Chapter 14
Anaesthesia of the pig
Introduction
Although surgery may be performed on the farm using either local analgesia or general anaesthesia, whenever possible pigs requiring more than minor surgery should be transported to a veterinary clinic having the equipment and personnel needed should complications arise. If the surgery is to be performed using local analgesia, sedation can be added to facilitate restraint. General anaesthesia employed under farm conditions will be dictated by financial concerns, availability of anaesthetic agents, and considerations for the animal such as adequacy of oxygenation. Pigs used in research studies must be anaesthetized using protocols that are appropriate for the particular study. Consequently, these anaesthetic protocols may be simple or complex but always should be selected after careful consideration of analgesia and animal welfare.
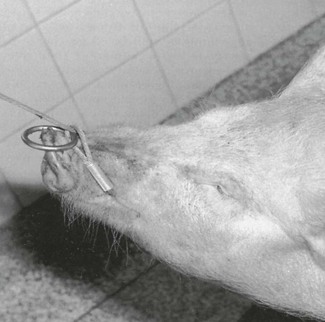
Pigs range in size from small newborn piglets to adults weighing 350 kg or more. As a result, the methods of restraint and administration of anaesthetics vary accordingly. While small pigs are easily restrained, large sows and boars may prove both difficult and dangerous. Small pigs can be simply held in arms or restrained on their sides by grasping the undermost legs and leaning on the pig’s body. A pig in a pen can be restrained for injection by placing a wooden board between the pig and the person effectively to squeeze the pig against the pen wall or in a corner. Boards used specifically for this purpose should be larger than the pig and may have handles attached on one side to facilitate manipulation of the board. Restraint of large sows and boars is also easy if a weighing crate is available, especially one with a ‘head catcher’. Large pigs can be restrained by a rope or wire snare placed around the upper jaw, caudal to the canine teeth (Fig. 14.1). Commercial snares have a metre-long metal handle to facilitate snaring the jaw and controlling the pig once it is caught. In most cases, the pig will try to escape by pulling back against the snare and thus immobilizes itself.
In research studies, a pig may be immobilized by placing it in a webbed sling in sternal position with all four limbs passed through holes in the sling. Although Vietnamese potbellied pigs kept as pets have been handled frequently and are easy to approach, they often resist restraint. The caudal edge of the tusks are razor-sharp and care should be taken to avoid lacerations when handling the jaws of pigs with untrimmed tusks.
Most anaesthetists consider pigs to be good subjects for general anaesthesia, provided due care is exercised. Although they resent restraint, as evidenced by the struggling and loud squeals they produce, they often respond predictably to anaesthetic agents. Recovery from anaesthesia is usually calm, unless the pigs are in pain or disoriented. As they have little body hair, pigs are liable to develop hypothermia when sedated or anaesthetized, or hyperthermia in a hot environment. Pigs tend to be fatter than most other farm animals and this can make accurate IM injections difficult. The shape of the pig’s head, together with the fat in the pharyngeal region (especially in Vietnamese potbellied pigs) coupled with a small larynx and trachea, increases the likelihood of respiratory obstruction in sedated and anaesthetized animals. Results of physiological studies of breathing patterns in non-anaesthetized obese potbellied pigs have identified upper airway syndrome with high inspiratory resistance and progressive limitation of inspiratory airflow. In those studies, mild laryngeal adduction occurred during expiration and a light plane of sleep. During deep sleep, the laryngeal adduction was absent and the pigs became hypoxic and aroused. The authors postulated that the laryngeal adduction identified in the potbellied pigs is a form of autopositive end-expiratory pressure (auto-PEEP) that provides expiratory braking to preserve end-expiratory lung volumes (Tuck et al., 1999, 2001). This effect would be lost during heavy sedation and anaesthesia, thereby resulting in hypoventilation.
Without endotracheal intubation, patency of the airway in pigs is best maintained by keeping the head and neck extended, pushing the lower jaw forward by applying pressure behind the vertical ramus of the mandible (Fig. 14.2), and by pulling the tongue out between the incisor teeth. Salivation, even if not excessive, can contribute to airway obstruction and promote laryngospasm. Consequently, anticholinergics are frequently given before general anaesthesia unless there are specific contraindications.
The anaesthetic agents listed in this chapter may not be available in all countries and many are not licensed for use in pigs. Choice of agents used will also depend on the pig as a food animal or not, its health, the procedure to be performed, and where the procedure will be performed (e.g. owner’s farm, veterinary clinic, or research facility). In some countries, pigs given unapproved drugs cannot be used for food.
Porcine malignant hyperthermia
Some breeds (particularly Poland China, Pietrain, and Landrace) and strains of pigs suffer from a biochemical myopathy which manifests itself during general anaesthesia. Malignant hyperthermia (MH) was first reported in Landrace cross pigs following the use of succinylcholine during halothane anaesthesia (Hall et al., 1966). The syndrome bears a close resemblance to a condition described in the early 20th century in pigs with pale, soft, exudative muscle (PSE) and to the Porcine Stress Syndrome. We now know that sudden death may occur in MH-susceptible humans in response to high environmental temperatures, exercise, or stress. Since retrospective studies of several generations of human family trees prior to 1970 identified multiple sudden deaths within families, it is thought that the syndrome had been present but unrecognized; 1970 was the turning point in the history of MH and much research has been published since that time. The MH syndrome is inherited in pigs in an autosomal recessive pattern, while in humans it is autosomal dominant. Only two causative genes have been identified thus far, with mutations of the type 1 ryanodine receptor of skeletal muscle being most commonly involved. All inhalation anaesthetic agents, except nitrous oxide, and the neuromuscular blocking agent, succinylcholine, are triggering agents. Injectable anaesthetic agents do not trigger the syndrome but MH may develop in pigs anaesthetized with injectable agents when the stress of handling and induction of anaesthesia has already initiated muscle metabolism changes. If identified early in pigs anaesthetized with injectable agents, ventilation with oxygen and cooling the pig may abort the syndrome. It is possible that some injectable agents, for example thiopental and alfaxalone, may delay the onset of MH during inhalation anaesthesia (Hall et al., 1972). The MH syndrome does not always occur at the onset of anaesthesia, but may develop at any time during anaesthesia and in the first few hours after anaesthesia.
Individuals suspected of susceptibility to MH can be tested using a muscle biopsy and a laboratory test that measures the degree of contracture induced in muscle fibres exposed to halothane or caffeine (the in vitro contracture test or IVCT). Although the test is sensitive, its specificity varies according to European and North American interpretations of the results and some false positives and false negatives are possible (Rosenberg et al., 2007).
Clinical signs
Triggering of the MH syndrome initiates release of calcium from the sarcoplasmic reticulum of skeletal muscle resulting in muscle contracture and increased cellular metabolism with production of heat and lactate (Rosenberg et al., 2007). Increased production of carbon dioxide results in hypercarbia, rapid deep respirations, and increased end-tidal CO2 concentrations. The carbon dioxide absorber in a rebreathing anaesthetic circuit becomes very hot to touch and the granules rapidly develop intense indicator colour. Release of catecholamines causes an initial increase in blood pressure with tachycardia and multifocal ventricular dysrhythmias. Hyperkalaemia and metabolic acidosis develop and, in some cases, the pH can decrease to less than 6.80. The skin of white pigs becomes blotchy red and white. Signs of advanced MH in most pigs include generalized muscle rigidity with spreading of the digits and a severe and sustained rise in body temperature up to 42.2°C (108°F) (Fig. 14.3). Ultimately, the animal becomes hypoxic and without treatment usually dies. In pigs, the signs observed during early development of MH syndrome are likely to be caused by MH, however, in dogs, there are several differential diagnoses that must be considered (see Chapter 2, Table 2.9). Accurate identification of MH relies on the presence of clinical signs and the more signs present the more likely that the patient has MH (Box 14.1).

Figure 14.3 Rigidity of the limbs and spreading apart of the digits in a pig developing porcine malignant hyperthermia syndrome.
Treatment of MH
Treatment involves eliminating anaesthetic agent from the anaesthetic circuit, hyperventilation with oxygen to aid elimination of CO2, aggressive cooling by covering the patient with ice, countering metabolic acidosis with injection of sodium bicarbonate and balanced electrolyte solution, and blocking the trigger by administration of dantrolene (Box 14.2). Dantrolene is a hydantoin derivative that was synthesized in 1967 (Krause et al., 2004). It binds to a specific ryanodine protein site and blocks the release of calcium, thereby interrupting the trigger and allowing signs of MH to reverse. Dantrolene is generally administered IV for rapid effect. The oral preparation of dantrolene is less costly. The bioavailability of dantrolene in animals after oral administration is less than decribed for humans. However, when MH susceptibility is suspected, administration of dantrolene orally before anaesthesia may prevent development of the syndrome. Azumolene, a more water-soluble analogue of dantrolene, has been investigated for treatment of MH but is not recommended due to unacceptable hepatic toxicity reported in humans. Dysrhythmias may be treated as needed but calcium channel blockers should be avoided when dantrolene has been administered. Hyperkalaemia should be treated in the usual recommended way with calcium, sodium bicarbonate, glucose and insulin, and hyperventilation.
Sedation
The pig’s reaction to restraint (struggling and vocalization) is sufficiently vigorous that sedation is widely used to facilitate handling and minor procedures, as well as for restraint prior to local or general anaesthesia. Intramuscular injections can be administered in the neck immediately behind the base of the ear at the level of the second cervical vertebra, or in the triceps, quadriceps, gluteal, or longissimus dorsii muscles. The neck is the preferred site in pigs intended for food to avoid damage in the other muscles. The skin should be clean before injection to avoid production of an abscess. The impact of the site of injection on drug action was evaluated in a study involving IM injection of azaperone, 1 mg/kg, and ketamine, 5 mg/kg (Clutton et al., 1998). Mature gilts were injected using 19 gauge 5 cm needles at four sites: in the neck muscle 5 cm caudal to the base of the ear; in the triceps muscle midway between the point of the shoulder and elbow; in the middle gluteal muscle at the centre of a triangle between midline, tuber coxae and tail-head; and in the quadriceps muscle in the cranial third of an imaginary line connecting patella and tuber ischii The degree of sedation at 16 minutes after injection was not significantly different between the injection sites, although the range of times to recumbency was narrower after injection in the quadriceps muscle. In a separate experiment, the sedative agents were either refrigerated, at room temperature, or warmed, and no differences in response to injection from the pigs were observed.
Agents employed for sedation in pigs
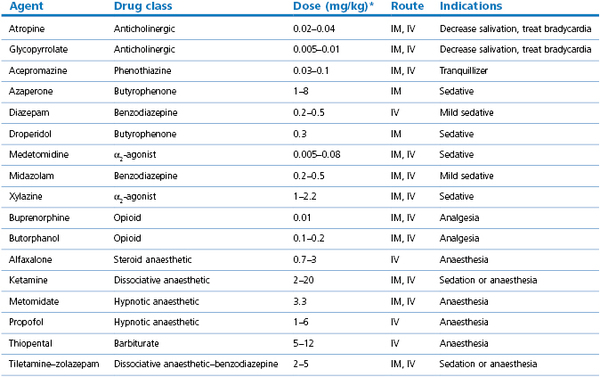
Some of the agents listed in this section may be administered in low or high doses according to whether the desired effect is sedation or anaesthesia (Table 14.1). Commonly, these agents are used in combinations with other agents, and more details may be found in the section on general anaesthesia.
Acepromazine
Phenothiazine derivatives are not as effective in pigs as they are in other species. However, administration of acepromazine, 0.03–0.1 mg/kg IM, renders pigs that are more easily restrained for IV injection, much less likely to dislodge the IV needle by head-shaking, and vocalize less. Acepromazine should be allowed 30 minutes to produce its full effects. Acepromazine has been commonly used with ketamine for anaesthesia in young pigs.
α2-Agonists
Xylazine is not as an effective sedative as in other species. Recommended dose rates for xylazine are 1–2 mg/kg by IM or IV routes and these will induce a calming effect for about 30 minutes, however, the pigs arouse easily when disturbed and little sedative effect will be apparent. Xylazine induces a transient increase in mean arterial pressure (MAP) and decreased heart rate (HR) for about 15 minutes. Although ineffective when administered alone, xylazine has an additive effect when added to other agents, such as ketamine, butorphanol or midazolam, and increases the degree of sedation.
Medetomidine produces a dose-dependent sedative effect in pigs that is substantially greater than from xylazine. The effect varies from mild to moderate sedation with medetomidine, 0.005–0.02 mg/kg IM, to heavy sedation produced by medetomidine, 0.08 mg/kg IM. The depth and predictability of sedation can be improved by the addition of butorphanol, 0.2 mg/kg IM, and midazolam, 0.2 mg/kg IM. Medetomidine induces bradycardia and clinical and experimental studies have included atropine, 0.02–0.04 mg/kg IM, or glycopyrrolate, 0.005–0.01 mg/kg IM, to increase heart rates. The addition of atropine to combinations that include (dex)medetomidine is not recommended in dogs because these sedatives induce peripheral vasoconstriction and increased blood pressure and atropine may promote severe hypertension by increasing HR. In pigs, medetomidine has a greater vasoconstrictive effect on the pulmonary circulation and, although increases in pulmonary arterial pressure are not severe when compared with the cardiovascular effects of ketamine or tiletamine, the consequences of these effects should be taken into consideration when anaesthetizing pigs for cardiovascular research or pigs with cardiac disease (Sakaguchi et al., 1996).
When pigs are stressed, their response to anaesthetic agents may be less predictable. In one study, a combination of medetomidine, 0.08 mg/kg, butorphanol, 0.2 mg/kg, and atropine, 0.025 mg/kg, administered to young pigs did not provide sufficient sedation to allow blood sampling in all animals (Ugarte & O’Flaherty, 2005). The addition of ketamine or tiletamine–zolazepam may induce anaesthesia. Dexmedetomidine is twice as potent as medetomidine and, therefore, dose rates for dexmedetomidine are half that used for medetomidine. Administration of medetomidine and dexmedetomidine produce similar cardiovascular effects, including bradycardia, decreased cardiac output, increased systemic vascular resistance and increased MAP.
Azaperone
Azaperone is a licensed and commonly used pig sedative in Europe and is available for use in wildlife in the USA. Azaperone must be given by deep IM injection and the pig should be left undisturbed for 20 minutes for best effect. The dose range is 1–8 mg/kg depending on the effects sought, but it is recommended that a dose of 1 mg/kg is not exceeded for adult boars as higher doses can cause prolapse of the penis.
Azaperone causes vasodilation resulting in a small fall in arterial blood pressure, and some slight respiratory stimulation. Hypothermia may develop in sedated pigs in a cold environment.
Dissociative agents
Ketamine and tiletamine (in combination with zolazepam) can be used to provide sedation. Ketamine, 2–10 mg/kg IM, combined with acepromazine, or azaperone, or medetomidine, or midazolam may be used to induce relaxation and sedation.
Tiletamine–zolazepam is available as a commercial preparation containing equal parts of each drug and is usually prepared to achieve 500 mg of total drug per ml. Zolazepam is similar to diazepam and is included to provide muscle relaxation. Following IM injection in pigs, the highest plasma concentrations of tiletamine and zolazepam occur within 60 minutes, however, zolazepam is more slowly eliminated than tiletamine (Kumar et al., 2006). Tiletamine–zolazepam, 2–5 mg/kg IM, can be used alone to induce sedation or be combined with xylazine or butorphanol. Recovery can be slow and lethargy and incoordination may persist for several hours.
Droperidol
The butyrophenone compound, droperidol, has been used in pigs and doses of 0.1–0.4 mg/kg give similar sedation to that produced by azaperone. Droperidol, 0.3 mg/kg IM, produced sedation by 15–45 minutes after administration with a duration of 2 hours in young pigs and minimal effect on heart rates and systolic blood pressures (Bustamente and Valverde, 1997). Butyrophenone/analgesic drug mixtures have been used and droperidol, 20 mg/ml, and fentanyl, 0.4 mg/ml, dosed at 1 ml/50 kg IM, produces better sedation than droperidol alone. Sedation develops rapidly and lasts approximately 15 minutes.
Opioids
Opioids administered alone are unlikely to induce sedation in healthy pigs. Butorphanol, 0.2 mg/kg, with xylazine, 1–2 mg/kg, or medetomidine, 0.01–0.02 mg/kg, usually with addition of atropine, 0.02–0.04 mg/kg, is a popular combination for IM administration. The addition of midazolam, 0.2–0.5 mg/kg, to these combinations increases the quality of sedation. Increasing the dose rate for medetomidine results in heavy sedation or anaesthesia. Pet potbellied pigs may be sedated at lower dose rates for medetomidine than is usually used in domestic pigs. Other opioids are used in pigs for analgesia during and after general anaesthesia.
General anaesthesia
Preparation
Pigs should undergo a physical examination for evidence of ill-health. Clinical patients with abnormalities or suspected systemic disease should be evaluated further using haematological and clinical chemistry laboratory tests. Normal physiological values have been published and reveal that reference values for minipigs may differ from domestic pigs (Hannon et al., 1990; Clark & Coffer, 2008). Potbellied pigs have lower rectal temperatures than domestic pigs (Lord et al., 1999). Other appropriate diagnostics that require patient cooperation, such as radiology and ultrasound, may have to be facilitated by sedation or anaesthesia. Specific patients, such as those with a history of vomiting or recent trauma, may require other precautions for anaesthesia. Rabies is not a common disease in pigs but may occur where rabies is endemic and has been reported in Vietnamese potbellied pigs. Clinical signs of depression and difficulty walking are non-specific for rabies.
Food is generally withheld for 8–12 hours and water for 2 hours before anaesthesia. Although vomiting at induction of anaesthesia or during recovery is uncommon in pigs, it can occur and introduces risk for aspiration, and ventilation is decreased when a full stomach exerts pressure on the diaphragm. Fluid deficits present before anaesthesia should be corrected by fluid therapy. Details of existing drug therapy such as antibiotic food additives, or anthelmintics, should be noted as drug interactions with anaesthetic agents may occur.
Premedication
Salivation may be increased in anxious pigs and by administration of dissociative agents. Laryngospasm is easily induced in pigs and saliva on the arytenoids can be an inciting cause. Atropine, 0.02–0.04 mg/kg, or glycopyrrolate, 0.005–0.01 mg/kg, IM or IV may be included in the premedication to minimize salivation.
Anaesthesia can be induced and maintained with isoflurane or sevoflurane, however, in dogs, use of an inhalant as the sole anaesthetic agent is associated with increased risk of death (Brodbelt et al., 2008). In certain circumstances, use of an inhalant agent alone may be expedient, however, in general, preanaesthetic sedation in pigs is recommended to reduce the subsequent dosages of anaesthetic agents and thereby increase the safety of anaesthetic administration. The degree of sedation required depends, in part, on the anaesthetic technique which is to follow. Agents may be administered IM to induce moderate to severe sedation and anaesthesia induced by titration IV of anaesthetic agents, such as ketamine, ketamine–diazepam (or midazolam), or propofol, or by administration of isoflurane or sevoflurane by facemask. Alternatively, agents may be administered in larger doses IM to induce general anaesthesia sufficient to accomplish endotracheal intubation. Anaesthesia is then maintained with an inhalation agent or injectable agents.
Analgesia
Some anaesthetic agents, like medetomidine, provide some analgesia, while others, such as propofol, thiopental, or isoflurane, provide very little. Analgesia may be provided by several methods, including administration of non-steroidal anti-inflammatory agents (NSAIDs, such as flunixin meglumine, carprofen, meloxicam, or ketoprofen), opioids, and local anaesthetic agents. Keita et al. (2010) reported that meloxicam administered in 5-day-old piglets as a single IM dose, 0.4 mg/kg, 10–30 minutes before castration decreased plasma cortisol concentrations and decreased pain related behaviours 2 and 4 hours after surgery. Butorphanol, 0.2 mg/kg IM or IV, is a frequently administered opioid to pigs in general practice. Advantages of butorphanol include increasing the sedation and analgesia provided by other agent combinations with minimal adverse cardiovascular effects. Disadvantages of butorphanol are that it has a short-lived duration and that the analgesia provided is inadequate for major surgery, particularly orthopaedic surgery. Buprenorphine, 0.01 mg/kg IM or IV, has been used as part of anaesthetic protocols in pigs, with an apparent duration of action of 4 hours. A higher dose rate of buprenorphine, 0.1 mg/kg IM, has been advocated by others (Harvey-Clark et al., 2000; Malavasi et al., 2008). Buprenorphine administration during anaesthesia decreases the concentration of isoflurane required for anaesthesia (Malavasi et al., 2008). Further studies are needed to define optimum dose rate and the extent of analgesia provided by buprenorphine in pigs.
Morphine and meperidine (pethidine) prolong analgesia when administered IM with azaperone and ketamine in pigs (Hoyt et al., 1986). Fentanyl IV infusions, 5–35 µg/kg/h, IV have been included in anaesthetic protocols for research surgery.
Transdermal fentanyl patches, 2 µg/kg/h, have been investigated for use in young growing pigs. Serum fentanyl concentrations were achieved by 12 hours after patch application, but large interindividual differences were measured and no true steady state concentrations were observed (Malavasi et al., 2005). Consequently, the authors recommended that additional agents should be administered to ensure adequate analgesia. Partial patch detachment will change absorption and use of an occlusive dressing over the patch is recommended to improve adhesion. No depression of normal behaviour was noted in healthy pigs after application of a patch. Pigs undergoing abdominal surgery that received both a morphine epidural block and a transdermal fentanyl patch applied at the end of surgery had lower cortisol concentrations at the end of surgery, showed immediate interest in food after anaesthesia recovery, and gained weight in the 2 days following surgery in contrast with pigs receiving no opioids (Malavasi et al., 2006b).
Opioid dependence can develop in pigs. Experimental pigs administered morphine free base daily for 23 days were challenged by an injection of naloxone, 0.02 mg/kg, IM (Risdahl et al., 1992). All the pigs demonstrated behaviour associated with opioid dependence, i.e. ‘wet-dog’ shakes, crawling and dragging bellies, constant posture changes, increased vocalization, salivation and increased defaecation frequency. A clinical patient, a young 5.9 kg potbellied pig, developed similar signs after discontinuation of transdermal fentanyl after 9 days (Trim & Braun, 2010).
Local analgesic techniques, such as incisional infiltration with bupivacaine, up to 2 mg/kg, will contribute long-term analgesia to an anaesthetic protocol. Other nerve blocks applicable to specific surgeries can be incorporated, such as intercostal nerve blocks and interpleural nerve block for thoracotomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree