kg) or rebreathing system. IPPV should be performed in all reptiles. The concentration of the inhalational agent necessary to maintain a surgical plane of anesthesia depends on the health status of the patient. Minimum anesthetic concentrations (MAC) of isoflurane and sevoflurane have been determined in Green Iguanas and are 1.8% to 2.1% and 2.1% to 4.1%, respectively.54,55 Maintenance requirements for a surgical plane of anesthesia have been reported to be 2% to 3% for isoflurane and 3.5% to 4.5% for sevoflurane.5 Like all anesthetic agents, both isoflurane and sevoflurane will cause cardiopulmonary depression as shown in Green Iguanas, in which isoflurane at 3% induced severe hypotension.56 Therefore the concentrations of both agents would have to be reduced in debilitated patients. A study in Green Iguanas has shown that cardiopulmonary effects of both inhalational agents are similar; however, sevoflurane will produce faster induction and recovery times.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree