Chapter 20
Anaesthesia for intrathoracic procedures
The primary concern for anaesthesia of intrathoracic procedures is management of the pneumothorax that is necessary for surgical access created by opening the chest wall and/or the diaphragm. Ventilation must therefore be controlled and monitored closely by the anaesthetist or an individual on the anaesthesia team to maintain appropriate oxygenation and gas exchange (see Chapter 9). Manual ventilation can be satisfactory but the use of a mechanical ventilator ensures accurate delivery of minute ventilation, a rhythmic cycle of breathing that the surgeon can anticipate during the procedure, and more time for the anaesthetist to devote to patient monitoring. Clinical intrathoracic procedures are most commonly performed in dogs and cats; specific management details are included in latter sections of this chapter. Some surgical or medical procedures may inadvertently penetrate the pleural cavity and create a pneumothorax, for example, ventral cervical surgery near the thoracic inlet (tracheal ring repair, ventral cervical decompression, or mass excision), oesophageal endoscopy, cranial abdominal surgery resulting in a crural tear, mass excisions over the thorax, and trauma disrupting the integrity of the thoracic wall (fractured ribs, pulmonary bullae, or penetrating wound). Experimental thoracotomy is frequently performed in pigs and sheep. The general principles for anaesthetic management are the same for all species.
General principles
Preanaesthetic evaluation and preparation
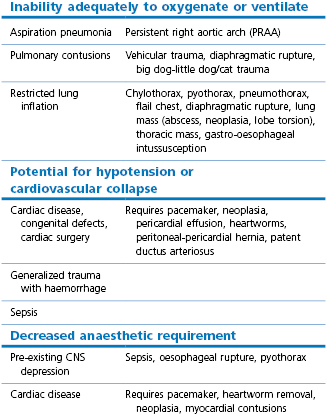
The incidence of postoperative pulmonary complications is high in human patients after thoracic and upper abdominal surgery (Duggan & Kavanagh, 2010). Factors associated with such complications are a history of smoking, increasing age, degree of preoperative dyspnoea, increasing ASA score, extent of an intrathoracic tumour, presence of cardiac disease, and duration of surgery exceeding 3 hours. Low serum albumin has also been found to be an important predictor of postoperative pulmonary complications and 30-day mortality (Duggan & Kavanagh, 2010). Thus, the physical status of the veterinary patient scheduled for thoracic procedures should be evaluated with a thorough physical examination. Some patients may be relatively healthy, others will have specific abnormalities that must be considered in the anaesthetic management (Table 20.1). These might include pneumonia, diffuse pulmonary neoplasia, a space-occupying mass(es), pulmonary or myocardial contusions, restricted lung inflation from pulmonary or external causes, cardiac disease, pre-existing central nervous system (CNS) depression, or sepsis.
Lung function should be evaluated when respiratory disease is present by assessing the degree of exercise intolerance (ability to walk without dyspnoea), the pattern of breathing, auscultation of the lungs for abnormal sounds, arterial blood gas analysis (PaCO2 and PaO2), and thoracic radiography (Miller, 2007). An electrocardiogram (ECG) should be obtained to identify any dysrhythmias. Lung function may be evaluated in dogs and horses with spirometry. Flow-volume loops are obtained with a pneumotachometer and a tightly fitting mask over the patient’s muzzle. Further information may be obtained from computerized tomography (CT) in some patients. Whenever possible, medical treatment should be instituted before anaesthesia to improve the patients’ physical status, for example, removal of air or drainage of pleural fluid.
The surgical approach varies depending on the specific lesion and location, and the surgical techniques utilized – abdominal, left or right thoracotomy, and median sternotomy are most common; thoracoscopy may be performed instead of or in addition to thoracotomy. Requirements for management of the various approaches may differ, for example, analgesic techniques for a lateral thoracotomy will be different from a thoracoscopy.
Preanaesthetic preparation of both patient and equipment is key to avoid or prevent complications. Basic needs include one or more often two venous catheters sufficient for rapid fluid therapy, warming devices as heat loss can be severe, and a thermometer, a pulse oximeter, and blood pressure monitor. Controlled ventilation, either manual or by mechanical ventilator, is necessary. Blood for transfusion should be available for some procedures and blood typing or cross-matching before anaesthesia may also be advisable for procedures where blood loss is common. Suction equipment should be available for procedures involving surgery of pulmonary tissue so that blood and fluid can be removed from the airway as needed. Suction technique of the trachea should be intermittent rather than continuous to avoid collapsing the lung from excessive suction.
Ideally, facilities should be available for intensive monitoring that include capnography, blood gas analysis, and invasive pressure measurements, however, management of an acute diaphragmatic rupture in a dog or cat is likely to be much less complex than a thoracotomy for pericardectomy or experimental cardiac surgery. Many procedures in dogs, such as lobectomy, pericardectomy, thoracic duct ligation, and exploratory, can be performed by thoracoscopy when the equipment is available. Although this technique has the benefit of decreased postoperative pain for the patient, it presents some difficulties for the anaesthetist particularly involving provision of adequate ventilation and oxygenation. One-lung ventilation (OLV) requires additional equipment preparation and lengthens the time from induction to start of the surgical procedure.
The species of the patient will have a significant impact on choice of anaesthetic agents and anaesthetic management. Anaesthesia of a sheep for experimental thoracotomy must include plans for management of bloat and prevention of aspiration in the event of regurgitation. Maintenance of adequate PaCO2 and PaO2 is often difficult in a horse with a diaphragmatic rupture, and management of recovery is more difficult than in a dog.
Ventilation and oxygenation
The effects of artificial ventilation and of opening the chest on cardiopulmonary function have been discussed in Chapter 9. Controlled ventilation (IPPV) with cyclical stretching of the lungs may cause lung damage that results in complications postoperatively. Toll-like receptors are thought to play a key role by activating a series of complex signalling pathways that initiate inflammation (Curley et al., 2009). The impact of IPPV strategy on healthy lungs may be minimal since several studies comparing ventilation at tidal volumes of 12–15 mL/kg with 6 mL/kg for relatively short times (5 hours) have not identified differences between ventilation protocols when measuring plasma tumour necrosis factor α (TNFα) and interleukin concentrations (Curley et al., 2009; Beck-Schimmer & Schimmer, 2010). It has been suggested that a two-hit process may be required for ventilator-induced injury. Also recommended is further research into the effect of anaesthetic agents on the mechanisms of lung injury as downregulation of these processes offering a degree of protection has been documented with some anaesthetic agents (Curley et al., 2009).
Use of low tidal volumes of 6 mL/kg of ideal body weight is recommended for ventilation of human patients with acute lung injury and acute respiratory distress syndrome (ARDS) of various aetiologies (Bigatello & Pesenti, 2009). Injury in the lung is not homogeneous but occurs in small areas. Delivery of a fixed volume breath to the lung results in areas of pressures within the lung that are different to the measured tracheal pressure. Alveoli that are closed with fluid-filled terminal airways will not be ventilated and air is then directed at a higher pressure into alveoli that are already ventilated (compliant alveoli) resulting in their overventilation. A disadvantage of restriction of tidal volume to avoid barotrauma is that, despite an increase in respiratory rate, it may result in an inability effectively to maintain PaCO2 within normal limits. In this instance, an option is to allow ‘permissive hypercapnia’ where a higher than normal PaCO2 (<55 mmHg) is purposely allowed to avoid high airway pressure or tidal volume. The body of information on ventilator-induced lung trauma has largely been acquired from investigations involving humans and mice, and research involving the varied domestic species is needed for accurate veterinary relevance.
It is usual to modify the parameters used for ventilation of healthy lungs for ventilation of animals with damaged lungs, such as pulmonary contusions, pneumonia, and collapsed lung lobes, by limiting the peak inspiratory pressure to ≤15 cmH2O whenever possible. The decrease in tidal volume achieved by this low pressure is compensated for by an increase in respiratory rate. Similarly, tidal volume must be decreased after lung lobectomy and at the change from two-lung ventilation (2LV) to OLV to avoid over distension of the one lung being ventilated, barotrauma, and pneumothorax.
Lung collapse will occur even in healthy patients without thoracic disease during anaesthesia. Three areas for potential collapse are: (1) the alveoli (atelectasis, loss of aeration of a whole acinus) caused by resorption of oxygen, inadequate distending pressure, or physical compression in the dependent lung; (2) bronchiolar collapse (airway closure) in areas of lung with low ventilation; and (3) capillary collapse for a number of reasons including absolute or relative hypovolaemia (Tusman & Böhm, 2010). Administration of 100% oxygen will potentiate atelectasis because of the absence of nitrogen to keep the alveoli open after O2 is absorbed. However, reducing the inspired oxygen % increases the risk of hypoxaemia in patients with acute lung injury; 100% oxygen may be needed for OLV, in the event of cardiovascular collapse, and during colonic surgery.
Lung recruitment manoeuvre
A lung recruitment manoeuvre is a ventilator strategy designed to reverse lung collapse, restore more uniform expansion of the lungs, and improve arterial oxygenation (see Chapter 9). It is a brief and controlled increment in airway pressure to open alveoli and bronchioles and is generally followed by the application of positive end-expiratory pressure (PEEP) to maintain the improvement in oxygenation (Tusman & Böhm, 2010). The optimal PEEP varies with the individual patient and does not always increase PaO2. Natural recruitment manoeuvres in awake subjects include coughing, sighing, sneezing, and postural changes, and they may play a part in quick reversal of lung collapse in healthy patients after anaesthesia. The recommended technique for recruitment in human patients involves increasing PEEP at 5 cmH2O increments every 5 breaths up to 20 cmH2O and then increasing the airway pressure to 40 cmH2O to deliver a larger tidal volume for 10 breaths before a stepwise decrease of PEEP in increments of 2 cmH2O (Tusman & Böhm, 2010). PEEP may have a significant adverse effect on cardiovascular function, usually starting at 10 cmH2O PEEP, that can be recognized by decreases in heart rate (HR) or mean arterial pressure (MAP) or decreased MAP to below 55 mmHg. The procedure is then halted and PEEP decreased. Blood volume is expanded by infusion of crystalloid or colloid solution before the next attempt at recruitment.
A gradual progressive decrease in SpO2 to below 90% in dogs with diseased lungs could have one of several origins, such as progressive lung collapse, decreased cardiovascular function, or developing pneumothorax. Recruitment may be indicated when cardiovascular failure and pneumothorax have been ruled out and lung collapse is suspected. A simple recruitment technique is to increase the peak inspiratory pressure over several breaths to 30–40 cmH2O, holding inspiration for 7–15 seconds. The lung inflation should be performed gently.
One-lung ventilation
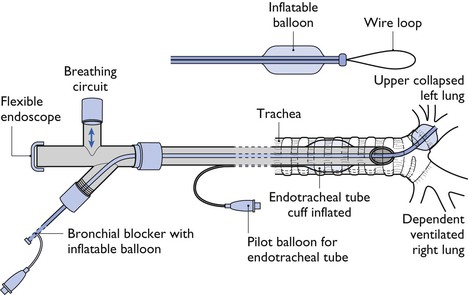
Bilateral lung ventilation (2LV) is most frequently used for thoracic surgery or thoracoscopy in veterinary patients and is satisfactory for most procedures (Leasure et al., 2011). However, OLV may be advantageous in some cases. The main indications for OLV are: (1) to isolate diseased lung to prevent contamination of the healthy lung during extirpation of an abscess or when excessive haemorrhage is anticipated; and (2) to control distribution of ventilation to a selected lung. This situation is desirable when lung collapse would improve surgical exposure, to prevent ventilation of a lung with a major bronchial or alveolar tear, or to improve visibility or surgical access during thoracoscopy. Lung separation can be accomplished by using a bronchial blocker or a double lumen endotracheal tube (Brodsky, 2009). Occlusion of the bronchus to the right cranial lobe may be difficult to achieve (Caccamo et al., 2007).
A bronchial blocker (BB) is a narrow-diameter tube with a cuff or balloon at the end that is inserted into the bronchus of the lung to be collapsed (Fig. 20.1). Inflation of the BB balloon after exhalation allows the lung distal to the inflated balloon to remain collapsed. The BB can be inserted through the lumen of a standard endotracheal tube or the BB may be an integral moving part in the wall of a special endotracheal tube. Several BBs with different designs are manufactured for use in humans (Narayanaswamy et al., 2009). The Arndt endobronchial blocker (Cooke Critical Care) is a balloon-tipped catheter with an inner lumen that contains a flexible wire. The wire exits the end of the catheter as a flexible wire loop that can be guided into a selected bronchus by the tip of a flexible endoscope. Once the blocker is positioned, the wire loop is withdrawn from the catheter and the balloon is inflated. The endoscope is then used to observe the accuracy of balloon inflation. The lumen of the BB can be used to insufflate O2 into the collapsed lung. One problem is that a BB may slide around within the bronchus during surgical manipulation and is at risk of being displaced into the trachea when the patient is moved or during surgery. Some catheters intended for other uses have been used as BB but complications have been seen, such as damage to the bronchial mucosa from a very high pressure created by the balloon (Brodsky, 2009).
A double lumen endotracheal tube (Carlens and Robertshaw/Roberts-Shaw) is designed to allow selective ventilation of the left and right lungs in human patients. The main body of the endotracheal tube has a cuff that resides within the trachea. One lumen extends beyond the tip of the other and can be inserted into one main bronchus and the cuff of that extension can be inflated to isolate the lung. Double lumen endotracheal tubes are available as right or left sided depending on which main bronchus is to be isolated. The tubes can be effectively positioned in large dogs but not so easily in smaller patients. The position of the tube can be checked using a flexible endoscope. One lung can then be ventilated as usual and the other either deflated or insufflated with oxygen to achieve continuous positive airway pressure (CPAP) (see Chapter 9). Problems associated with use of double lumen tubes include difficulty in placement, difficulty in occluding the bronchus to the right cranial lung lobe, and the need to change (ideally) to a single lumen tube for recovery to allow total lung inflation. A recent report described the use of a 37 Fr right double lumen endobronchial tube (Portex Ltd) in a 30 kg Pointer anaesthetized in left lateral recumbency for right middle lung lobectomy (Jiménez Peláez & Jolliffe, 2012). During surgery, the left lung and right cranial lung lobe were ventilated. Intraoperative PaO2 was not reported.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree