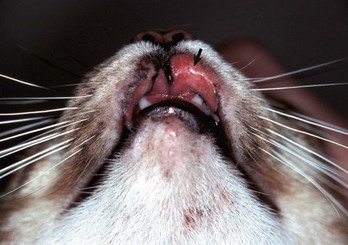
CHAPTER 7 There are limited numbers of ways that pathogenic agents gain entry into the alimentary system (Box 7-1). The most common, of course, is through ingestion. However, under certain circumstances, pathogens may be coughed up from the lungs into the pharynx and swallowed. Systemic blood-borne infections of viruses (viremia), bacteria (bacteremia), and systemic toxins (septicemia and toxemia) may make their way through the bloodstream and attach to specific receptors on the epithelial lining cells of the alimentary system. Parasites may migrate through various regions of the body to find a home within the mucosa or roam free in the lumen of the alimentary tract. Considering the types of materials that are ingested by domestic animals, it is significant that they are not constantly ill. This resistance to disease occurs because the alimentary system is well suited to protect itself against most potentially pathogenic insults (Box 7-2). These protective mechanisms include oral secretions, such as saliva; “normal” resident flora and fauna; the gastric pH; opening of tight junctions between intestinal cells to allow macromolecules, such as immunoglobulins, into the lumen; vomiting; secretions from the liver and pancreas; intestinal proteolytic enzymes, macrophages, and other effector cells, such as neutrophils, within the submucosa, which are exuded into the alimentary lumen; the high rate of epithelial turnover; increased peristalsis resulting in diarrhea; Paneth cells; and the immune system. Paneth cells produce antimicrobial peptides and proteins, including lysozyme and secretory phospholipase A2. They also produce α-defensins (cryptdins). Defense mechanisms of the oral cavity include the stratified epithelial surface that is resistant to trauma and some irritants; taste buds, which reject potentially toxic materials based on taste and tongue feel; an indigenous bacterial flora that occupy attachment sites that would otherwise be available to pathogens; and saliva (Box 7-3). Saliva provides a flushing action so potential pathogens are cleared from the oropharynx and swallowed. Saliva also forms a protective coating of the mucosa and contains antimicrobial lysozyme in the zymogen granules of serous cells and immunoglobulins, especially immunoglobulin A (IgA), in a manner analogous to cryptal enterocytes of the intestine, through the production of a secretory component. Migration through the alimentary tract, including the oral cavity, eliminates neutrophils at the end of their lifespan. In their absence, stomatitis results. Palatoschisis, or cleft palate, and cheiloschisis, or cleft lip, are among the most common developmental abnormalities of the oral cavity. Cheiloschisis is sometimes referred to as hare lip because this is a normal feature of the rabbit. It is a failure of fusion of the upper lip along the midline or philtrum. Palatoschisis can be genetic or toxic in origin. It results from a failure of fusion of the lateral palatine processes. It can be caused by steroid administration during pregnancy in primates, including humans. Depending on the size of the defect, which may involve only the soft palate or both the soft and hard palates (Fig. 7-1), the lesion may be surgically correctable. It is a matter of some ethical concern whether to correct such defects without also sterilizing the patient because of the potential for cleft palate to have a genetic cause. Important sequelae to the host from cleft palate are starvation, as the result of the inability of the nursing animal to create a negative pressure in the mouth with a resultant failure to suckle, and aspiration pneumonia, since no effective separation is present between the oral and nasal cavities. Fig. 7-1 Palatoschisis and cheiloschisis, hard and soft palate, puppy. Although vesicular stomatitis correctly refers to oral vesicles and blisters, the term is generally reserved for those lesions caused by epitheliotropic viruses. The vesicular stomatitides are listed in Table 7-1. Their genesis is from virus-induced epithelial cytolysis attended by fluid accumulation and subsequent rupture of the resultant vesicle. Blistering or vesiculation of the oral epithelium is present early in the course of these diseases. All of these diseases are virus-induced, and all have identical appearances at gross and histopathologic examination. None of these conditions is fatal. They produce great economic loss because of poor weight gain in affected animals and sometimes abortions in gravid females. The exact cause of the abortions is unknown, but it is probably related to the stress induced by the painful oral, cutaneous, and pedal (hoof or foot) lesions. Secondary bacterial invaders, both Gram-negative and Gram-positive, of these lesions can result in endotoxemia. Several diseases, such as foot-and-mouth disease and vesicular exanthema, affect the coronary bands of the digits and interdigital clefts, resulting in lameness. Some of these diseases (foot-and-mouth disease, vesicular exanthema, and swine vesicular disease) are exotic to the United States (US) and thus are reportable to state or federal authorities, or both, if the clinician or pathologist suspects the disease. This requirement is due to the great expense involved in eradicating these diseases from the US and their potential use as agents of agroterrorism. Nontariff export/import barriers designed to prevent the introduction of highly contagious agents, such as foot-and-mouth disease, into animal populations of countries with which we trade are often put in place. The gross lesions of the vesicular stomatitides are epithelial. Fluid-filled vesicles are present in the oral cavity, lips, rostral palate, and tongue (Fig. 7-2, A). Entry of virus in these cases is most likely oral into areas of temporary loss of mucosa as the result of normal mastication and trauma. The viruses are cytolytic, and the resultant release of virus from cells infects neighboring cells. The lesions enlarge centripetally, forming vesicles. Bullae result from coalescence resulting in erosions and ulcers. These ulcers are typically hyperemic (Fig. 7-2, B). Viremia, often transient, sometimes occurs. Fig. 7-2 Cutaneous vesicles, vesicular exanthema, snout, pig. Vesicular exanthema is a specific disease of pigs that is indistinguishable clinically and pathologically from foot-and-mouth disease. This disease is uniquely American and was believed eradicated from pigs in 1956 through enactment of federal laws requiring the cooking of garbage fed to pigs. The evidence indicates that vesicular exanthema of swine serovars are variants of San Miguel sea lion virus. This latter marine calicivirus occurs in coastal sea lion and fur seal populations from California to Alaska (Web Fig. 7-1). Web Fig. 7-1 Cutaneous vesicles, San Miguel sea lion virus infection, foreflippers, northern fur seal. Erosions are defined by a loss of part of the thickness of the surface epithelium, whereas ulcers are full-thickness epithelial losses exposing the basement membrane. Thus erosions may progress to ulcers, which in hollow organs may become perforating ulcers. Erosive and ulcerative stomatitis can have a variety of causes. Agents responsible include the viruses of bovine viral diarrhea (BVD) (Fig. 7-3), rinderpest, malignant catarrhal fever (Fig. 7-4), feline calicivirus, and bluetongue, and in equids, nonsteroidal antiinflammatory drugs (NSAIDs). Other causes include uremia (Fig. 7-5); ingested foreign bodies, such as foxtail awns; the feline eosinophilic granuloma complex; and vitamin C deficiency in primates and guinea pigs (Web Fig. 7-2). Often, the oral lesions must be evaluated in the context of the clinical signs, together with histopathologic findings and ancillary testing, to arrive at a definitive diagnosis. Additionally, the vesicular stomatitides can progress to ulceration secondary to abrasion to the point that they cannot be distinguished from the ulcerative stomatitides. Fig. 7-3 Erosions and ulcers, bovine viral diarrhea virus infection, hard palate, cow. Fig. 7-4 Erosions and ulcers, malignant catarrhal fever, hard palate, dental pad and buccal papillae, cow. Fig. 7-5 Uremic ulcers, hard palate, dog. Web Fig. 7-2 Ulcerative gingivitis secondary to scurvy (vitamin C deficiency), gingiva, monkey. The two major diseases in this category, bovine papular stomatitis and contagious ecthyma, are zoonotic. Bovine papular stomatitis is recognized by papules on the nares, muzzle, gingiva, buccal cavity, palate, and tongue (Fig. 7-6). Lesions also occur in the esophagus, rumen, and omasum. Microscopically, acantholysis is responsible for the macule and ballooning degeneration of these cells, which may contain intracytoplasmic eosinophilic parapoxvirus inclusions at a later stage (Fig. 7-7; also see Fig. 1-12). Erosion of the infected cells accompanied by a neutrophilic infiltrate heals readily from the unaffected basal epithelium. The disease is more common in immunosuppressed animals such as those persistently infected with BVD virus. In humans, the disease is called milker’s nodules and is characterized by papules of the hands and arms. Fig. 7-6 Epithelial plaques, papular stomatitis, hard palate mucosa, calf. Fig. 7-7 Hydropic change, papular stomatitis, hard palate mucosa, cow. Contagious ecthyma, sore mouth or infectious pustular dermatitis, is a condition of sheep and goats characterized by progression of the stages typical of pox viruses—macules, papules, vesicles, pustules, scabs, and scars in areas of skin abrasions, including the corners of the mouth (Fig. 7-8; also see Fig. 17-43), mouth, udder, teats, coronary bands, and anus. Occasionally, the mucosa of the esophagus and rumen also can be affected. The virus is quite hardy and can survive for 50 to 60 days in the summer and longer in cold weather. At room temperature, scabs containing virus can be infective after 10 years. Eosinophilic cytoplasmic inclusion bodies are visible at microscopic examination of lesions early in the course of disease. The condition in humans is called orf. Fig. 7-8 Contagious ecthyma, oral mucous membranes, lamb. Necrotizing stomatitis occurs in cattle, sheep, and pigs. In cattle, it is sometimes referred to as calf diphtheria (Fig. 7-9). Necrotizing stomatitis is the end-stage of all other forms of stomatitis when they are complicated by infection with Fusobacterium necrophorum, a filamentous-to-rodlike-to-coccoid, Gram-negative anaerobe. Bacterial toxins are responsible for the extensive lesions. Necrotizing stomatitis is characterized by yellow-gray, round foci surrounded by a rim of hyperemic tissue in the oral cavity, larynx, pharynx, or tongue. Well-demarcated foci of coagulation necrosis typify the histologic appearance of necrotizing stomatitis. As might be expected in foci of inflammation, there is a circumferential rim of leukocytes and hyperemia. Clinical signs include swollen cheeks, inappetence, pyrexia, and halitosis. Infection may become systemic if severe, resulting in lesions throughout the alimentary system and associated lymphoid tissue. Fig. 7-9 Necrotizing stomatitis, calf diphtheria, tongue, calf. In cats, lip lesions are commonly visible near the philtrum and may extend through the adjacent haired skin. Oral lesions may occur anywhere in the mouth, including the gingiva, hard and soft palates, oral and nasal pharynx, tongue, and occasionally draining lymphoid tissues, excluding the tonsils, which do not have afferent lymphatic vessels (Fig. 7-10; also see Fig. 17-21, C). In dogs, eosinophilic granulomas typically are raised, fungating masses on the ventral and lateral lingual epithelium and palate. Collagenolysis (because collagen is acellular, it cannot undergo necrosis) is characteristically central in the lesion. The surrounding inflammatory tissue contains mixed inflammatory cells with increased numbers of eosinophils, mast cells, and multinucleated giant cells (see Web Fig. 3-14). Lesions grouped as the eosinophilic granuloma complex of cats include eosinophilic ulcer, linear (collagenolytic) granulomas, and eosinophilic plaques. The latter two lesions are strictly cutaneous and do not affect the oral cavity. No proven etiologic link has been established between these cutaneous conditions (linear granulomas and eosinophilic plaques) and oral eosinophilic granulomas. The cause of the canine lesions is unknown. Lymphoplasmacytic stomatitis is an idiopathic condition of the cat named on the basis of the histologic appearance of the lesions (Fig. 7-11). Associations have been hypothesized between this condition and the presence of bacteria or calicivirus associated with feline leukemia virus (FeLV) and/or FIV infection. It is a chronic condition characterized by red, inflamed gums, a fetid breath, and inappetence. The oral mucosa may be hyperplastic and ulcerated. An inefficient immune response may be responsible for the persistence of oral bacteria and the accumulation of lymphocytes and plasma cells. Fig. 7-11 Lymphoplasmacytic stomatitis, gingiva, cat. Hyperplastic Diseases: Gingival hyperplasia is a simple overgrowth of gum tissue, principally the fibrous submucosa. The hyperplasia can become so severe as to bury incisor teeth (Fig. 7-12). Gingival hyperplasia is most common in brachycephalic dog breeds and is present in 30% of boxer dogs older than 5 years. Fig. 7-12 Gingival hyperplasia, gingiva, dog. Grossly, gingival hyperplasia can be indistinguishable from an epulis (Fig. 7-13). Epulis is a nonspecific term that designates a growth of the gingiva. The several kinds of epulides can only be distinguished by histopathologic examination. These include fibromatous epulis of periodontal ligament origin—a benign tumor of dental mesenchyme. This distinction is not just an academic exercise because, although all epulides are considered benign, one form, acanthomatous epulis or acanthomatous ameloblastoma, invades bone and can be quite destructive. This growth arises from the epithelial rests of Malassez or epithelial tooth germ. Fortunately, this type of epulis can be managed therapeutically. Whether the epulides represent fibrous and epithelial hyperplasia or benign neoplasms of tooth germ is controversial. Fig. 7-13 Fibromatous epulis, left mandible, molar teeth, dog. Neoplasia: In the dog, 70% of tumors of the alimentary system are in the oral cavity and oropharynx. These tumors run the gamut of biologic behavior from simple epithelial hyperplasia to malignant neoplasms with metastases to distant sites. Squamous cell carcinomas occur in the oral cavity, particularly in old cats, in which they account for 60% of oral neoplasms. They generally occur on the ventrolateral surface of the tongue and tonsils. Lingual squamous cell carcinomas occur more commonly in felids, and tonsillar squamous cell carcinomas are more common in canids. Although often appearing histologically aggressive, only a small percentage of lingual neoplasms metastasize, most commonly to draining lymph nodes, the mandibular and medial retropharyngeal. Unfortunately, most tonsillar carcinomas metastasize, initially to regional lymph nodes and then to distant sites. Squamous cell carcinomas vary both in size and in gross appearance—from flat to proliferative (Fig. 7-14). These tumors are often quite aggressive locally, invading subjacent tissues. Some tumors contain more differentiated cells, keratin, often in whorls (keratin pearls) and visible desmosomes (intercellular bridges), whereas others are less well differentiated but with significant mitotic activity. In these latter cases, intracellular immunohistochemical markers for cytokeratin are useful in determining a definitive diagnosis. The amount of fibrous tissue within an individual tumor is variable. Some carcinomas induce a scirrhous response, whereas others have areas of necrosis caused by rapid tumor growth, “collision necrosis,” of the tightly packed proliferating cells and loss of contiguity with the blood supply. Fig. 7-14 Squamous cell carcinoma, palate, woodchuck. Ninety percent of melanomas of the oral cavities of dogs are malignant. A breed predilection exists for Scottish terriers, Airedales, cocker spaniels, golden retrievers, Bedlington terriers, Duroc pigs, and others. Most melanomas contain copious intracellular pigment and are visibly black. Some melanomas without pigment, termed amelanotic melanomas, present a greater diagnostic challenge to both the clinician and pathologist (Fig. 7-15). Immunohistochemical staining for tryrosinase-related proteins (TRP-1, TRP-2), Melan-A, and melanocytic antigen PNL2 are useful for immunohistochemically identifying amelanotic tumors. Melanomas are composed of melanocytes and are of neural crest origin. Cellular morphology within melanomas varies from spindloid to epithelioid. Thus some neoplasms are histologically difficult to differentiate from squamous cell carcinomas and others from fibrosarcomas. Fig. 7-15 Amelanotic melanoma, mandibular symphysis, dog. Oral extramedullary plasmacytomas may occur anywhere in the mucous membranes of the oral cavity, esophagus, or intestine. In the oral cavity, they are slow-growing neoplasms and in spite of often-recognized anisokaryosis, mitoses, and multinucleate cells, they rarely invade surrounding tissues and have not been reported to metastasize. Histologic examination is required for accurate diagnosis (see Fig. 13-33). Abnormal development and positioning of the teeth may affect dental function. Malocclusion refers to a failure of the upper and lower incisors to oppose properly. This feature is “normal” for some dogs, particularly the brachycephalic breeds. In the extreme, malocclusions can lead to difficulty in the prehension and mastication of food. Malocclusions are named according to the position of the mandible. Protrusion of the lower jaw is termed prognathia, whereas a short lower jaw with resultant protrusion of the upper jaw is termed brachygnathia and sometimes hypognathia (Fig. 7-16). Sometimes these terms are incorrectly used, referring to brachygnathia as superior prognathia and prognathia as superior brachygnathia. Fig. 7-16 Prognathia, head, horse. Malocclusions result from abnormal jaw conformation or rarely from abnormal tooth eruption patterns. In some animals, such as rodents and rabbits, the teeth continue to grow throughout the animal’s lifetime. If these animals are not provided with sufficient roughage in their diets, the teeth (both incisors and cheek teeth) overgrow and either “lock” the jaw or because of a lack of occlusal grinding surfaces, prevent the animal from receiving proper nutrition (Web Fig. 7-3). Web Fig. 7-3 Overgrown teeth, head, guinea pig. In simple-toothed animals and rarely in other animals, agenesis of a tooth or teeth occurs and is generally of no clinical significance (see Fig. 17-35). Supernumerary tooth development is less common than tooth agenesis and is similarly of little clinical significance. Some animals, such as elasmobranches (sharks), continue to produce row on row of teeth as the outermost rows are lost. Dental dysgenesis may be primarily due to dysplasia of the enamel-forming organ or secondary to trauma, infection and hyperthermia, toxicosis, or other metabolic irregularities during odontogenesis. Segmental enamel hypoplasia occurs before eruption of the permanent teeth of dogs as a result of hyperthermia and viral infection, most often by canine distemper virus infection. Enamel is fully formed when the teeth erupt; therefore virus infection of ameloblasts must occur during enamel formation, which is before the dog is 6 months of age, if enamel hypoplasia is to occur. Canine distemper virus infection causes necrosis and disorganization of the enamel organ. After the virus is cleared, structure and function of the enamel organ return to normal. Thus segmental enamel hypoplasia results from the lack of enamel formation during the period of virus infection (Fig. 7-17). A similar condition in calves is caused by in utero BVD virus infection. Fig. 7-17 Enamel hypoplasia, permanent incisor teeth, dog. Chemicals, most notably tetracycline antibiotics ingested during the process of mineralization, can cause yellowish, permanent discoloration (see Fig. 1-58). Congenital porphyria, a defect in red blood cell production, may result in incorporation of porphyrins into dentin, resulting in pink discoloration of the teeth (pink tooth) (Web Fig. 7-4). Both tetracycline and porphyrins fluoresce under ultraviolet light, dramatically demonstrating these lesions. Web Fig. 7-4 Pink tooth, congenital porphyria teeth, adult ox. Fluoride incorporation into the enamel and dentin occurs in fluoride toxicosis, particularly in cattle and sheep. A relationship exists in beef cattle between fluorosis and selenium supplementation, with selenium supplementation being protective in high fluoride areas such as those downwind from aluminum smelters or with high levels of fluoride in ground water. Excessive dietary concentrations of fluorine during odontogenesis (from 6 to 36 months of age) may result in incorporation of the fluoride in the enamel and dentin of the permanent teeth. The result is soft, chalky, discolored enamel, usually yellow, dark brown, or black (Web Fig. 7-5). Occlusal grinding of affected soft teeth against more normal enamel results in rapid dental wear to the extent that severely affected sheep may have almost completely worn down their incisors. One wonders therefore about the cumulative effect of fluoride supplementation in municipal drinking water, vitamins with added fluoride, fluoride-supplemented toothpaste, fluoride treatment of teeth, reconstituted and bottled soft drinks made with fluoridated water, and so forth. It is difficult to calculate the total fluoride load ingested by individuals or what the effects may be of that fluoride supplementation. Web Fig. 7-5 Fluorosis, cheek teeth, cow. Loss of normal dental structure and function often results from rapid and irregular and/or abnormal wear of occlusal surfaces in many species of domestic animals. In those species with hypsodont teeth, attention to the dentition as the animal ages is often a major factor in overall body conditioning and health (Fig. 7-18). Aggressive treatment of occlusal surface irregularity by filing of high points in the dental arcade (floating) can notably prolong an animal’s life. Rock chewing or other compulsive oral behaviors in dogs may result in accelerated dental wear. Similarly, cribbing in horses and herbivorous animals grazing on sandy soils can cause premature dental wear. In all species, exposure of dentin or the pulp canal may lead to dental infection with serious consequences. Fig. 7-18 Dental attrition, molar teeth, antelope. Feline External Resorptive Neck Lesions: Cats suffering from feline external resorptive neck lesions often have pain upon chewing that may be reflected by inappetence and/or abnormal masticatory movements. External neck resorption of the cheek teeth of otherwise dentally normal cats is caused by odontoclastic resorption of cementum, particularly in the neck area or root of the tooth. Osteoclast ingrowths partially or completely line the resorption cavity. The resultant cavity may harbor bacterial plaque, resulting in intense inflammation and further osteoclastic resorption of dental tissue, including dentin and the root canal. The primary cause of this condition is not known. Odontomas are hamartomas originating in the enamel organ and are usually seen in puppies and foals (Fig. 7-19). They usually contain well-recognizable dentin and enamel, as well as ameloblasts, odontoblasts, and dental pulp. Fig. 7-19 Odontoma, incisor teeth, cow. The palatine tonsils are pharyngeal lymphoid structures covered by stratified squamous epithelium. Their function is uncertain, although it is likely they serve in lymphocyte production and antibody formation (see Chapter 13). In carnivores, they are found in crypts or recesses at the dorsolateral aspect of the caudal oropharynx. In pigs, they are flat and recognized by tiny pores in the surface epithelium of the caudal soft palate. Equids, ruminants, and pigs have lingual tonsils in addition to palatine tonsils. Tonsils do not possess afferent lymphatic vessels and do not serve as lymph filters. Therefore only primary (or direct) or hematogenous infections occur (tonsillitis) (Fig. 7-20), as well as primary neoplasms of either the lymphoid (lymphoma) (Fig. 7-21) or epithelial (squamous cell carcinoma) (Fig. 7-22) components. In many viremias of mammals, such as pseudorabies of pigs, virus may be isolated from the tonsils. Fig. 7-20 Necrotizing tonsillitis, tonsils, dog. Fig. 7-21 Lymphoma (lymphosarcoma), tonsil, dog. Changes in the salivary glands are uncommon in domestic animal species. A ranula is a cystic saliva-filled distention of the duct of the sublingual or submaxillary salivary gland that occurs on the floor of the mouth alongside the tongue (Fig. 7-23). It is thus epithelial lined. The cause is generally unknown, although some cases are due to sialoliths. A salivary mucocele, in contrast, is a pseudocyst not lined by epithelium but filled with saliva. The cause of this lesion is also unknown, but it may occur secondary to traumatic rupture of the duct of a sublingual salivary gland with resultant leakage and encapsulation of saliva by reactive connective tissue. Fig. 7-23 Ranula, mandibular salivary duct, dog. Sialoliths are rare in domestic animal species. When they do occur, they are considered to be caused by inflammation of the salivary gland with sloughed cells or inflammatory exudate forming a nidus for mineral accretion (Fig. 7-24). Thus they are one cause of ranula formation.
Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity*
Introduction
Portals of Entry
Defense Mechanisms
Oral Cavity
Defense Mechanisms
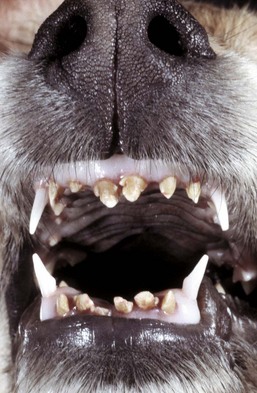
Developmental Anomalies
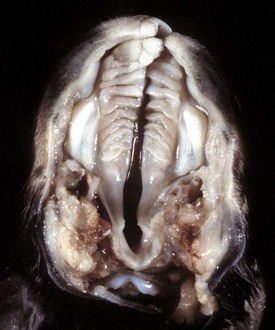
The lateral palatine processes have failed to fuse during the first trimester of gestation (palatoschisis). In dogs, palatoschisis has been attributed to genetic abnormalities, excessive intake of vitamin A during gestation, and the administration of cortisone during gestation. The upper lip is also cleft (cheiloschisis). (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
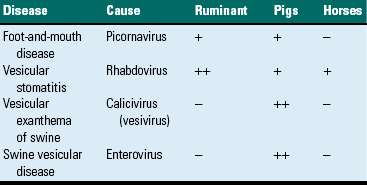
Vesicular Stomatitides
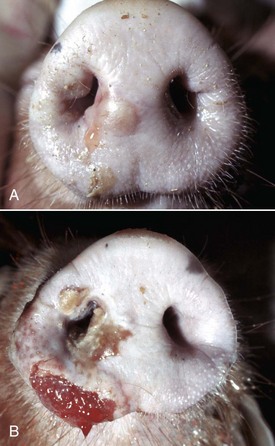
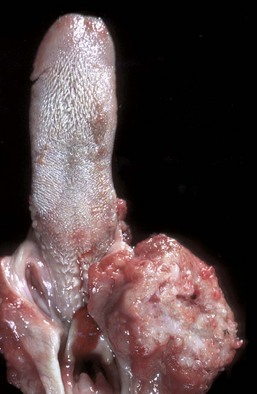
A, Vesicles, both intact (upper vesicle) and ruptured (lower vesicle), are present on the planum rostrale and are caused by the infection of injured mucosal epithelial cells with vesicular exanthema of swine virus, a calicivirus (vesivirus). B, Ruptured vesicles with cutaneous ulceration, vesicular exanthema (later stage of the disease). Note the ruptured vesicles which can cause pain resulting in inappetence. (A from Gelberg H, Lewis RM: Vet Pathol 19:424-443, 1982. B courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Specific Vesicular Diseases

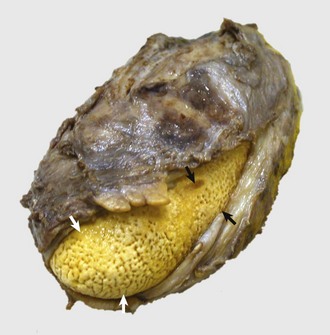
On the nonhaired portion of the foreflipper are vesicles both intact (arrow) and ruptured, caused by the infection of injured mucosal epithelial cells with San Miguel sea lion virus, a calicivirus (vesivirus) These vesicles will rupture with trauma, resulting in cutaneous erosion and ulceration. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Erosive and Ulcerative Stomatitides
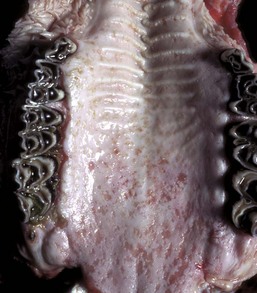
Erosions and ulcers caused by this pestivirus are particularly evident on the mucosal epithelial surface of the caudal hard palate. These lesions are characteristic of the ulcerative stomatitides, which, unlike the vesicular disease viruses, do not form vesicles. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
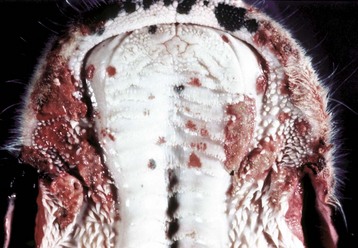
The erosions and ulcers (red areas on mucosal surface) are due to malignant catarrhal fever virus, a herpes virus, but are characteristic of many ulcerative stomatitides. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
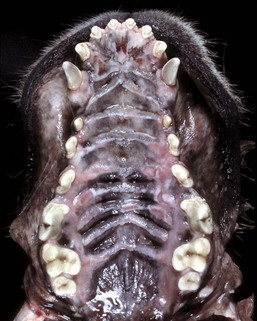
Ulcers present on the transverse palatine ridges and periodontal gingiva are secondary to vascular damage associated with increased concentrations of plasma blood urea nitrogen and creatinine from kidney failure. Affected animals often have an ammoniacal or uremic odor to the breath. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
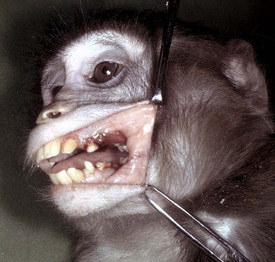
There is a deep ulcer at the commissure of the mouth and smaller ulcers periodontally. Vitamin C deficiency in primates and guinea pigs can result in gingival erosions and ulcers, and even tooth loss. (Courtesy College of Veterinary Medicine, University of Illinois.)
Parapox Stomatitides
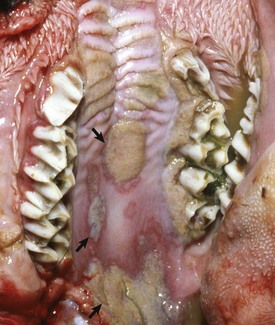
Virus-induced (parapoxvirus) epithelial plaques and papules are present on the mucosal epithelium of the hard palate and adjacent gingiva (arrows). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
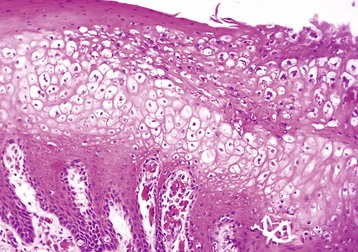
There is massive cytoplasmic swelling of the epithelial cells of the stratum spinosum. At a later stage, these cells may contain intracytoplasmic eosinophilic parapoxvirus inclusions (not visible here). H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
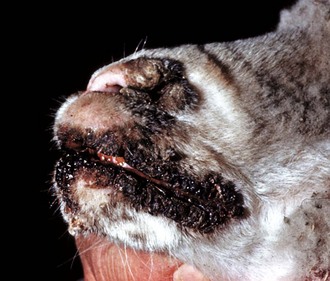
Note crusts around nose and lips. Multiple pustules and coalescing ruptured pustules covered by scabs are present on the skin. The parapoxvirus induces epithelial proliferation (acanthosis), followed by vesicle formation. These vesicles rupture and are quickly covered by scabs. Lesions develop at the sites of trauma, such as occur with a nursing lamb, where damage to the superficial oral epithelium allows entry of the virus into skin. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Necrotizing Stomatitides
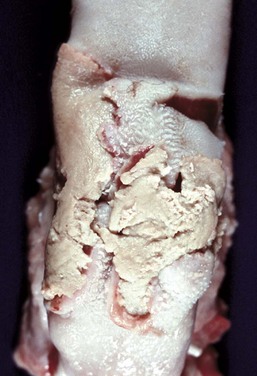
The dorsal surface of the tongue is ulcerated, and the ulcers are covered by a diphtheric membrane. Calf diphtheria is caused by infection with the bacterium Fusobacterium necrophorum secondary to abrasion and/or trauma to the mucosal epithelium of the oral cavity or larynx. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Eosinophilic Stomatitides
Lymphoplasmacytic Stomatitis
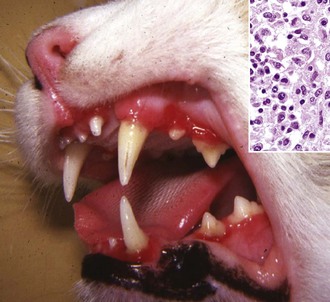
This chronic condition of cats is characterized by red, inflamed gums, fetid breath, and inappetence. The oral mucosa can also be hyperplastic and ulcerated. Inset, There is a florid infiltrate of mixed inflammatory cells, including many lymphocytes and plasma cells in the submucosa beneath the epithelium. H&E stain. (Figure courtesy Dr. C. Patrick Ryan, Veterinary Public Health, Los Angeles Department of Health Services; and Noah’s Arkive, College of Veterinary Medicine, University of Georgia. Inset, Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Oral Mucosal Hyperplasia and Neoplasia
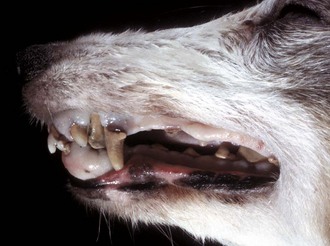
Hyperplastic gingiva has enveloped the lower incisor teeth. Dental calculus (tartar, brown) is also present on both upper and lower incisor, canine and molar teeth. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
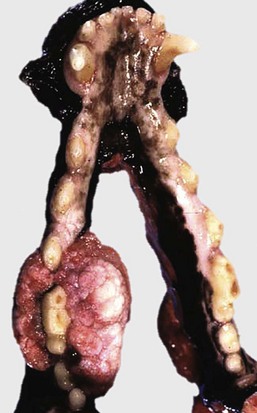
This growth is an epulis (fibromatous type); however, epulides are often grossly indistinguishable from gingival hyperplasia. Epulis is a term used to designate a growth of the gingiva that is firm, periodontal, and usually solitary, in contrast to gingival hyperplasia. This distinction is not just an academic exercise because, although all epulides are considered benign, one form, acanthomatous ameloblastoma, is locally invasive. It invades bone and can be quite destructive. (Courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
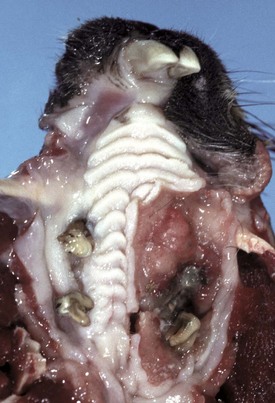
A mass of proliferating neoplastic squamous epithelial cells has displaced and replaced the mucosa and underlying tissue of the left hard palate and gingiva. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
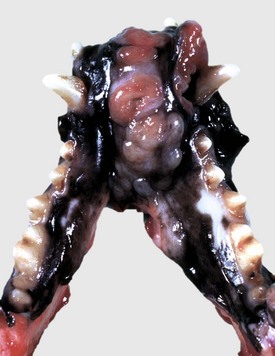
A proliferative, ulcerated, nonpigmented mass is present on the oral mucosa at the mandibular symphysis and protrudes into the oral cavity, likely resulting in malocclusion. Incisor teeth have been lost. Note the absence of pigmentation (melanin) in this tumor. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Teeth*
Malocclusions

The mandible is elongated compared with the maxilla. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
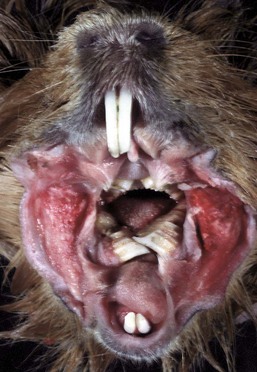
The incisors and molars are longer than normal and the tongue is entrapped by the lower molar teeth, which will lead to starvation unless corrected. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
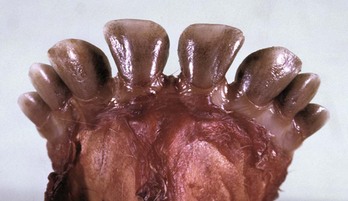
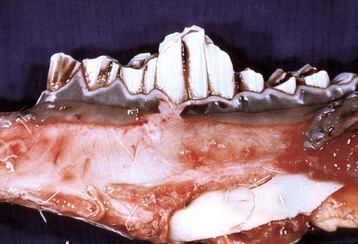
Anomalies of Tooth Development
There is a lack of enamel formation with resultant discrete deep pits and exposure of the dentin (light yellow to beige areas of the teeth), the result of infection with canine distemper virus and necrosis of the ameloblasts during enamel formation. Permanent adult teeth (shown in illustration) are infected with virus before their eruption and while they are still within their sockets (dental alveoli). (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
The teeth are discolored brown from the accumulation of porphyrins in the dentin. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
The enamel is chalky and weak, and the teeth are rapidly worn down. (Courtesy Dr. L. Krook, College of Veterinary Medicine, Cornell University.)
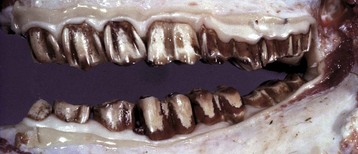
Lesions Caused by Attrition and Abnormal Wear
Age-associated dental wear results in improper mastication of feedstuffs and malnutrition. This condition occurs most commonly in horses and is referred to as “step-mouth” or “broken mouth.” (Courtesy College of Veterinary Medicine, University of Tennessee.)
Miscellaneous Dental Lesions
Dental Neoplasia
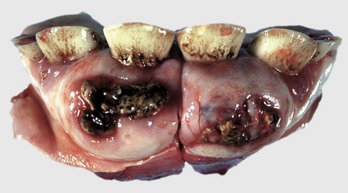
This is a hamartoma (a benign tumorlike nodule) of the enamel organ that in this case has expanded bilaterally on the rostral mandibles. There is extensive hemorrhagic ulceration over the tumor. Diagnosis can be confirmed by radiographic and histopathologic examination. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Tonsils
Portals of Entry
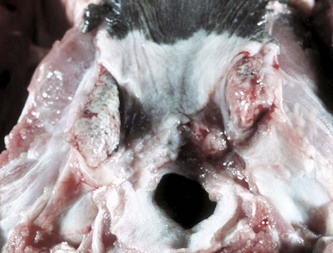
The palatine tonsils are enlarged and discolored. The right tonsil is covered by a diphtheritic membrane, and the left tonsil is extensively ulcerated. Because there are no afferent lymphatics to the tonsils, infection is either primary (by direct spread) or hematogenous. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
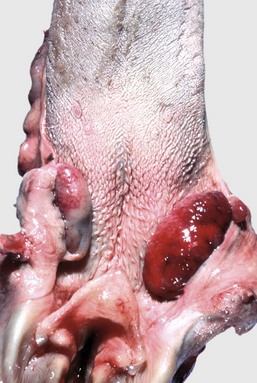
Proliferation of malignant lymphocytes has expanded the tonsils so that they now protrude beyond their crypts. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Salivary Glands
Miscellaneous Diseases or Conditions
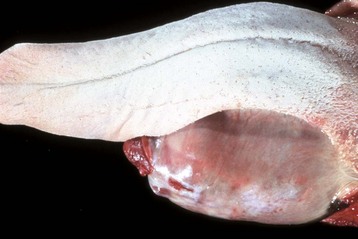
This is a cystic distention of the left mandibular salivary duct along the ventral-lateral aspect of the tongue. (Courtesy Dr. P. Stromberg, College of Veterinary Medicine, The Ohio State University.)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity