Chapter 11
Anaesthesia of the horse
Additional routes of administration of opioid agents
Other drugs used as analgesics
Caudal epidural injection and catheterization
Subarachnoid injection and catheterization
The complete desensitization of the forelimb below the carpus
The complete desensitization of the distal hind limb
Local analgesia for castration
Preventive methods and/or treatments
Methods of control at anaesthetic induction
TIVA for short procedures (up to 30minutes)
Agents used to extend the duration of anaesthesia
TIVA for medium duration procedures (30–90minutes)
α2-Agonist/guaifenesin/ketamine – the ‘Triple Drip’
α2-Adrenoceptor agonist/benzodiazepine/ketamine
General points in relation to maintenance of anaesthesia using volatile agents
Increase in circulating fluid volume
Introduction
Probably no other species of animal presents as many special problems to the veterinary anaesthetist as the horse. Perioperative (7 day) mortality rate in relation to general anaesthesia in apparently healthy horses is around 1% (Johnston et al., 1995) and has remained constant to that time over at least 30 years (Hall, 1983) despite increasing sophistication of anaesthetic techniques and monitoring. The situation may be improving as a number of more recent surveys have posted lower mortality rates. Of particular note, Bidwell et al. (2007), in a retrospective survey of nearly 18 000 anaesthetics from a referral practice, reported a mortality rate of 0.12 % directly related to anaesthesia, which increased to 0.24 % taking 7-day survival into account. Limited improvement in survival rates results mainly from the longer duration of many surgical procedures that, without the advances that have occurred, would have been impossible to perform. Increased duration of anaesthesia significantly increases the risk.
The veterinary anaesthetist is faced with numerous disturbances of cardiopulmonary and skeletal muscle function in the equine patient associated with general anaesthesia, many of which are only very incompletely understood. Their certain prevention is currently impossible, and measures designed to overcome one problem often only result in exacerbation of another. Developments in the provision of reliable sedation has enabled a wider range of procedures to be carried out under local analgesia than was once considered practicable, but general anaesthesia still remains the only option in many cases.
To the time of Johnston’s et al.’s studies (1995), prolonged anaesthesia was usually maintained with inhalation agents and, although in the USA isoflurane was often used, halothane was still the agent in routine use in Europe. Over the past 15 years, new inhalation agents are used, but also a very wide variety of injectable drug combinations have been employed, both on their own as total intravenous anaesthesia (TIVA) and as supplements to inhalation anaesthesia. Some of these combinations have a good evidence base for their use; others have not. Certainly, there is need for a new epidemiological study to see if improvements in mortality and morbidity really have taken place.
There is no ideal method of anaesthesia in the horse – even the two authors do not always agree on which is ‘best’. This chapter will attempt to give the reader the background knowledge to understand some of the major causes of the problems that occur, how to avoid or correct them and to assess whether regimens suggested in publications may or may not have a part to play in improving safety. It will then suggest some regimens which have a good evidence base for use and/or that the authors have found useful.
Sedation of the standing horse
It frequently is necessary to sedate horses to enable procedures to be carried out easily and safely. Horses are not good subjects for sedation for if they experience a feeling of muscle weakness or ataxia they may panic in a violent manner. Historically, the most effective sedative was, for many years, chloral hydrate. Introduction of the mood-altering ‘neuroleptic’ agents, in particular the phenothiazine, acepromazine, followed in 1969 by xylazine (Clarke & Hall, 1969), and more recently other α2– adrenoceptor agonists (α2-agonists) has revolutionized equine sedation and, with the addition of local analgesic techniques, many procedures can now be performed in the standing animal. However, even with modern agents, sedated horses must be handled with caution for they may be aroused by stimulation and when disturbed can respond with a very well aimed kick.
Currently, sedation of the standing horse usually is achieved with combinations of acepromazine, and/or an α2-agonist plus a small dose of an opioid (see sedative/opioid combinations below).
Phenothiazines
Acepromazine
Acepromazine is the phenothiazine derivative most widely used in horses for both its mood-altering and its sedative actions. Intravenous (IV) doses of 0.03 mg/kg or intramuscular (IM) of 0.05 mg/kg exert a calming effect within 20–30minutes of injection and, although at these dose rates obvious sedation may only be apparent in 60% of horses, they become much easier to handle. Doses may be doubled, but the level of sedation does not always increase, although the duration will. Acepromazine (in paste or tablet forms) also may be given orally at maximally recommended doses of 0.1 mg/kg. Oral availability in horses is high and can be equal to that of the IM route (Hashem & Keller, 1993). Acepromazine is very long acting, and elimination may be further delayed in old or sick animals, particularly in those with even mild liver disease.
Acepromazine at the doses recommended has little effect on ventilation, but causes hypotension through vasodilation (Kerr et al., 1972). Hypovolaemic horses may faint. Tachycardia may result from the fall in arterial blood pressure, but sometimes first degree atrioventicular block is seen. A very small proportion of horses (the authors have seen two such cases) show aberrant reactions, and may become recumbent without any apparent cardiovascular cause; these reactions were more common with other phenothiazine agents. In stallions and geldings, effective sedation with phenothiazine derivatives is associated with protrusion of the flaccid penis or, on very rare occasions, priapism. In either case, physical damage to the penis must be avoided. In a very small proportion of horses, prolonged prolapse occurs. Treatment of this complication is by manual massage, compression bandage and replacement in the prepuce followed by suture of the preputial orifice. It is the opinion of the authors, and of many veterinarians (Driessen et al., 2011) that the low incidence of this complication coupled with the possibility of immediate treatment means that where a phenothiazine agent is the drug of choice its use is not contraindicated in stallions or geldings.
Acepromazine has proved a very safe agent in the horse. The calming and low-level sedative effects it produces make it the agent of choice for interventions such as shoeing, and when used in young animals it appears to assist in training the horse to tolerate many future procedures without sedation. When acepromazine is combined with opioid agents, deep sedation is achieved and such combinations may be used in cases where α2-agonists would be inappropriate. Acepromazine is an excellent premedicant before general anaesthesia; it calms the horse prior to the insertion of catheters, lengthens the action of the anaesthetic agents, reduces pulmonary ‘shunt’ fraction (Marntell et al., 2005a) and statistically reduces the overall risk of anaesthesia and surgery (Johnston et al., 1995). Its prolonged duration of action means that it contributes to a quiet recovery.
α2-adrenoceptor agonists
Three α2-agonists, xylazine, detomidine and romifidine are marketed for equine sedation and anaesthesia. Two α2-agonists marketed for small animals have also been used in this species. The general properties of all these agents are described in Chapter 4.
Xylazine
Following the introduction of xylazine, it rapidly gained in popularity because of the reliable sedation produced in horses. Doses of 0.5–1.1 mg/kg IV are followed within 2 minutes by obvious signs of effect. The horse’s head is lowered and the eyelids and lower lip droop (Clarke & Hall, 1969; Kerr et al., 1972). Although the horse may sway on its feet, cross its hind legs or knuckle on a foreleg, with xylazine alone, it will remain on its feet and show no panic. Sedation is maximal after about 5 minutes and lasts 30–60 minutes depending on the dose. Doses of 2–3 mg/kg IM give similar effects, maximal sedation being achieved 20 minutes after injection. Xylazine has analgesic properties, particularly in colic (reviewed by England & Clarke, 1996) and, in colic cases, analgesia is associated with the marked reduction in gut movement caused by drugs of this class. Horses sedated with xylazine alone remain very sensitive to touch and the apparently well-sedated horse may, if disturbed, respond with a very sudden and accurate kick.
Investigations into the effects of xylazine in horses have been reviewed (England & Clarke, 1996). There is a transient rise in arterial blood pressures. Mean arterial pressure (MAP) peaks 1–2 minutes after IV injection; the pressure then slowly falls to below resting values and remains depressed for at least one hour (Fig. 11.1). Concurrent with the hypertensive phase, there is profound bradycardia coupled with both atrioventricular and sinoatrial heart block. Heart block is at its most intense in the first few minutes and, in many cases, disappears as the heart rate increases. Changes in blood pressure are dose dependent in intensity and duration and, following IM injection, changes are similar but less marked. Cardiac output (CO) is significantly reduced, probably because of the bradycardia; IV doses of 1.1 mg/kg cause falls of 20–40% of normal resting values of CO. The changes in MAP result from a balance between peripheral vasoconstriction and the centrally mediated fall in heart rate and CO (see Chapter 4). At doses of up to 1.1 mg/kg, xylazine does not cause severe respiratory depression although there may be a small rise in PaCO2 and a slight decrease in PaO2. However, some upper airway obstruction may occur; horses may snore if left in the head down position for any length of time. Heavy coated horses may sweat as sedation is waning – most commonly seen if atmospheric temperatures are high. Other side effects are those typical of α2-agonists (see Chapter 4) and include hyperglycaemia, diuresis, gut stasis and increase in uterine tone; this latter makes it theoretically preferable not to use xylazine in pregnant mares. Changes in insulin production and hyperglycaemia do not appear to be a feature of xylazine action in neonatal foals (Robertson et al., 1990).
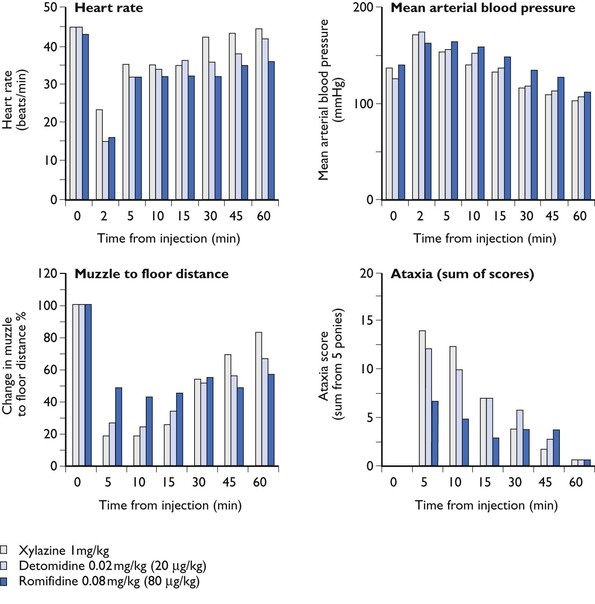
Figure 11.1 Comparison of some of the sedative and cardiovascular effects of IV doses of xylazine (1 mg/kg), detomidine (20 µg/kg) and romifidine (80 µg/kg). Data from 5 ponies. SE bars are omitted for the sake of clarity Data as reviewed by England and Clarke.
When used as a premedicant, xylazine greatly reduces the amount of both injectable and volatile anaesthetic agents subsequently required. In the 30 years since its launch, xylazine has become the ‘gold standard’ for equine sedation and premedication, particularly in North America. In Europe, until the advent of generic forms and alternative agents forced prices to reduce to a realistic level, its use was limited by much higher pricing.
Detomidine
Detomidine, a very potent α2-agonist was first used as a sedative and analgesic agent in 1982 and, since its introduction, has gained great popularity for sedation and premedication of all types of horse (Vainio, 1985; Alitalo, 1986; Clarke & Taylor, 1986; Short, 1992). Initially, very high doses (up to 160 µg/kg) of detomidine were recommended but it soon became obvious that maximal sedative effects were obtained with IV doses of 10–20 µg/kg (0.01–0.02 mg/kg) and that higher doses increased the duration rather than depth of sedation. Slightly higher doses appear necessary to provide good analgesia (Hamm et al., 1995). The concentrated but non-irritant form of 10 mg/ml in which detomidine (as Domosedan or Dormosedan, and now generics) is provided makes it very suitable for IM injection, doses approximately twice those given IV being required to produce the same effect.
Detomidine has some effect when injected subcutaneously (SC) and is also absorbed through mucous membranes and there is now a gel form available for this purpose (Gardner et al., 2010; Kaukinen et al., 2011). When given in food, the effect is less reliable as the drug is extensively metabolized by first pass through the liver. The property of easy absorption across mucous membranes must, for reasons of personal safety, be taken into account when handling the drug.
The type of sedation produced by detomidine is identical to that produced by xylazine; in blind trials comparing IV xylazine (1 mg/kg) IV and detomidine 20 µg/kg IV (England & Clarke, 1996), all parameters assessed and measured except duration of action were identical (see Fig. 11.1). As with xylazine, horses under detomidine sedation are sensitive to touch and may kick. Lower doses of detomidine do not always result in maximal sedation, but may prove useful where a short period of action is required. Detomidine premedication reduces the dose of anaesthetic agents subsequently required.
The cardiovascular properties, bradycardia, peripheral vasoconstriction and changes in arterial blood pressure (see Fig. 11.1) are also as described for xylazine above (Short, 1992). With higher doses, the hypertensive phase is considerably more prolonged (Short et al., 1986). At clinically used doses, respiration is slowed and PaO2 is slightly decreased. Some horses snore. The authors have noted occasional horses (usually but not always those suffering from toxaemic conditions) becoming tachypnoepic for some 10–15minutes after the administration of detomidine and similar reactions have been reported after other α2-agonists (Bettschart-Wolfensberger et al., 1999; Kendall et al., 2010). The condition appears self-limiting and does not require treatment. Other side effects include reduction in gut motility, hyperglycaemia, sweating and an increase in urination. Doses above 60 µg/kg may cause swelling of the head; this problem can be prevented by propping the head in a normal position. Occasionally urticarial reactions have been noted; these are self-limiting and regress without treatment. Moderate doses of detomidine are claimed to be safe in pregnancy (Jedruch et al., 1989) and many mares have received multiple doses throughout gestation without any maternal or fetal harm resulting.
The actions of detomidine (and of other α2-adrenoceptor agonists) can be reversed by the specific antagonist, atipamezole. Dose rates from 60 to 200 µg/kg have been used, that required depending on the degree of residual sedation (Nilsfors & Kvart, 1986; Bettschart-Wolfensberger et al., 2001b; Hubbell & Muir, 2006). At the doses of detomidine recommended, it is rare that antagonism is required, but the authors have been grateful of its availability in cases of overdosage of α2/opioid combinations where a horse has become severely ataxic or even recumbent, and in one case of a massive overdose of detomidine (Di Concetto et al., 2007).
Romifidine
The type of sedation produced by romifidine differs from that produced by the other α2-agonists; the horse’s head does not hang so low, and there is considerably less ataxia (Voetgli, 1988; England & Clarke, 1996), although (Ringer et al., 2013) found no difference from xylazine when comparing the drugs given by constant rate infusion (CRI). Despite the apparent reduced sedation, romifidine at doses of from 40 to 120 µg/kg (0.04–0.12 mg/kg) IV enables a range of clinical procedures to be performed; the effect (and the ataxia) is enhanced by combination with opioids. In clinical practice, romifidine has proved very popular where ataxia is particularly unwelcome, e.g. for shoeing. The pharmacological actions of romifidine other than sedation are similar to all α2-agonists. Romifidine reduces gut motility, the duration of this effect being dose dependent (Freeman & England, 2001). Self-limiting urticarial reactions may occur occasionally. The degree of analgesia produced by romifidine has been questioned; the results appear to depend on the nociceptive stimulus used (England & Clarke, 1996). Spadavecchia et al. (2005) found good reliable antinociception, Voetgli (1988) that antinociception was not dose related or consistent, and Hamm et al. (1995) claimed there was no analgesic activity. Rohrbach et al. (2009) compared the antinociceptive effects of romfiidine, xylazine and detomidine; all three α2-agonists provided equal antinociception, although duration was drug dependent. In equine practice, romifidine is widely used for premedication and, as part of anaesthetic combinations, it reduces the dose of anaesthetic agents subsequently used.
Other α2-agonists
Medetomidine’s use has been investigated in horses (Bryant et al., 1991; Bettschart-Wolfensberger et al., 1999). Doses of 5–7 µg/kg (0.005–0.007 mg/kg) IV are sufficient to cause very deep sedation with severe ataxia, and higher doses may result in recumbency. The marked hypnotic properties make the agent unsuitable for routine use as a sedative in horses. However, it appears to be short acting, having a half-life of elimination of 29 minutes (Grimsrud et al., 2012) and its excellent analgesic and muscle relaxant properties mean that it has become quite widely used as a CRI during anaesthesia (see Box 11.1). Bettschart-Wolfensberger et al. (2005) showed that dexmedetomidine at 3.5 µg/kg IV in the horse had similar effects as medetomidine.
Practical use of α2-adrenoceptor agonists
The majority of the sedative, cardiopulmonary and other side effects appear similar with all three α2-agonists commonly used in horses (see Fig. 11.1). The exceptions are the different manifestation of sedation and less of ataxia with romifidine, and the fact that xylazine appears to have the greatest ecbolic effect, so is not the preferred choice for pregnant mares. Otherwise, the choice will depend on the duration of action required, the route of administration, and on personal preferences. For example, all three agents provide excellent analgesia in cases of colic, although the bradycardia and lack of gut motility induced must be considered in the subsequent assessment. Before a definitive diagnosis has been made in a colic case, it is preferable to use xylazine or a low dose (up to 15 µg/kg) of detomidine for sedation and analgesia, as high doses may mask signs indicating the requirement for surgery. Following a decision that surgery is required, the longer acting romifidine with its lack of ataxia, may be the most suitable analgesic for transportation. Although there are no scientific studies of combining different α2-agonists, in practical clinical use, there are many reports of changing from one to another at subsequent dosing; the effects appear to be additive as expected.
α2-Agonists have marked cardiopulmonary side effects, and it is not surprising that occasional cases of collapse, or even of death have been reported following their use. Some reported cases may have resulted from intracarotid injection, but where there is a delay between injection of the drug and the unexpected event, then such a reaction is probably drug induced. In dogs, xylazine sensitizes the heart to adrenaline-induced arrhythmias but, in horses, such sensitization has not been proved (W.W. Muir, personal communication). However, there are anecdotal reports of collapse in horses which have been given an α2-agonist when in a high state of excitement. Unfortunately, immediate sedation is necessary in many such situations and, in these cases, sufficient time must be left after drug administration before further stimulation is applied. In such situations, the authors prefer combinations with acepromazine, which may reduce the risk. Clinical reports have suggested that combinations of α2-agonists and potentiated sulphonamides should be avoided, although any scientific basis for these observations is unproven.
Some veterinarians premedicate horses with an anticholinergic agent (atropine 0.01 mg/kg or glycopyrrolate 0.01 mg/kg) prior to the administration of an α2-agonist, but use of such combinations is controversial. Anticholinergic drugs will prevent or reverse the bradycardia caused by α2-agonists and will improve CO but will result in hypertension (Pimenta et al., 2011). Anticholinergic drugs, if used, must be given an adequate time prior to sedation; giving the two drugs together is not a rational choice.
Infusions of α2-agonists
Where α2-agonists are being used to provide sedation for surgery, sometimes it is necessary to increase their duration of action. Intermittent dosing, each increment being 25–50% of the original dose administered, is effective, but a constant rate infusion (CRI) provides a more level and controllable plane of sedation. Usually, the agent used is diluted in a saline drip then, following the initial loading dose, is infused to effect. Infusions have been used alone, with opioids (see opioid combinations below) and as an adjunct to general anaesthesia (see PIVA below). Some doses that have been used in these circumstances are summarized in Box 11.1. Of these, the most thorough exploration of suitable dose rates were those of Bettschart-Wolfensberger et al. (1999) for medetomidine and Ringer et al. (2012a,b) for xylazine and romifidine, as in each case a dose that would give a steady level of sedation was elucidated, then the result confirmed with pharmacokinetic studies. However, doses from experimental studies are only a guide in the clinics; sometimes they are inadequate or too high for the individual clinical case, and the infusions should always be given ‘to effect’.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



