Anaesthesia and Analgesia
4.1 Challenges encountered when anaesthetizing rabbits
Anaesthetizing rabbits is perceived by many vets and owners as a high-risk procedure; however, recent advances in anaesthetic drugs and monitoring are challenging that paradigm. It is usually the anaesthetic agent that is blamed for any problems that occur, despite the inherent safety of most of the newer drugs. There are other factors that can affect anaesthetic risk, especially in rabbits. Stress, hypoxia and pre-existing disease are the biggest threats, especially if more than one of these factors is present in the same animal (see Box 4.1). Anaesthetic safety can be improved by considering the risk factors and taking steps to minimize them.
Loud noises, unfamiliar surroundings and the sight, smell or sound of predators is stressful to rabbits awaiting or recovering from surgery. Restraint causes endogenous catecholamine release that can cause cardiac arrhythmias. Pain is especially stressful. It reduces appetite, slows gut motility and can lead to gut stasis and the ultimate development of fatal hepatic lipidosis. Rabbits appear to be particularly susceptible to the effects of pain after surgical intervention, especially after abdominal surgery and incisor removal.
Hypoxia can easily develop in rabbits. Their lung capacity is small (see Figures 8.9 and 11.7). Endotracheal intubation is difficult. The anatomy of the mouth, nasopharynx and trachea does not lend itself to visualization of the mucous membranes, nasopharynx or larynx, so it is more difficult to notice and visualize problems with mucous membrane colour as well as the opening of the glottis (see Figure 1.20). The conformation of the short-nosed breeds, such as the Netherland dwarf, can impair respiratory function and gas exchange in much the same way as occurs in brachycephalic dogs. The tidal volume of rabbits is only 4–6 mL/kg (Gillett, 1994) compared to 10–15 mL/kg in dogs and cats, and the lungs occupy a small volume in comparison with the abdominal contents. Inspiratory and expiratory movement is primarily due to movement of the diaphragm rather than the actions of the intercostal muscles, so the degree of ‘fullness’ of the abdomen has a significant effect on the ease of breathing. Positioning anaesthetized rabbits with the weight of the abdominal viscera against the diaphragm can interfere with respiratory movement, as can conditions causing abdominal effusion or dilation of the gut. Pre-existing lung disease, e.g., from Pasteurella multocida infection, can compromise alveolar gas exchange. In addition to these problems, the response of a lightly anaesthetized rabbit to the smell of an anaesthetic vapour is to hold its breath. Apnoea is associated with bradycardia and hypercapnia. In a study by Flecknell et al. (1996), a period of apnoea lasting between 30 seconds and 2 minutes was the response to both isoflurane and halothane delivered either by mask or in an induction chamber. Some anaesthetic agents (e.g., medetomidine/ketamine) induce a fall in arterial oxygen tension (Hellebrekers et al., 1997) and add to the risk of hypoxia.
Many rabbits undergoing general anaesthesia are not healthy and it is sensible if possible to ensure that any such individual is properly stabilized first (see Box 4.2 and Box 4.3). Dental problems or gastrointestinal disturbances can result in malnourishment and debility. Some rabbits with dental problems salivate profusely, which results in dehydration and electrolyte loss. Obese rabbits have high resting heart rates and can develop hypertension and cardiac hypertrophy (Carroll et al., 1996). Hyperinsulinaemia, hyperglycaemia and elevated serum triglycerides occur in obese rabbits and hepatic lipidosis develops readily after short periods without food, especially if the rabbit is stressed. Obese rabbits are poor surgical candidates.
Even apparently healthy rabbits can be suffering from latent infections such as pasteurellosis or encephalitozoonosis. Congenital heart defects, such as ventricular septal defects, occur and cardiomyopathy is sometimes present, especially in giant breeds such as the French lop (see Figure 11.10). Repeated anaesthesia with ketamine/xylazine infusion has been linked with heart disease and death. Marini et al. (1999) postulated that repeated episodes of hypoxaemia lead to cell death and necrosis, resulting in myocardial fibrosis. The rabbit has limited collateral myocardial circulation and is therefore predisposed to ischaemia by vasoconstriction.
4.2 Reducing anaesthetic risk in rabbits
Clinical examination prior to anaesthesia gives an idea of the general health status of the rabbit. Baseline data specific to the individual should be collected at this time: for instance, an accurate weight, heart rate, respiratory rate, assessment of gut motility and an assessment of hydration. Clinical examination can only provide some of the information required, particularly as rabbits tend to hide any health problems, and a pre-anaesthetic blood profile should be considered to complement the examination. The information that the clinician needs to gather prior to planning a suitable anaesthetic protocol includes:
• Is the heart working effectively?
• Does the blood have sufficient oxygen-carrying capacity?
• Is the kidney working effectively?
• Is the liver working effectively? and
These areas all have a major impact on anaesthetic safety and will dictate the drugs and protocols used. Some debilitated patients need nutritional support prior to anaesthesia. Dehydrated, shocked or hypotensive patients require intravenous or intraosseous fluid therapy. Rabbits cannot vomit, so pre-anaesthetic fasting is not required, although a short starvation period of 1–2 h is required to ensure the mouth is empty and the stomach is not overfull. Except in emergency cases, a short period of stabilization is frequently the difference between a successful and unsuccessful anaesthetic intervention.
An accurate weight is required to calculate the dosages of drugs to be used. The amount of digesta in the gastrointestinal tract fluctuates throughout the day and influences bodyweight. Rabbits can have large amounts of food in their digestive tract, especially in the caecum. Fasting reduces bodyweight but does not necessarily reduce the amount of medication required. Fat animals require lower dose rates than thin ones. Because of these considerations, some authors advocate calculating dosages on metabolic body size, i.e., Wkg0.75 (Aeschbacher, 1995).
Stress levels can be reduced by the provision of a quiet, secluded kennel both before and after anaesthesia. Long periods without food and water, as well as being confined in a carrier, can be stressful. If facilities are available, a cage with familiar bedding (i.e., hay) is beneficial postoperatively and may be necessary preoperatively if there is a long delay between admission and surgery. Ideally, rabbits awaiting or recovering from anaesthesia should be kept away from predators such as dogs, cats, ferrets or raptors. Quiet, gentle handling reduces stress levels in rabbits. A tight grip around the chest or throat can compromise respiratory function.
Good anaesthetic equipment, the correct range of drugs and an observant anaesthetist improve anaesthetic safety. Although anaesthetic monitoring equipment is advantageous, it cannot replace a human being who is closely observing the patient. During anaesthesia, the risk of hypoxia is reduced by positioning the patient so the airway is unimpeded and the weight of the abdominal viscera is not against the diaphragm. Extending the neck and pulling the tongue out of the mouth not only allows the anaesthetist to observe the colour of the mucous membranes but also moves the base of the tongue away from the epiglottis and opens the airway. Careful observation by the anaesthetist ensures an unimpeded airway during surgical procedures. If possible, an endotracheal tube should be placed. Respiratory effort is largely diaphragmatic and observation of shallow respiratory movements can be difficult. Drapes and surgeons can obscure the anaesthetist’s view of the patient. If respiration monitors are not available, it can be difficult for the anaesthetist to be certain that the animal is breathing and cooperation between the surgeon and anaesthetist is required. Clear plastic drapes facilitate observation of respiratory movements during surgery.
Anaesthetized rabbits require the administration of oxygen throughout the anaesthetic period. A period of preoxygenation before masking down decreases the risk of hypoxia should breath holding occur. Oxygen can be delivered through a facemask, endotracheal tube, nasal tube or even a tube placed in the pharynx through the mouth.
As in other species, a balanced anaesthetic is required to permit surgery but can be difficult to achieve in rabbits. A light plane of anaesthesia not only causes breath holding in response to the volatile agent but also results in movement or even ‘screaming’ in response to surgical stimuli. Screaming is an alarm response of rabbits to unpleasant stimuli and can occur during light anaesthesia. The patient maintains expiration for an alarming length of time and can become hypoxic or even cyanosed. The actual scream may not be audible, especially if the rabbit is intubated. The anaesthetist’s reaction to the prolonged period of respiratory arrest is often to turn down the concentration of anaesthetic agent. The result is a lightening of anaesthesia and increased response to surgical stimuli. An increased concentration of the volatile agent is then required to deepen anaesthesia. If the rabbit is not intubated, the smell of the anaesthetic agent stimulates breath holding and failure to inhale the anaesthetic vapour and surgical anaesthesia is then difficult to achieve. The administration of additional quantities of injectable agents is possible, but undesirable because of the length of time for it to take effect and uncertainty about the required dose. This type of unsatisfactory anaesthetic can be overcome by using an injectable induction agent and maintaining anaesthesia with a volatile agent. The slow introduction of volatile gases prevents breath holding if a facemask is used. Some individuals appear to dislike the feeling of a facemask even when sedated and will stop breathing in response to this. If anaesthesia is induced with a facemask, an effective premedicant is required. Endotracheal intubation overcomes the problem of breath holding. It also permits the continuous administration of oxygen and gives greater control of the depth of anaesthesia. Endotracheal intubation allows intermittent positive pressure ventilation if it is needed.
Good postoperative care and routine analgesia are important to reduce pain and stress, restore appetite and prevent the development of hepatic lipidosis.
4.3 Anaesthetic equipment
Specialized but simple anaesthetic equipment is required for rabbit anaesthesia. Clear facemasks permit observation of the colour of the nose and mucous membranes. A range of small, uncuffed endotracheal tubes (1.5–5.0 mm) are required for endotracheal intubation. Soft 3–4 F nasogastric catheters can be used for nasal intubation. Rabbits have a small tidal volume (4–6 mL/kg) and anaesthetic circuits with low dead space are required. Paediatric connectors can be used to make up a Bain circuit or T-piece (Portex, Arnolds). Although a gag is not necessary for intubation, purpose-made rodent gag and cheek dilators are useful to visualize the oral cavity and pharynx. A laryngoscope with a narrow, long blade (0 or 1, a Wisconsin paediatric blade) can be used to visualize the larynx and aid intubation. Purpose-made rabbit laryngoscopes are now available.
Pulse oximetry can be used as an adjunct to anaesthetic monitoring. The tongue is the best site for the sensor but is not always accessible, e.g., if the rabbit is undergoing dentistry or anaesthesia is maintained with a facemask. A satisfactory signal is sometimes found in either the pinna or the base of the tail if the hair is clipped off and a suitable sensor is available. A rectal probe may also be used. Poor peripheral perfusion in rabbits anaesthetized with medetomidine or ketamine may prevent a satisfactory signal. Respiration monitors can be used in larger rabbits but can prove unreliable in small breeds. Electrocardiography can be used for cardiac monitoring. Newer monitoring systems may combine both pulse oximetry and electrocardiography in the same unit. In recent years capnography (a measurement of end tidal pCO2) has become more commonly used in veterinary medicine and because the veterinary units are designed to deal with species that have small tidal volumes, it is very useful for rabbit anaesthetic monitoring. Capnography provides information on arterial CO2 concentration as well as lung perfusion (Stanford, 2004; Swenson et al., 2008).
Rectal temperature is monitored with a standard thermometer or a digital thermometer with a remote sensor. Many monitoring units include a digital thermometer as part of their information gathering. A maximum/minimum digital thermometer with a remote sensor designed for measuring household indoor and outdoor environmental temperatures can be used. The sensor is lubricated and carefully inserted into the rectum, permitting remote monitoring of body temperature throughout the anaesthetic period.
4.4 Drugs, analgesics and anaesthetic agents used in rabbit anaesthesia
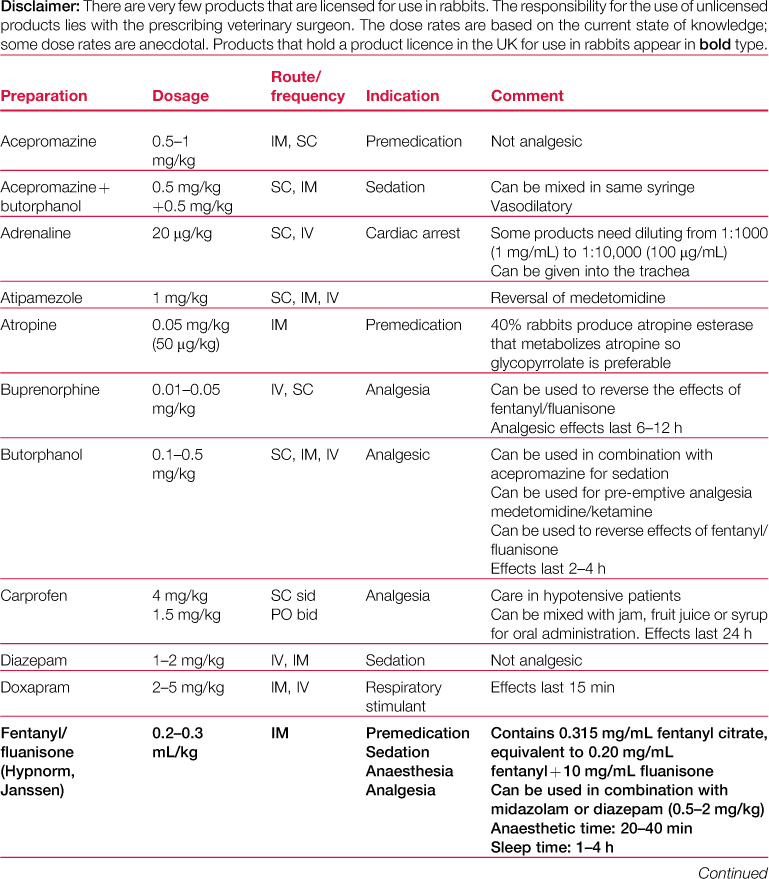
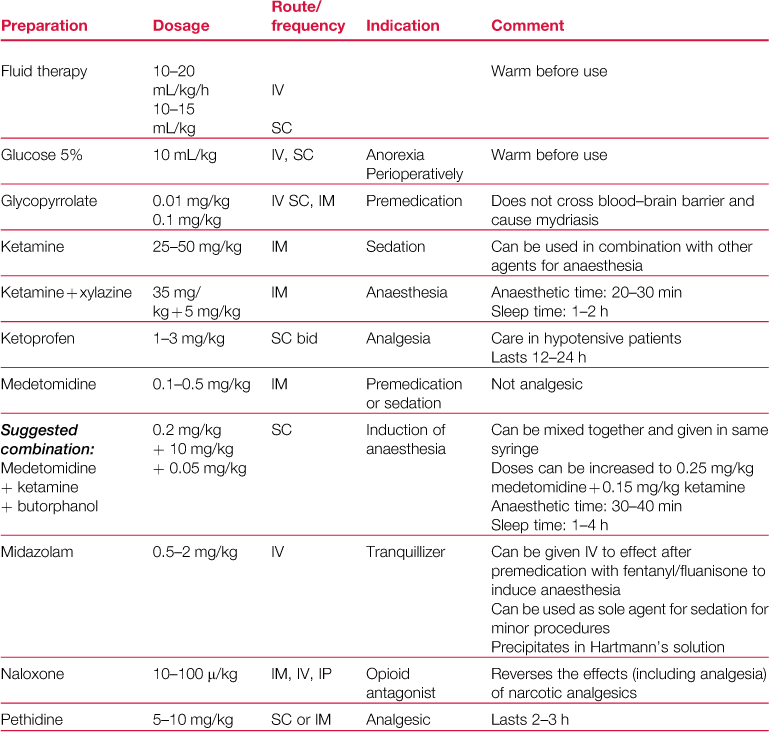
A formulary of drugs used during rabbit anaesthesia is given in Table 4.1.
4.4.1 Controlled drugs
Many of the narcotic analgesics used in rabbits are drugs capable of being abused by humans and are classed as controlled drugs that are scheduled under UK law. Schedule 1 includes drugs such as cannabis and LSD that are not used therapeutically in either veterinary or human medicine. Schedule 2 drugs include fentanyl, pethidine and morphine and require a written requisition signed by a veterinary surgeon to obtain the preparation from the wholesalers. A register recording the purchase and supply of these drugs must be maintained, and they must be kept in a locked, immovable cabinet. Schedule 3 drugs include buprenorphine and barbiturates and also require a written requisition but transactions do not need to be recorded. Buprenorphine needs to be kept in a locked cupboard. Some benzodiazepines, such as temazepam or diazepam, require a written requisition. It is advisable to keep all such preparations, including butorphanol and ketamine, which are not included on the controlled drugs list, in a locked cupboard.
4.4.2 Atropine and glycopyrrolate
Atropine and glycopyrrolate are anticholinergic agents used to reduce bronchial and salivary secretions and protect the heart from vagal inhibition. In rabbits, anticholinergics are not routinely used for premedication, although they can be used to counteract the cardiovascular effects of xylazine seen during anaesthesia with a combination of xylazine and ketamine (in this instance, atropine should be administered a few minutes prior to the xylazine to avoid prolonged hypertension caused by a combination of peripheral vasoconstriction and an elevated heart rate). About 40% of rabbits produce atropine esterase, which rapidly breaks down atropine, so if an anticholinergic drug is required, glycopyrrolate is preferable. A potentially undesirable effect of anticholinergic agents is the reduction of gastrointestinal motility.
4.4.3 ‘EMLA’ cream
EMLA cream (AstraZeneca) is a topical preparation containing 2.5% lidocaine and 2.5% prilocaine that is applied to the skin to provide local anaesthesia. It is supplied with an occlusive dressing to place over the cream while the local anaesthetic takes effect. The cream can be applied to the marginal ear vein to provide local anaesthesia of full-thickness skin and prevents the rabbit shaking its head and dislodging the needle in response to venepuncture (Flecknell, 1998a). EMLA cream takes 45–60 min to become effective.
4.4.4 Phenothiazine derivatives: acepromazine
Acepromazine is a phenothiazine derivative that has a depressant action on the central nervous system. It is a dopamine inhibitory, α-adrenergic blocking agent with weak antimuscarinic activity (Bishop, 1998). Acepromazine is a sedative that potentiates the effects of other anaesthetic agents and facilitates a smooth recovery. It is used routinely as a premedicant in dogs and other species. Acepromazine is hypotensive and does not have analgesic properties. In rabbits, acepromazine can be used for premedication prior to induction with volatile agents. It can also be combined with butorphanol to provide sedation.
4.4.5 Benzodiazepines: diazepam and midazolam
Diazepam and midazolam are effective sedatives in rabbits. They produce good muscle relaxation and potentiate the effect of anaesthetics and narcotic analgesics. Cardiovascular and autonomic side effects are negligible (Green, 1975). Midazolam and diazepam have a similar spectrum of effects except that midazolam is more potent (Longley, 2008) and has a shorter duration of action (Flecknell, 1984). Diazepam is poorly soluble in water and is therefore prepared in an oily solvent (Valium, Roche) that is locally irritant and can cause tissue damage and skin sloughing if administered perivascularly. The inflammation caused after intramuscular injection can also affect the drug uptake, making dosing less reliable. A water-soluble diazepam preparation that requires dilution before use is available (Diazemuls, Actavis). Midazolam is water soluble (Hypnovel, Roche) and does not cause a tissue reaction if it is administered perivascularly. Intramuscular or intravenous midazolam has been recommended as a routine short-acting sedative for diagnostic procedures (Ramer et al., 1999). It is absorbed across mucous membranes and can be given intranasally if required.
Both diazepam (licensed use, drug data sheet for Hypnorm) and midazolam (suggested but not licensed on the Hypnorm data sheet) can be given after premedication with fentanyl/fluanisone (Hypnorm, Janssen) to induce anaesthesia (see Box 4.4).
4.4.6 α2-Adrenergic agonists
4.4.6.1 Xylazine
Xylazine (Rompun, Bayer) produces moderate sedation and minimal analgesia in rabbits. It is seldom used as a sole agent but is given in combination with ketamine. The combination causes cardiovascular and respiratory depression, and cardiac arrhythmias are produced at high doses. Xylazine and ketamine have been associated with a high mortality rate (Flecknell et al., 1983). Atipamezole, an α-adrenergic blocking drug used to reverse the effects of medetomidine, can be used to reverse the effects of xylazine. Xylazine is no longer commonly used to sedate rabbits in the UK, as newer, safer, products are readily available.
4.4.6.2 Medetomidine
Medetomidine (Domitor, Pfizer) is a more specific α2-agonist than xylazine and has a lower incidence of side effects. It is relatively expensive and rabbits require comparatively larger doses than other species. Medetomidine can be used on its own as a premedicant or it can be combined with ketamine to provide surgical anaesthesia. Medetomidine causes peripheral vasoconstriction, which gives mucous membranes a slight mauve appearance that may be mistaken for cyanosis. The vasoconstriction is not dangerous but the poor colour of the mucous membranes could mask a true cyanosis should it occur. Hypoxia occurs during anaesthesia with medetomidine and oxygen should be administered throughout the anaesthetic period (Flecknell, 2000). Vasoconstriction can prevent satisfactory pulse oximetry and venepuncture for blood collection or intravenous fluid therapy. Medetomidine can cause hypothermia and diuresis.
Medetomidine has some advantages. It can be given by subcutaneous rather than intramuscular injection. It provides good laryngeal relaxation for endotracheal intubation. It is not a respiratory depressant and recovery is usually complete within 3 h. Recovery can be hastened by reversal with atipamezole. The use of medetomidine in combination with ketamine is described in Box 4.5.