Anatomy and Physiology of Reproduction in the Female Llama and Alpaca
Female Reproductive Anatomy
The ovaries of the llama are ellipsoid to globular (1.3–2.5 × 1.4–2.5 × 0.5–1.0 cm), while those of the alpaca are more globular (1.3–1.9 × 0.9–1.3 × 0.9–1.3 cm) varying the sizes according to the structures present on the ovaries (Figure 14-1).1 Each ovary weighs between 1.9 and 2.4 grams (g). In nulliparous females, the ovaries are flattened laterally and have an irregular surface because of the many small follicles present on it. In adults, numerous follicles, varying in size from 2 to 5 millimeters (mm), may be observed on the surface of the mature, normal ovary. They are not easily detected by transrectal palpation, especially in young animals, but are visually detectable by ultrasonography once they have reached a size of 3 mm.2 Larger follicles and corpora lutea that project from the surface of the ovaries can be easily detected by palpation and ultrasonography (Figure 14-2).3,4 Llama and alpaca cumulus oocyte complexes (COCs) are dark and easily distinguished through the follicle wall by trans-illumination. In llamas, the COC is located in the follicle hemisphere containing the expected ovulation point. Oocytes collected from follicles 2 to 11 mm in diameter range from 172 to 200 micrometers (µm). Immature oocytes had a very distinct and large germinal vesicle with a dark nucleolus. Mature oocytes display a metaphase plate surrounded by a dark area easily observable with low magnification.5 The ovarian bursa (a long conical, pocketlike fold of mesosalpinx) completely surrounds the ovary (Figure 14-3). Its apex forms a large circular orifice within which lie the fimbriae of the oviduct.
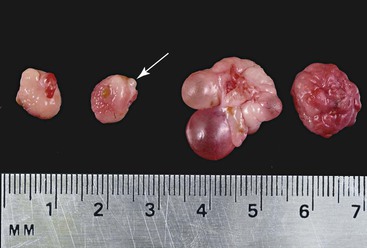
Figure 14-1 Morphology of the alpaca ovary. Left pair of ovaries from a lactating anestrous female (Note old corpus albicans, arrow). Right set of ovaries from a cyclic female. Note the follicular wave on the same ovary. (Reprinted with permission, Tibary A and Anouassi A. Theriogenology in Camelidae, Second Edition, Actes Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.)
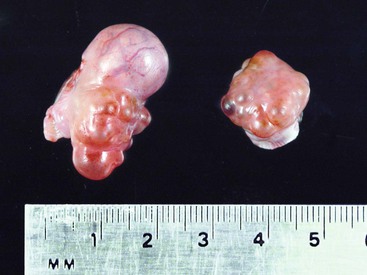
Figure 14-2 Pair of ovaries from a female alpaca at 8 days after ovulation. Note the protruding large CL (15 mm) and the dominant follicle on the left ovary. (Reprinted with permission, Tibary A and Anouassi A. Theriogenology in Camelidae, Second Edition, Actes Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.)
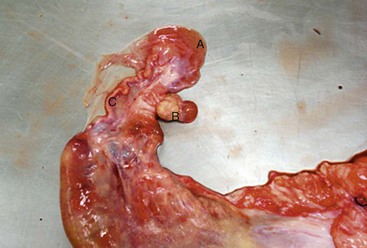
Figure 14-3 Ovary (B) exteriorized from its large bursa (A). Note the tortuous uterine tube (oviduct, C). (Reprinted with permission, Tibary A and Anouassi A. Theriogenology in Camelidae, Second Edition, Actes Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.)
The uterine tubes are rather long (10.5–18.3 and 20.4 ± 4.2 centimeters [cm] in llamas and alpacas, respectively), tortuous, quite firm, and embedded within the mesosalpinx. At the uterotubal junction, a papilla with a well-defined sphincter prevents the retrograde flow of fluid into the oviduct from the uterus, but the reverse is possible (Figure 14-4).1
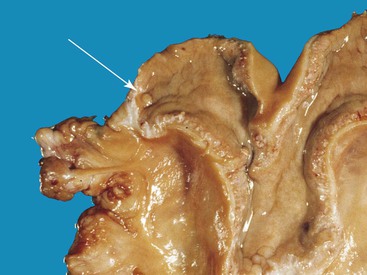
Figure 14-4 Open left uterine horn. Note the protruding uterotubal junction papilla (arrow) at the tip of the horn. (Reprinted with permission, Tibary A and Anouassi A. Theriogenology in Camelidae, Second Edition, Actes Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.)
The uterus of llamas and alpacas is bicornate, and in situ resembles the letter “Y” (Figure 14-5). The body is short (3–5.5 cm) and has approximately the same diameter. The left uterine horn may be slightly larger than the right one, even in nulliparous females. The length of the uterine horn of a llama, from the end of the septum to the tip of the horn on the convex surface, varies from 20 to 22.5 cm.1 The appearance of the uterine horns changes according to ovarian status. On ultrasonography, the uterus and the cervix appear heterogeneous because of an increase in intercellular fluid during follicular dominance. Meanwhile, cervical folds become more echogenic and prominent during luteal dominance. In general, the uterine horns are relatively straight and the uterine tone maximal during the estrogenic phase, whereas curling becomes maximal and the tone minimal during progesterone dominance.3,5 The uterus is located in the pelvic area in nongravid females, reaching the abdominal cavity during pregnancy. Considering that the majority of pregnancies are carried out in the left horn, asymmetry between both horns becomes extremely pronounced in multiparous females.4
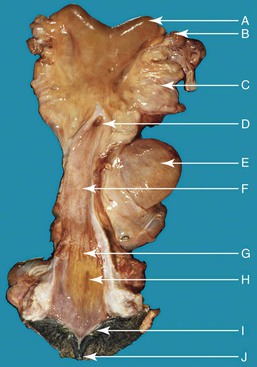
Figure 14-5 Anatomy of the reproductive tract in a female alpaca. uterine horn (a), note the absence of clear bifurcation externally; left ovary (b), brad ligament (c), fornix and external os of the cervix (d), urinary bladder (e), vagina (f), vestibule-vaginal sphincter (g), urethral orifice (h), vulvar lip (i), clitoris (j). (Reprinted with permission, Tibary A and Anouassi A. Theriogenology in Camelidae, Second Edition, Actes Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.)
The cervix is 2 to 5 cm long and 2 to 4 cm in diameter and has been described in two ways. One description indicates that two or three cervical rings are present (Figure 14-6). However, it has also been described as a single spiral fold with two or three turns, giving the appearance of two or three rings.1
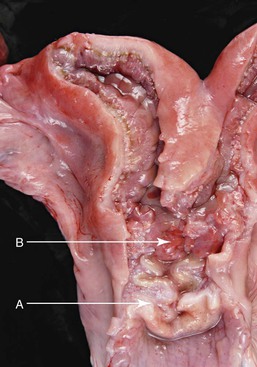
Figure 14-6 A, Cervix; note the three cervical spiraling rings. B, Body of the uterus is very small because of the long septum separating the uterine horns. (Reprinted with permission, Tibary A and Anouassi A. Theriogenology in Camelidae, Second Edition, Actes Edition, in press, Actes Edition, Institut Agronomique et Vétérinaire Hassan II, Rabat Morocco.)
The llama’s vagina, from the hymen to the cervix, varies in length from 15 to 25 cm and is approximately 5 cm in diameter.1 In alpacas, the vagina has an approximate length of 13.4 ± 2.0 cm and a diameter of 3.4 ± 0.7 cm. The vulva is small, with an opening of 2.5 to 3 cm in llamas, and has well-defined external labia that lie in a slightly slanted to vertical position approximately 4 to 6 cm ventral to the anal orifice.1 The vulva remains virtually unaltered despite changes in ovarian structure or sexual receptivity.
Female Reproductive Physiology
Puberty
The precise age at onset of puberty in female llamas and alpacas remains undetermined. Age at first ovulation depends on age at first mating as camelids are induced ovulators.6 Current knowledge suggests that puberty is greatly influenced by nutritional status and environmental conditions. In their natural habitat, the puna, age at puberty is reflected by forage availability and time of birth relative to the wet season. Nutritional deprivation during early development delays puberty. An increased production of ovarian hormones has been observed at approximately 6 months of age in well-fed llamas. Ovarian activity has been reported to begin around 10 months of age in alpacas, by which time the diameter of follicles is 5 mm or larger. In addition, it has been demonstrated that the age at which breeding maturity is reached depends partly on body weight. The first successful mating in female alpacas may occur at 12 to14 months of age and a body weight of approximately 40 kilograms (kg). Furthermore, a relationship seems to exist between body weight at mating and ability to maintain pregnancy. For each kilogram of gain in body weight, a 5% increase in parturition rate is seen. However, pregnancy rate becomes independent of body weight beyond 33 kg. Under conditions of good nutrition, a body weight of 33 kg is easily achievable in most yearlings. The usual practice in the puna is to breed llamas and alpacas for the first time at 2 years of age, although pregnancies have been observed in 6-month-old females. In any case, it is recommended that breeding be delayed until the female has reached two thirds of her anticipated adult body weight to reduce risks of stunted growth of the dam and dystocia.4
Ovarian Activity
Llama and alpaca females are induced ovulators, requiring copulation to trigger the luteinizing hormone (LH) surge responsible for ovulation.7 Therefore, they do not have regular estrous cycles, as described in other domestic species. Instead, it is advisable to refer to ovarian activity in terms of follicular phase and luteal phase. Studies using laparoscopy, ultrasonography, and hormone profiles in llamas and alpacas have demonstrated the occurrence of repetitive cycles (waves) of ovarian follicular growth and regression without ovulation in absence of copulation.8,9 In fertile, nonmated females, during each follicular wave, a cohort of follicles is recruited, followed by the synchronous emergence of a group of antral follicles, which grow to 4 to 5 mm in diameter. Of these, one follicle is selected to become the dominant follicle, and the others undergo atresia and regress.3 The dominant follicle grows to maturity and finally regresses if not ovulated. The dominant follicle exerts a negative effect on the rest of the follicles in both ovaries, thus regulating their number and diameter. Proposed mechanisms involved in the suppression of follicular growth by dominant follicles include reduction in pituitary gonadotrophin (follicle-stimulating hormone [FSH]) release because of high estradiol plasma concentrations, locally produced substances such as inhibin and androgens, or both, as it has been described in other species. Before final regression of the dominant follicle, a new follicle emerges and starts growing, producing the coexistence of regressing and growing follicles over several days, a phenomenon known as overlapping follicular waves. In most nonovulating llamas, waves overlap about 30% of their lifespan, that is, two successive follicular waves coexist for approximately 8 days. Because of this peculiarity, a follicle larger than 5 to 6 mm in diameter may be found at any given time in camelids, although it is not possible to discern whether the observed follicle is growing or regressing at a single examination.
In nonlactating llamas, the duration of follicular waves varies between 20 and 25 days.9,10 The follicular growth phase spans, on average, 9 days; the plateau phase takes approximately 5 days; and the regression phase lasts 8 days. The interwave interval is defined as the time elapsed between the beginnings of two consecutive waves. This period ranges from 16 to 20 days in llamas. However, because of the high variation observed in this interval in both species, it has been recommended that use of a mean value be avoided, as it does not accurately describe what is occurring in an individual animal, nor does it allow prediction of the optimal time for breeding.6 During the growth phase, follicles grow at rates of 0.5 to 0.9 mm/day.9 Ovulatory size is attained 6 to 8 days after the beginning of the growth phase. The maximum diameter of the dominant follicle averages 12 mm (range 9–16.5 mm), and this size is reached approximately 12 days after the beginning of growth. Thereafter, follicle regression proceeds at a rate of 0.8 mm/day. Lactation and pregnancy strongly affect follicular activity, and their negative effects are additive in llamas, conditioning shortened interwave intervals and reduced maximum diameter of dominant follicles. Thus, during gestation, waves begin at 15-day intervals, and dominant follicles reach a maximum diameter of 10 mm.10
In alpacas, maximum follicular diameters average 9 mm (range from 8 to 12 mm). On the basis of laparoscopic examinations of the ovaries, the average duration of follicle development, maintenance, and regression was consistently estimated at approximately 12 days.8 More recent studies carried out in Australia have reported interwave intervals ranging from 12 to 22 days.11 This indicates that the duration of follicular waves is widely variable (probably ranging from 12 to more than 20 days). Despite the high variation reported, the growth rate of dominant follicles seems to be consistent over the first 10 days after emergence in alpacas averaging 0.4 mm/day.11 In addition, longer interwave intervals were associated with the development of larger-sized follicles. It remains to be determined whether phylogenetic links exist between alpacas and vicuñas, a species in which the interwave interval averages 4.2 days for nonpregnant, nonlactating animals and if possible influence of crossbreeding with llamas explains this individual variation among alpacas.12 As in llamas, the mature phase lasts between 2 and 8 days, and the regression phase takes 3 to 8 days.9,11
In both species, the occurrence of large dominant follicles between ovaries is almost equal.11,13 No regular pattern of dominant follicles emerging between the left ovary and the right ovary has been demonstrated. The 65% alternation between ovaries reported in one study indicates that many successive waves occur in the same ovary.9 The view of the two ovaries as one unit controlled by systemic rather than local mechanisms has been proposed.
A close relationship between follicle size and estradiol-17β secretion has been reported.9 In addition, peak estradiol-17β plasma concentration occurs approximately 12 days after the beginning of the growing phase in llamas and 8 days in alpacas and is closely associated with the maximum size of the follicle.4 Plasma progesterone concentrations remain basal in anovulatory animals, offering an interesting animal model to study follicular dynamics in the absence of progesterone. Given that follicular waves usually overlap, the estrogen produced during those waves remains elevated, determining long periods of receptivity (≥30 days) interrupted by short stages of male rejection.8,10
The mechanism controlling growth and recruitment of follicles in SACs has not yet been elucidated. Studies in sheep indicate that follicle recruitment requires at least basal levels of gonadotropins. In cattle, the secondary surge of FSH following ovulation may play a role in initiating follicular recruitment in the next cycle, and a relationship has been proposed between FSH and the emergence of follicular waves. Thereafter, estrogens and inhibin secreted by a developing follicle inhibit further central FSH secretion, which becomes inadequate for the growth of subordinate follicles but stimulates growth and cell differentiation within the same follicle. No temporal association between FSH secretion and follicle development has been observed in llamas.13 Further studies, including development of sensitive methods for FSH measurement, are needed for better understanding of the process of follicular recruitment in llamas and alpacas.
Anovulatory follicles characterized by a larger diameter and a longer lifespan have been described in llamas and alpacas.14 Structures exceeding 12 or 14 mm in alpacas and llamas, respectively, and lasting 25 to 30 days have been referred to as cystic follicles. Follicular development appears to be normal through initial growth and maturity, with the follicle continuing to grow over the expected maximum diameter without any transition period between maturity and cystic status. In studies where the gross and ultrasonic morphologies of persistent follicles were evaluated, similar structures were identified as hemorrhagic follicles. They appeared grossly as a large fluctuating structure, filled with a dark red content, and ultrasonically they showed free floating echogenic content.15 Occurrence of anovulatory, oversized follicles appears increased in nonmated animals, and some individuals seem to have a higher tendency to develop them. Whether cystic and hemorrhagic follicles are the same structures or not is still debated. These structures do not seem to secrete estrogen. They are refractory to, and their presence is not associated with, any alteration or abnormality of ovarian dynamics.
Copulation and Mating Behavior
Behavior related to the establishment of dominance hierarchies is observed among males when they are first placed together with females. This behavior consists mainly of challenging and is characterized by spitting and jumping. Dominance is usually established quickly. The initiation of breeding behavior is marked by the pursuit of females by males. Some females become recumbent immediately, but most move away from the male until he mounts and puts pressure on the hindquarters in an attempt to force the female to lie down in a seated position. It is important to point out that sitting position is also assumed by females in response to the stress of being chased by the male. Once the female is recumbent, the male mounts her and moves forward to achieve intromission. Males penetrate the cervix during copulation and deposit semen into both uterine horns.16 During copulation, males show their excitement with trembling ears, tail flipping up and down, nostrils dilating, and constantly vocalizing by making a guttural sound. Females assume a very passive attitude and, in some instances, may change position and lie in lateral recumbency. Copulation generally lasts from 3 to 65 minutes, with an average time of 20 to 30 minutes.17 Ejaculation is a continuous process during mating. Receptive females generally sit near a mating couple. Nonestrous females strongly reject the males by spitting, kicking, and running away, but some may assume a sternal posture and submit to mating by a dominant male.1 Sexual activity is particularly intense during the first week following the introduction of males into a herd of nonpregnant females. More than 70% of the females are mated at least once during this time. Thereafter, the continuous association of males and females inhibits the sexual activity of the males in spite of the presence of estrous females. Although the physiologic basis for the decline remains unknown, it has been shown that if the males are allowed to rest for 4 to 5 days, they resume normal sexual activity. By taking such simple measures, conception rates have been increased from the usual 50% to 70%.18 The presence of a functional corpus luteum (CL) is usually associated with a nonreceptive status. During the luteal phase, the female strongly rejects the male by spitting, kicking, screaming, and attempting to escape.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


