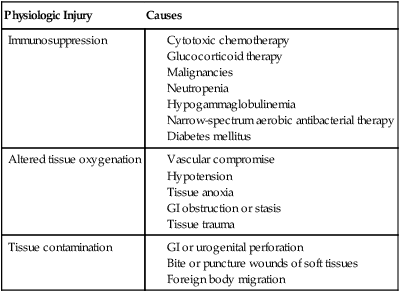
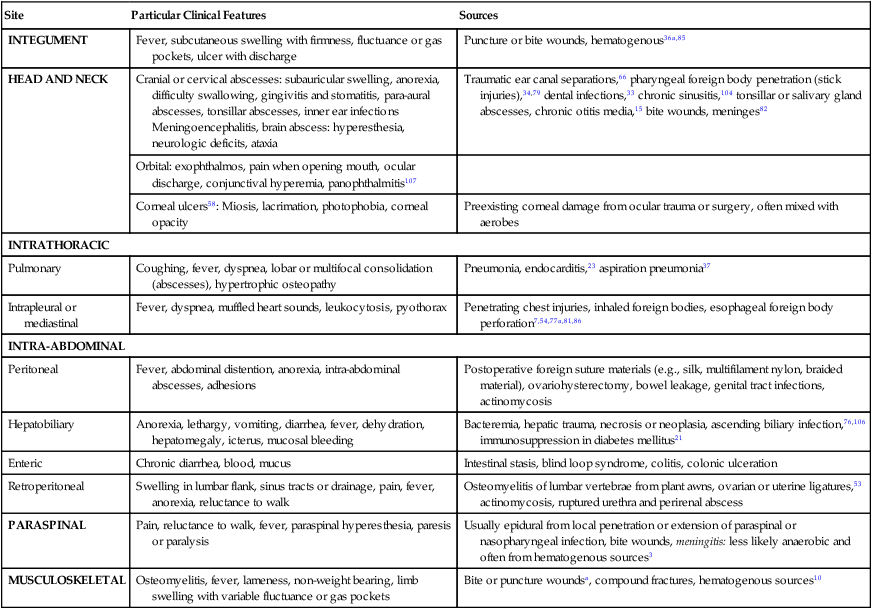
Obligate anaerobes can be gram-positive or gram-negative rods or cocci (Table 39-1). They cannot grow in the presence of molecular oxygen because they do not make superoxide dismutase, and most do not produce catalase. These enzymes are necessary for the breakdown of reactive oxygen intermediates (e.g., superoxide anion, hydrogen peroxide) that are normally generated when bacteria grow in the presence of oxygen. Only species of the genus Clostridium form endospores anaerobically, distinguishing them from the genus Bacillus, which forms spores under aerobic conditions. The most commonly encountered anaerobes are listed in Web Table 39-1. TABLE 39-1 Characteristics of Obligate Anaerobic Bacteria Most Commonly Isolated from Dogs and Cats WEB TABLE 39-1 Species of Obligate Anaerobic Bacteria Isolated from Dogs and Cats aB. fragilis was most commonly isolated in dogs and cats. bF. nucleatum was most commonly isolated in dogs and cats (F. canifelium not identified). cPorphyromonas spp. were most commonly isolated in dogs, and P. canoris was most commonly isolated in cats. dC. perfringens was most commonly isolated in dogs and cats. eP. heparinolyticus was most commonly isolated in dogs and P. bivia most commonly isolated in cats. Data compiled from cases from William R. Pritchard, Veterinary Medical Teaching Hospital, University of California, Davis, CA, 1999–2008. Anaerobes make up a significant portion of the normal bacterial flora of dogs and cats.16,63 The predominant microorganisms living on most mucosal surfaces are anaerobes, and metabolic by-products produced by this group are important in the regulation of the numbers of aerobic species (facultative and obligate) that also make up the normal flora. Thus, anaerobic bacteria play an important role in protection of mucosal surfaces from interactions involving other microorganisms with pathogenic potential. For example, approximately 1 million salmonellae are needed to produce disease in animals with intact anaerobic flora, whereas fewer than 10 salmonellae are needed to produce disease in animals with few anaerobes. The importance of this observation is seen clinically; dogs are more prone to develop salmonellosis when they are medicated with antibacterials, especially those effective in reducing the anaerobic component of the normal flora (e.g., ampicillin).102 Aside from being responsible for a significant portion of innate immunity (the normal flora), anaerobes are also opportunistic pathogens causing clinical illness.39,44,49,60,61 Compromise of a mucosal surface or an adjacent area by perforation or other trauma may provide a portal of entry for the normal anaerobic flora into a normally sterile site. At this point, these anaerobes in addition to other members of the normal flora become clinically relevant. These are important considerations because, to be successful in preventing anaerobic infection, antibacterial therapy should always be started before results of microbiologic analysis of exudative material are received. Knowledge of the normal flora at a specific site helps in making an educated guess about the microorganisms likely to be causing the infection. If anaerobic bacteria are involved in an infectious process, an average of two different species are almost always admixed with aerobic bacteria. The most commonly associated aerobic bacteria are enterics (mainly Escherichia coli), members of the genus Pasteurella, and coagulase-positive staphylococci (Web Table 39-2; see Bite Wound Infections, Chapter 51). WEB TABLE 39-2 Most Commonly Isolated Aerobic Microorganisms Associated with Anaerobic Infections in Dogs and Cats aMost common isolate was E. coli, accounting for 87% of the enteric isolates from dogs and 89% of the enteric isolates from cats. bMost common isolate was P. canis, accounting for 58% of the isolates from dogs, and P. multocida ss multocida accounting for 74% of the isolates from cats. cMost common isolate was P. aeruginosa accounting for 96% of the isolates from dogs. dMost common isolate was S. intermedius group (S. intermedius, S. pseudintermedius) accounting for 77% from dogs, and S. intermedius group accounting for 56% of the isolates from cats. eMost common isolate was A. canis from dogs and Actinomyces spp. from cats. fMost common isolate was E. faecalis, accounting for 50% from dogs and 57% from cats. gMost common isolate was S. canis, accounting for 85% from dogs and 82% from cats. Data compiled from cases from William R. Pritchard Veterinary Medical Teaching Hospital, University of California, Davis, CA, 1999–2008. If they contain the appropriate virulence factors, anaerobic bacteria may also produce mucosal infections. In addition to periodontal disease, anaerobes are associated with disease in other regions of the gastrointestinal tract (see Chapter 88); the most important of these anaerobes within the intestine are Clostridium difficile and Clostridium perfringens (see Chapter 37).96,100 Both of these species may be a part of the normal flora of dogs and cats, although evidence suggests that both may be passed to previously uncolonized animals.57,77,77 Infectious processes involving a normally sterile site are usually a consequence of “contamination” by members of the resident microflora. The composition of the normal flora of the contiguous surface usually mirrors what is found in the infectious process. In human patients, infectious processes involving structures above the diaphragm are more likely to contain anaerobes originating in the mouth, whereas disease below the diaphragm usually involves anaerobes from the intestinal tract.97 In animal patients, the various species of anaerobes do not seem to have different predilections for a particular site or location.51 Although disease produced by C. difficile is the result of its proliferation in the enteric tract of the host after a trigger event (e.g., certain antibacterial and chemotherapeutic agents), epidemiologic evidence from human hospitals suggests that the agent can be spread from patient to patient, resulting in outbreaks of C. difficile–associated disease (see Chapter 37).89 Such observations imply that the organism either is or has the potential to be contagious. However, determining whether C. difficile is truly a contagious pathogen is difficult because it is also found in the enteric tract of clinically healthy animals.83,96 It is unclear whether toxigenic C. perfringens is contagious. It likely causes disease by proliferating in the intestinal tract and producing enterotoxin. The trigger that stimulates the proliferation is unknown. It must also be determined whether the source of the agent is endogenous, acquired from another infected animal, or acquired from the environment. The report of outbreaks of C. perfringens–associated diarrhea in small-animal hospital settings suggests that it is contagious, although it is important to remember that some C. perfringens are present in the enteric tract of clinically healthy dogs.57,100 Detectable levels of C. perfringens enterotoxin are not often found in the feces of clinically healthy animals (see Chapter 37).100 The genus Fusobacterium includes numerous species, one of which—Fusobacterium nucleatum—has five subspecies.11 Fusobacterium canifelium is common in the oral cavity of dogs and cats and has been isolated from dog and cat bite wounds in people. Unfortunately, this organism is typically resistant to quinolones, unlike the human isolates. Obligate anaerobic bacteria cannot live in healthy tissue. On certain mucosal surfaces (e.g., intestinal tract, gingival crevices, genital tract), they live with other microorganisms (facultative anaerobes) that scavenge molecular oxygen, resulting in a local environment with very low redox potential (Eh, a measure of oxygen tension). Likewise, in compromised tissue, inflammatory cells and co-inoculated aerobic microorganisms lower the Eh sufficiently for anaerobes to grow. Numerous factors that predispose the body to anaerobic infections are listed in Table 39-2. TABLE 39-2 Factors Contributing to Development of Anaerobic Infections GI, Gastrointestinal. Components of anaerobic bacteria have been shown to elicit potent inflammatory responses. Gram-negative anaerobes possess lipopolysaccharides with endotoxic activity, as do their aerobic counterparts. The peptidoglycan of gram-positive anaerobes incites the same inflammatory response as gram-positive aerobic microorganisms. Some anaerobic bacteria produce capsules (e.g., Bacteroides fragilis, pigmented Prevotella spp. and Porphyromonas spp.), which incite inflammatory responses that result in abscess formation; in other words, capsules without viable bacteria can also induce abscess formation.101 Some evidence suggests that co-inoculated aerobic microorganisms induce anaerobe capsule formation. Capsules also play a more traditional role by inhibiting phagocytosis.9,42 Aerobic and anaerobic microorganisms can have a synergistic relationship.9 Anaerobes act with facultative aerobes such as E. coli to induce abscess formation more rapidly than either bacterial group alone. Actinomyces, a genus of facultative anaerobes, is commonly found in association with anaerobic infections from foreign body migrations and in association with pyothorax or peritonitis (see Chapter 47). The facultative bacteria lower the oxidation-reduction potential of the tissue environment, facilitating anaerobic proliferation. The necrotic and abscessed tissue may protect the facultative organisms from antimicrobials and body defenses such as phagocytosis. In addition to helping trigger capsule formation, aerobic organisms scavenge oxygen and curtail phagocytosis of the anaerobic component of the sample (and vice versa).103 Enzymes such as β-lactamase produced by one member of the aerobic-anaerobic partnership protect susceptible microorganisms in the vicinity from being killed by β-lactam antibacterials. These synergistic interactions are important to keep in mind when designing therapeutic regimens, because antibacterial therapy should be directed at both populations for optimal resolution of the infection. Some anaerobic bacteria produce toxins. Fusobacterium necrophorum produces a toxin that forms pores in leukocyte membranes.42 C. difficile produces toxins (A and B) that disrupt the cytoskeletal elements of the intestinal epithelial cell, resulting in cell death.1,87 C. perfringens produces an enterotoxin that interacts with the target cell membrane, resulting in the formation of pores that are made partly by the enterotoxin and partly by the target cell.87 Electrolytes are lost through these pores, resulting in reversal of ion and water flow (diarrhea) and, finally, death of the cell. Some species of anaerobes produce adhesin molecules (pili or fimbriae).42 These proteins are usually associated with more virulent anaerobes. However, adhesins probably do not play a major role in pathogenesis other than association with specific sites or locations on mucosal surfaces. Adhesin molecules probably are not expressed and do not have an important role when exposed to phagocytic cells to which they might adhere—an event that leads to phagocytosis and the demise of the anaerobe. In keeping with this model, it has been shown that blood or abscess isolates of B. fragilis are rarely piliated, whereas those found on the mucosal surface almost always have pili.9 Conditions involving anaerobic bacteria infecting a normally sterile site vary according to location, but all involve the formation of a pyonecrotic process. This process stems primarily from the inflammatory response triggered by anaerobic capsule material, cell wall constituents of the anaerobic and aerobic microorganisms that may be present, and thwarted attempts at phagocytosis, leading to deposition of lysosomal contents into surrounding tissue. The most commonly encountered sites of anaerobic isolation are listed in Web Table 39-3. The clinical findings of such infections are listed in Box 39-1 and Table 39-3. Exudates from infectious processes that contain anaerobes are often malodorous and may be darkly discolored. TABLE 39-3 Comparative Clinical Features of Anaerobic Infections WEB TABLE 39-3
Anaerobic Infections
Etiology
Microorganism
Gram Reaction
Shape
Bacteroides spp.
Negative (pale)
Rod
Prevotella spp.
Negative (pale)
Rod (coccobacillus)
Porphyromonas spp.
Negative (pale)
Rod (coccobacillus)
Fusobacterium spp.
Negative (pale)
Rod (usually thin)
Peptostreptococcus anaerobius, anaerobic gram-positive cocci
Positive
Coccus
Clostridium spp.
Positive
Rod (spores)
Eubacterium spp.
Positive
Rod
Actinomyces spp.
Positive
Rod
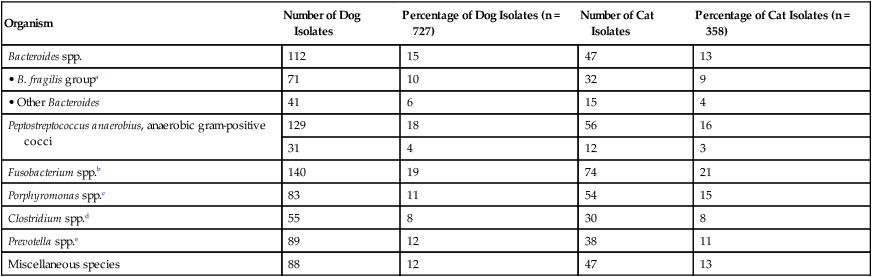
Organism
Number of Dog Isolates
Percentage of Dog Isolates (n = 727)
Number of Cat Isolates
Percentage of Cat Isolates (n = 358)
Bacteroides spp.
112
15
47
13
• B. fragilis groupa
71
10
32
9
• Other Bacteroides
41
6
15
4
Peptostreptococcus anaerobius, anaerobic gram-positive cocci
129
18
56
16
31
4
12
3
Fusobacterium spp.b
140
19
74
21
Porphyromonas spp.c
83
11
54
15
Clostridium spp.d
55
8
30
8
Prevotella spp.e
89
12
38
11
Miscellaneous species
88
12
47
13
Microorganism
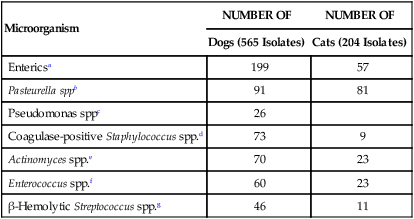
NUMBER OF
NUMBER OF
Dogs (565 Isolates)
Cats (204 Isolates)
Entericsa
199
57
Pasteurella sppb
91
81
Pseudomonas sppc
26
Coagulase-positive Staphylococcus spp.d
73
9
Actinomyces spp.e
70
23
Enterococcus spp.f
60
23
β-Hemolytic Streptococcus spp.g
46
11
Epidemiology
Pathogenesis
Physiologic Injury
Causes
Immunosuppression
Altered tissue oxygenation
Tissue contamination
Clinical Findings
Site
Particular Clinical Features
Sources
INTEGUMENT
Fever, subcutaneous swelling with firmness, fluctuance or gas pockets, ulcer with discharge
Puncture or bite wounds, hematogenous36a,85
HEAD AND NECK
Cranial or cervical abscesses: subauricular swelling, anorexia, difficulty swallowing, gingivitis and stomatitis, para-aural abscesses, tonsillar abscesses, inner ear infections
Meningoencephalitis, brain abscess: hyperesthesia, neurologic deficits, ataxia
Traumatic ear canal separations,66 pharyngeal foreign body penetration (stick injuries),34,79 dental infections,33 chronic sinusitis,104 tonsillar or salivary gland abscesses, chronic otitis media,15 bite wounds, meninges82
Orbital: exophthalmos, pain when opening mouth, ocular discharge, conjunctival hyperemia, panophthalmitis107
Corneal ulcers58: Miosis, lacrimation, photophobia, corneal opacity
Preexisting corneal damage from ocular trauma or surgery, often mixed with aerobes
INTRATHORACIC
Pulmonary
Coughing, fever, dyspnea, lobar or multifocal consolidation (abscesses), hypertrophic osteopathy
Pneumonia, endocarditis,23 aspiration pneumonia37
Intrapleural or mediastinal
Fever, dyspnea, muffled heart sounds, leukocytosis, pyothorax
Penetrating chest injuries, inhaled foreign bodies, esophageal foreign body perforation7,54,77a,81,86
INTRA-ABDOMINAL
Peritoneal
Fever, abdominal distention, anorexia, intra-abdominal abscesses, adhesions
Postoperative foreign suture materials (e.g., silk, multifilament nylon, braided material), ovariohysterectomy, bowel leakage, genital tract infections, actinomycosis
Hepatobiliary
Anorexia, lethargy, vomiting, diarrhea, fever, dehydration, hepatomegaly, icterus, mucosal bleeding
Bacteremia, hepatic trauma, necrosis or neoplasia, ascending biliary infection,76,106 immunosuppression in diabetes mellitus21
Enteric
Chronic diarrhea, blood, mucus
Intestinal stasis, blind loop syndrome, colitis, colonic ulceration
Retroperitoneal
Swelling in lumbar flank, sinus tracts or drainage, pain, fever, anorexia, reluctance to walk
Osteomyelitis of lumbar vertebrae from plant awns, ovarian or uterine ligatures,53 actinomycosis, ruptured urethra and perirenal abscess
PARASPINAL
Pain, reluctance to walk, fever, paraspinal hyperesthesia, paresis or paralysis
Usually epidural from local penetration or extension of paraspinal or nasopharyngeal infection, bite wounds, meningitis: less likely anaerobic and often from hematogenous sources3
MUSCULOSKELETAL
Osteomyelitis, fever, lameness, non-weight bearing, limb swelling with variable fluctuance or gas pockets
Bite or puncture woundsa, compound fractures, hematogenous sources10
Specimen Source
Species (Total No.)
Ranking
Number with Positive Culture Results (%)
Number from Which Anaerobes Were Isolated (%)
Draining tract
Dog (114)
2
85 (75)
16 (14)
Cat (22)
3
9 (41)
4 (18)
Wound
Dog (269)
2
131 (49)
37 (14)
Cat (31)
1
14 (45)
9 (29)
Abscess
Dog (244)
1
93 (38)
62 (25)
Cat (72)
1
27 (38)
21 (29)
Bone
Dog (278)
6
77 (28)
8 (3)
Cat (17)
6
9 (53)
2 (12)
Respiratory tract
Dog (1213)
5
321 (26)
103 (8)
Cat (476)
4
121 (25)
68 (14)
Subcutaneous mass/tissue
Dog (431)
4
107 (25)
50 (12)
Cat (95)
2
25 (26)
19 (20)
Abdominal fluid
Dog (355)
3
79 (22)
45 (13)
Cat (144)
5
22 (15)
18 (13)
Pleural fluid
Dog (194)
5
33 (17)
22 (8)
Cat (159)
7
30 (19)
14 (9) ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anaerobic Infections
