Chapter 1
An introduction to anaesthesia and general considerations
The ‘classic’ signs of anaesthesia
Computer control in anaesthesia
Minimum Alveolar Concentration (MAC) and Minimum Infusion Rate (MIR)
General considerations in the selection of the anaesthetic method
Significance of conditions found by preanaesthetic examination
Cardiovascular and respiratory disease
Drug metabolism and disease states
Factors affecting transport of drugs in the body
Influence of pre-existing drug therapy
Introduction
Anaesthesia is one of the greatest ‘discoveries’ there has ever been – there are few scientific advances which have reduced pain and suffering in so many people and animals. It is difficult to remember that the first anaesthetic was administered only in the 1840s (there is argument as to who was first to administer it clinically), although analgesics (for example opiates) have been available for many centuries. The term anaesthesia was coined by Oliver Wendell Holmes in 1846 to describe using ether to produce insensibility in a single word and it comes from the Greek ‘without feeling’. The term ‘analgesia’ is Greek for ‘without pain’.
While anaesthesia has precisely the same meaning as when it was first coined, i.e. the state in which an animal is insensible to pain resulting from the trauma of surgery, it is now used much more widely. Starting with the premise that ‘pain is the conscious perception of a noxious stimulus’, two conditions may be envisaged: general anaesthesia where the animal is unconscious and apparently unaware of its surroundings, and analgesia or local anaesthesia where the animal, although seemingly aware of its surroundings, shows diminished or no perception of pain. Perioperative analgesia, a subject once much neglected by veterinarians, is now recognized as an essential component of the process, and the physiology of pain and mechanisms of how it can be controlled and treated are discussed in Chapter 5, Analgesia.
Veterinary anaesthesia
The clinical discipline of veterinary anaesthesia is essentially a practical subject based on science. In addition to the scientific base for human anaesthesia, the veterinarian has to contend with species differences, particularly in anatomy and in metabolism that effects the actions and elimination of drugs.
In clinical veterinary anaesthesia, the major requirements of the anaesthetist are:
▪This includes prevention of awareness of pain, relief of anxiety and sympathetic animal handling
▪This includes adequate immobility and relaxation
▪Ensuring neither the animal, nor the personnel are injured in any way.
All patients require an adequate standard of monitoring (Chapter 2), and of general care throughout the anaesthetic process. However, other than this, there is a myriad of acceptable methods from which the anaesthetist can choose to satisfy the above aims. Certain drugs and/or systems may be put forward as ‘best practice’ but, in veterinary anaesthesia, there is rarely the ‘evidence base’ to prove that they are so. Choice of methods used may be limited by a number of factors. Legal requirements, which will depend on the country concerned, need to be observed. Examples include laws involving the control of dangerous drugs, or the choice of drugs in animals destined for human consumption. In the European Union, currently, the ‘cascade’ is a major barrier to anaesthetists’ choice. This law implies that if there is a drug licensed for a species, it is criminal to use another unlicensed agent for the same purpose, unless the veterinary surgeon can prove that their alternative choice was justified for welfare of the specific individual animal concerned. Facilities may be limited, and while expense should not be the governing factor, it does need to be considered; animals throughout the world require anaesthesia and if the owners cannot afford the cost, the animal will be denied treatment. The objective of this book is to give the reader the information enabling them to make an informed choice of the best method of anaesthesia and care for their patient in their circumstances.
General anaesthesia
General anaesthesia is and has been given many different definitions (reviewed by Urban & Bleckwenn, 2002), but a simple practical one that has been used in the previous editions of this book is ‘the reversible controlled drug induced intoxication of the central nervous system (CNS) in which the patient neither perceives nor recalls noxious or painful stimuli’. Professors Rees and Grey (1950) introduced the concept that the requirements from general anaesthesia were analgesia, muscle relaxation and ‘narcosis’, these being known as the ‘Liverpool Triad’. This idea has been expanded to add suppression of reflexes (motor and autonomic) and unconsciousness or at least amnesia and, most importantly, that these requirements should be achieved without causing harm to the patient. For over 100 years anaesthesia was achieved mainly with a single drug, most commonly ether. Now, as well as a number of anaesthetic drugs which are administered by inhalation as is ether (see Chapter 7), single-agent drugs that are given by injection (see Chapter 6) are also employed. However, in clinical practice, it is now usual to use many different agents, which act at multiple receptors, in the CNS and peripherally, in order to achieve the goals required to provide good anaesthesia.
Currently, when considering single-agent anaesthetics, many authorities now believe that the state of general anaesthesia requires only two features: immobility in response to noxious stimulation and amnesia (the latter often taken as unconsciousness) (Eger et al., 1997; Urban & Bleckwenn, 2002). The argument is that the immobility is required for surgery; if the patient is unconscious they cannot perceive pain (although the autonomic system may still react to noxious stimuli), and if they don’t remember the pain, it is similar to lack of perception. This theory ignores evidence and theories relating to pain and hypersensitization as described in Chapter 5. However, it considers that analgesia is desirable but is not an essential feature of the state of ‘general anaesthesia’. Thus the ‘definition’ of a single-agent anaesthetic drug, such as the injectable agent, propofol or the volatile anaesthetic agents is that they have these two actions of preventing movement and causing amnesia (Franks, 2006). Some compounds which from structure and lipophility might be expected to be anaesthetics can cause amnesia without immobility; these are sometimes termed ‘non-anaesthetics’ (Johansson & Zou, 2001), their major interest being related to studies of mechanism of anaesthetic action.
Mechanisms of action of general anaesthetic agents
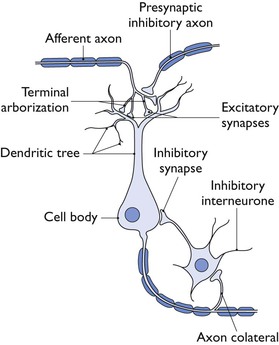
The central nervous system control of the functions altered by anaesthesia and related drugs is incredibly complex. Sherrington in 1906 pointed out the importance of the synapse in the CNS in providing connections between multiple neuronal systems (Fig. 1.1). At synapses, transmission involves release of transmitter that will ‘trigger’ the action of the next neuron. Receptors sensitive to the transmitter may be postsynaptic, or presynaptic feeding back on the original nerve terminal and modulating further action. Transmitters act through two main types of receptor: ionotropic and metabotropic. With inotropic or ‘ligand-gated’ receptors, the transmitter binds directly with the ion-channel proteins, allowing the channel to open and the ions to pass. Binding of a transmitter to metabotropic receptors involves G-proteins as secondary messengers. Recent roles for voltage-gated channels, where changes in cellular membrane potential triggers a response, such as two-pore potassium channels have also been identified.
In the CNS, three major transmitters are considered most directly relevant to general anaesthesia. Gamma-amino-butyric acid (GABA) is inhibitory and decreases the excitability of neurons. Glycine is inhibitory in most circumstances and is the most important inhibitory transmitter at the spinal cord. The main excitatory transmitter in the CNS is glutamate. Anaesthetic drugs that are thought to act at the N-methyl d-aspartate (NMDA) receptor, one of at least three types of ligand-gated glutamate receptor, inhibit the effect of glutamate, thus again inhibiting the CNS. However, there are many other relevant transmitters, for example acetylcholine, dopamine, norepinephrine, endogenous opioids and others (Sonner et al., 2003) and their resultant actions may influence (modulate) the actions, directly or indirectly, of the GABA, glycine and glutamate pathways.
In the 1900s, for the inhalation agents (no injectable had been discovered), Meyer and Overton independently noted the correlation between anaesthetic potency and solubility in oil, which led to the ‘lipid theory’ that general anaesthetics acted through a non-specific mechanism by changing the lipid bilayer of nerve cells. There are exceptions which disprove the hypothesis but, nevertheless, for most the correlation is amazing, and this theory, with modifications, held sway until Franks and Lieb (1984) showed that inhalational general anaesthetics inhibited protein activity in the absence of lipids. This finding led to the explosion of studies on (a) where in the CNS anaesthetics act, (b) differences between motor and amnesic actions and, finally, (c) the molecular targets for action. (Franks (2006) quotes a review that cites 30 possible such targets.) All these three points are inextricably interlinked. A full discussion is beyond the remit of this book, but the following very simplified summary is based on reviews by Urban & Bleckwenn (2002), Sonner et al. (2003), Rudolph & Antikowiak (2004), Franks (2006) and Perouansky et al. (2012).
All anaesthetic agents do not act in the same way or in the same place. Classified by their mode and place of actions, there appear to be four main types of anaesthetic agent: (1) injectable agents such as propofol, etomidate and alfaxalone; (2) volatile anaesthetic agents such as halothane, isoflurane and sevoflurane; (3) the injectable dissociative agents such as ketamine; and (4) the gaseous agents, nitrous oxide and xenon.
It is now considered that the inhibition of motor actions occurs at the spinal cord, at least for the volatile anaesthetic agents, while amnesia and unconsciousness are the remit of the higher centres in the brain (Eger et al., 1997) – these two aspects of anaesthesia being separate. Most anaesthetics can cause amnesia at subhypnotic doses, although the relative dose for amnesia in relationship to that required for unconsciousness varies between drugs. The area of brain critical for amnesia appears to be the hippocampus and basal nucleus of the amygdala. Other centres are involved in the production of sedation and unconsciousness. For example, functional neuroimaging demonstrated that when propofol was administered at sedative doses and a noxious stimulus applied, evoked responses were attenuated only in the somatosensory cortex, but once doses reached hypnotic levels, thalamic and cortical responses ceased. Ketamine, a dissociative anaesthetic, however, did not depress sensory inflow through the thalamus. Perouansky et al. (2012) summarize by pointing out that anaesthesia is a very complex state, and that current evidence shows that general anaesthetics produce separate ‘agent specific’ substates, probably at different areas of the CNS.
Three very differing molecular targets have been suggested as the major sites of anaesthetic actions: GABAA receptors, NMDA receptors and glycine receptors. Anaesthetic drugs that act at the GABAA receptors are the classical IV anaesthetics; these potentiate the action of GABA, hence increase overall CNS inhibition. The volatile anaesthetic agents have similar actions at GABAA receptors in addition to actions at other targets, and their mode of action is more complex. Dissociative anaesthetics such as ketamine and the gaseous anaesthetic agents are thought to be antagonistic at the NMDA receptor, thus blocking the action of the excitatory glutamate, but again, this does not explain all their actions.
A great deal is known about the GABAA receptor (there are also GABAB and GABAC). It is a polymeric receptor with five subunits; there have been at least 30 types of subunit cloned so the potential for heterogenicity is enormous. Different arrangements of subunits are sensitive to different anaesthetic agents. For example, genetically engineered ‘knock-out mice’ in which one particular subunit was missing were insensitive to the anaesthetic effects of the steroid anaesthetic, alfaxalone, but not to propofol suggesting that these two apparently similar IV anaesthetics were working through different configurations of GABAA receptor. The mode of action of the volatile anaesthetic agents, in particular in the brain, is less proven (and more complex) than for the IV agents; the volatile anaesthetic agents do have some actions at the GABAA receptor but at a much lower potency. Subunits of GABAA essential for efficacy differ between the volatile and IV agents. It is thought that glycine receptors are involved in the actions of the volatile agents, in particular those in the spinal cord which inhibit movement. The reason for giving these examples (there are very many more) of differing modes of actions between apparently similar types of anaesthetics is to point out that to say ‘an anaesthetic acts at the GABAA receptor’ is certainly not the whole story.
Knowledge of the mode of action of the anaesthetics that work at the NMDA receptor is less well developed. The anaesthetics (ketamine and the gaseous agents) are thought to cause their effects on memory and consciousness at least partly through these receptors. Sonner et al. (2003) consider that NMDA receptors in the spinal cord might be a target for all inhalation anaesthetic agents (not just those those termed ‘gaseous’) in the production of immobility. However, once again, action at the NMDA receptor does not explain all the actions seen.
The methods of investigation used to study anaesthetic actions are multiple, and readers are referred to the reviews cited above. All reviews point out the number of other potential molecular, ion or voltage-gated possible sites which might be the target for anaesthetic action. Other routes of investigation have examined the modulating influence of alternative CNS pathways. Following the findings of Franks and Lieb (1984), it was anticipated that a (relatively) simple pharmacological pathway for anaesthetic action might be found to explain anaesthetic actions, as has been for many other systems (e.g. opioids, see Chapter 5, α2-adrenoceptor agonists, see Chapter 4). This has not happened, although our knowledge is greatly expanded. However, we have yet really to know how general anaesthetics work, and indeed Sonner et al. (2003) raise a question that, at least for volatile agents, some variant of the Overton–Meyer lipid theory may yet play a partial role.
Depth of anaesthesia
Many authorities consider that ‘depth of anaesthesia’ is impossible to define, but anaesthesiologists need some guidelines to ensure that the patient comes to no harm. Only two years after the first demonstration of general anaesthesia, John Snow (1847) stated, quite emphatically, that the point requiring most skill in the administration of anaesthetics is to determine when it has been carried far enough. Snow described five stages of anaesthesia produced by diethyl ether, the last stage in his experiments with animals being characterized by feeble and irregular respiratory movements heralding death – clearly a stage too far. A major problem faced by all anaesthetists since that time is to avoid both ‘too light’ anaesthesia with the risk of sudden violent movement, and the dangerous ‘too deep’ stage. Snow suggested guidelines whereby anaesthetists could reduce the risk of either too light or too deep ether anaesthesia. Guedel, in 1918, devised a scheme involving observation of changes in respiratory rate, limb movement and eye signs which formed the basis of his celebrated ‘Signs and Stages of Ether Anaesthesia’ which has been included until very recently in all text books of anaesthesia, and is the basis for that described in Chapter 2.
The introduction of neuromuscular blocking drugs, which remove all the somatic responses on which Guedel’s scheme is based, completely changed the picture and the emphasis swung from the danger of too deep anaesthesia to that of too light anaesthesia with the risk of conscious awareness and perception of pain. Cullen et al. (1972), in an attempt to produce new guidelines indicating depth of anaesthesia, were forced to conclude that it was difficult to categorize the clinical signs of anaesthesia for any one inhalation anaesthetic let alone for inhalation agents in general. The signs also differed markedly when the dissociative anaesthetic agents such as ketamine were used. Today a very much broader range of different drugs are employed during anaesthesia. These include agents to give analgesia, amnesia, unconsciousness and relaxation of skeletal muscles as well as suppression of somatic, cardiovascular, respiratory and hormonal responses to surgical stimulation. All may influence the classical signs of ‘depth’ of anaesthesia.
Electroencephalography (EEG)
It is only possible to describe the EEG changes related to anaesthesia in the most general terms. The responsive alpha rhythm associated with awareness changes on induction of anaesthesia in terms of frequency and amplitude. The most common pattern seen with light general anaesthesia has low amplitude and is dominated by high frequency activity; it is often referred to as desynchronized. Increasing concentrations of anaesthetics tend to produce increasing amplitude and decreasing frequency, a phenomenon known as synchronization. In addition, some anaesthetics produce periods of burst suppression where the EEG is isoelectric, repetitive high amplitude spikes and complexes or even the epileptoid activity characteristic of the anaesthetic ethers.
The majority of attempts to monitor the depth of anaesthesia objectively have focused on the EEG, but the raw data are of limited practical value to the clinical anaesthetist. To simplify the extraction of useful information from complex waveforms, a number of methods of compressing, processing and displaying EEG signals have been developed and these techniques have, in many cases, been applied to a limited number of channels of EEG rather than the 16 channels normally studied.
In this technique, the EEG signal, after being digitalized, is subjected to Fast Fourier Transformation (FFT) in which it is separated into a series of sine waves. The sum of these sine waves represents the original integrated signal. Breaking up the original waveform in this way makes it possible to compare one non-standard wave form with another and, in particular, to extract the distribution of components of different frequency within the EEG signal. The power in each frequency band is derived from the sum of the squares of the amplitude of the sine waves into which the FFT has separated the original signal. Power spectrum analysis has been used in a number of experimental situations related to veterinary anaesthesia (e.g. Otto & Short, 1991; Murrell et al., 2003; Johnson et al., 2005, 2009).
A number of monitors, including the Bispectral Index (BIS), the Cerebral Function Monitor (CFM) and the Patient State Index (PSI) feed the EEG signals from a limited number of leads into a ‘black box’ which, on the basis of an algorithm derived from analysis of EEGs from a large number of human patients, produces a number which is related to depth of anaesthesia. All these monitors have a number of limitations in clinical use. They may be influenced by other electrical ‘noise’ such as the electromyogram (EMG). The algorithms are based mainly on anaesthetic drugs that have their hypnotic effect through actions at the GABAA receptors. The monitors are ineffective for the anaesthetic agents that act at the NMDA receptors such as nitrous oxide, xenon or ketamine. Of great concern is the fact that when neuromuscular blocking drugs were given to conscious human volunteers, BIS reduced to values suggestive of very deep anaesthesia (Messner et al., 2003). The use of these monitors may reduce the incidence of awareness under anaesthesia but has not eliminated it. The most common of these monitors is the BIS; its use and limitations in veterinary anaesthesia are described in Chapter 2.
Evoked responses are changes in the EEG produced by external stimuli, surgical or otherwise. Anaesthetic depth is a balance between cerebral depression and surgical (or other) stimulation. Thus, cerebral function during anaesthesia is most easily assessed by putting in a stimulus – auditory or somatic or visual – and observing the EEG response. That response can then be compared for amplitude and latency with the response to the same stimulus in the presence of differing brain concentrations of any anaesthetic. Evoked responses can be used as monitors of anaesthetic depth when agents acting at the NMDA receptor are being employed.
The ‘classic’ signs of anaesthesia
Use of the term ‘depth of anaesthesia’ is now so ingrained in common usage that it must be accepted since it probably cannot be eradicated. It is important, however, to realize that it commonly refers to depression of brain function beyond that necessary for the production of ‘general anaesthesia’.
The so-called ‘classic signs’ of anaesthesia, such as described in Chapter 2 for convenience of newcomers to the subject, were provided by the presence or absence of response of the anaesthetized subject to stimuli provided by the anaesthetist or surgeon. Particular signs of anaesthesia were, therefore, equated with particular anatomical levels or ‘planes’ of depression of the central nervous system. These signs were often likened to a series of landmarks used to assess the progress made on a journey. Such empirical, traditional methods of assessing the progress of anaesthesia and the anatomical implications that went with these methods incorporated a fallacy, because they took no account of the fact that the changing function of any biological system can only be made in terms of magnitude and time. A depth of unconsciousness is really a particular moment in a continuous temporal stream of biological or neurological phenomena to be interpreted by the magnitude and quality of these phenomena obtaining to that moment.
In general, the volatile anaesthetic agents halothane, enflurane, isoflurane, sevoflurane and desflurane produce a dose-dependent decrease in arterial blood pressure and many veterinary anaesthetists use this depression to assess the depth of anaesthesia. The effect is not so marked during anaesthetic techniques involving the administration of opioid analgesics and nitrous oxide. If the depth of unconsciousness is adequate, surgical stimulation does not cause any change in arterial blood pressure. There are, however, many other factors which influence the arterial blood pressure during surgery such as the circulating blood volume, cardiac output and the influence of drug therapy given before anaesthesia. If ketamine or high doses of opioids are given, arterial blood pressure may change very little if the depth of unconsciousness is increased by the administration of higher concentrations of inhalation anaesthetics.
Changes in heart rate alone are a poor guide to changes in the depth of unconsciousness. The heart rate may increase under isoflurane and desflurane anaesthesia due to the agents’ effects. Arrhythmias are common during light levels of unconsciousness induced by halothane, when they are usually due to increased sympathetic activity. In general, however, tachycardia in the absence of any other cause may be taken to represent inadequate anaesthesia for the procedure being undertaken.
Anaesthetic agents affect respiration in a dose-dependent manner and this was responsible for the original classification of the ‘depth of anaesthesia’. In deeply anaesthetized animals, tidal and minute volumes are decreased but, depending on the species of animal and on the anaesthetic agents used, respiratory rate may increase before breathing eventually ceases once the animal is close to death. As inadequate anaesthesia also is often indicated by an increase in the rate and/or depth of breathing the unwary may be tempted to administer more anaesthetic agent to the deeply anaesthetized animal in the mistaken impression that awareness is imminent. Laryngospasm, coughing or breath-holding can indicate excessive airway stimulation or inadequate depth of unconsciousness.
All anaesthetic agents, other than the dissociative drugs such as ketamine, cause a dose-related reduction in muscle tone and overdosage produces complete respiratory muscle paralysis. In the absence of complete neuromuscular block produced by neuromuscular blocking drugs, the degree of muscle relaxation may, therefore, usually be used as a measure of the depth of anaesthetic-induced unconsciousness. However, even in the presence of muscular paralysis due to clinically effective doses of neuromuscular blockers, it is not uncommon to observe movements of facial muscles, swallowing or chewing movements in response to surgical stimulation if the depth of unconsciousness becomes inadequate.
When animals are breathing spontaneously, there are several signs which are generally recognized as indicating that the depth of unconsciousness is adequate for the performance of painful procedures, i.e. the animal is unaware of the environment and of the infliction of pain – it is anaesthetized.
Unfortunately, there are many differences between the various species of animal in the signs which are usually used to estimate the depth of unconsciousness. One fairly reliable sign is that of eyeball movement, especially in horses and cattle, although even this may be modified in the presence of certain other drugs, such as the α2-adrenoceptor agents (see Chapter 11). Unless neuromuscular blocking drugs are in use, very slow nystagmus in both horses and cattle and downward inclination of the eyeballs in pigs and dogs usually indicates a satisfactory level of unconsciousness and, at this level, breathing should be smooth although its rate and depth may alter depending on the prevailing severity of the surgical stimulation. Rapid nystagmus is usually a sign that anaesthesia is light but it is a common feature of ketamine anaesthesia and it also seen sometimes seen in horses just before death. Absence of the lash or palpebral reflex (closure of the eyelids in response to light stroking of the eyelashes) is another reasonably reliable guide to satisfactory anaesthesia. In dogs and cats, it is safe to assume that if the mouth can be opened without provoking yawning or curling of the tongue, central depression is adequate. In all animals, salivation and excessive lacrimation usually indicate a returning awareness.
Disappearance of head shaking or whisker twitching in response to gentle scratching of the inside of the ear pinna is a good sign of unawareness in pigs, cats, rabbits and guinea pigs. Pupil size is a most unreliable guide to anaesthetic depth as various ancillary agents (e.g. opioids, atropine) may influence it. The pupils do, however, dilate when an overdose of an anaesthetic has been given or when awareness is imminent.
The experienced anaesthetist relies most of the time on an animal’s response to stimuli produced by the surgeon or procedure to indicate adequate depth of unconsciousness. The most effective depth is taken to be that which obliterates the animal’s response to pain and/or discomfort without depressing respiratory and circulatory function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree