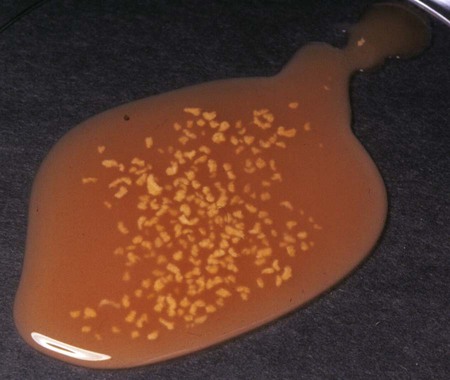
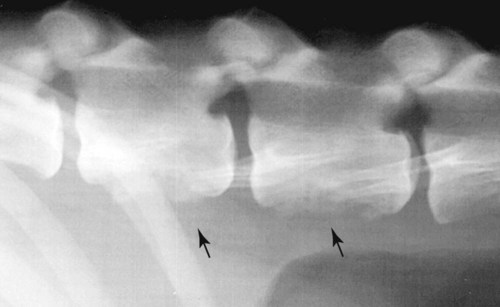
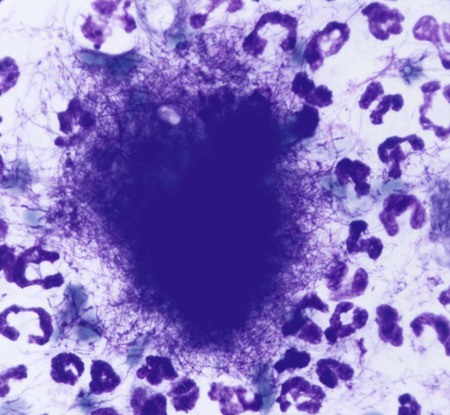
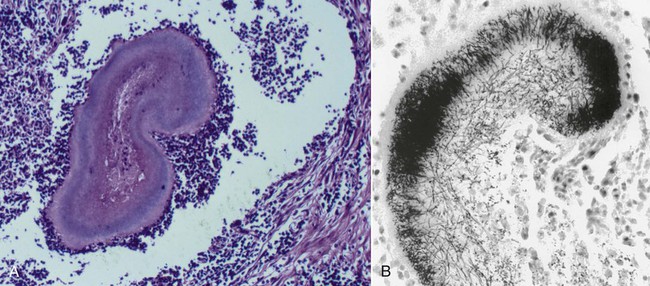
Actinomycosis is a slowly progressive infection characterized by pleural and peritoneal exudates, dense fibrous masses, frank abscesses, meningoencephalitis/meningitis, and fistulous tracts with draining sinuses. Actinomycosis may be refractory to or recur after short courses of antimicrobial therapy. Most Actinomyces infections are complicated by mixed infections with other bacteria. Diagnosis of actinomycosis may be challenging, because organisms may be difficult to isolate from lesions. Fibrous masses are frequently mistaken for neoplastic lesions, although occasionally neoplastic lesions can underlie Actinomyces infections.2 In the human literature, actinomycosis has been referred to as the “most misdiagnosed disease.”42 Actinomycosis is caused by anaerobic or microaerophilic bacteria, mostly belonging to the genera Actinomyces and Arcanobacterium, which are normal inhabitants of mucous membranes, especially of the oropharynx, but also the genital and gastrointestinal (GI) tracts. Some of the former members in the family Actinomycetaceae, such as A. pyogenes and A. bernardiae, have been reclassified into the closely related genus Arcanobacterium. The organisms have never been cultured from the environment.148 Together with other bacteria such as streptococci, actinomycetes colonize the periodontal mucosal surfaces and adhere to the tooth surface to form plaque.57,190 Actinomyces viscosus, Actinomyces hordeovulneris, Actinomyces canis, Actinomyces bowdenii, Actinomyces odontolyticus, Actinomyces coleocanis, Actinomyces israelii, Actinomyces bovis, and Actinomyces naeslundii have been cultured from the saliva and dental plaque of dogs, and Actinomyces coleocanis has been cultured from the canine vagina.57,81 A. viscosus, A. hordeovulneris, and Actinomyces denticolens have been cultured from normal feline gingiva.112 These organisms are not normally pathogenic, but if Actinomyces spp. are inoculated into tissues, along with associated bacteria, an insidious pyogranulomatous disease can develop in virtually any location. Isolates from dogs with actinomycosis have included A. bowdenii, A. canis, A. hordeovulneris, A. odontolyticus, A. viscosus, Actinomyces catuli, Actinomyces turicensis, and Arcanobacterium pyogenes.* A. bowdenii, A. viscosus, Arcanobacterium pyogenes, and Actinomyces meyeri have been recovered from infected cats.17,18,110,111,134 The most common species infecting humans is A. israelii.148 Actinomycosis occurs most commonly in young adult to middle-age large-breed dogs, especially retriever and hunting breeds, that have access to the outdoors.74,93 Both males and females may be affected, with the median age being approximately 5 years.170 Actinomycosis in outdoor dogs is related in large part to exposure to plant material, especially grass awns.24,59,61,84,130 Inhaled or ingested awns are contaminated in the oropharynx, after which they migrate to various sites and act as the nidus of infection. In cats, actinomycosis is often associated with bite wounds and can manifest as pyothorax, peritonitis, or cellulitis.110,170 As a result, actinomycosis is more commonly diagnosed in male cats. Because of the difficulty in culturing Actinomyces species and their susceptibility to many antibacterials, the prevalence of actinomycosis in dogs and cats has probably been underestimated. Actinomyces spp. are opportunistic pathogens that depend on mechanical disruption of the normal mucosal barrier, or inoculation into tissues at a site distant from the oropharynx through licking or biting. The disease characteristically spreads by direct extension and is unimpeded by normal tissue planes; however, hematogenous dissemination can also occur.85 The most common clinical forms of actinomycosis in cats and dogs involve the cervicofacial region, thorax, abdomen, and subcutaneous tissue. Infection of the cervicofacial region can develop from bite wounds, perforation of the oropharynx by a foreign body, or chronic periodontal disease. Pulmonary infections can develop by aspiration of oropharyngeal material, which may include inhalation of a contaminated grass awn. Preexisting lung disease, particularly neoplasia, can act as a nidus for infection.52 Alternative routes of thoracic infection include involvement of the mediastinum from esophageal perforation and direct extension of cervicofacial or abdominal disease. Central nervous system (CNS) infections develop from hematogenous dissemination from a primary site, or meningitis may result from extension of infection from a parameningeal focus, such as the middle ear, sinus, or cranial osteomyelitis.13,43 Risk factors for CNS actinomycosis in humans include dental caries; recent tooth extraction; head trauma; GI tract surgery; chronic otitis, mastoiditis, or sinusitis; tetralogy of Fallot; and intrauterine device infection with Actinomyces spp.158 CNS actinomycosis has been reported in dogs in association with concurrent otitis media/interna165 or head trauma,6 and in cats in association with retrobulbar abscessation13 and cutaneous abscesses of the tail base.17,164 Rarely, infections of the urinary bladder,18,49 gallbladder,75,133 and heart valve85,118,118 have been recognized in humans and also in cats and dogs. Actinomyces spp. are usually accompanied by so-called companion microbes, which help to undermine normal host defenses and reduce oxygen tension. As a result, the pathogenicity of Actinomyces spp. is dramatically increased in mixed infections. The associated bacteria are commensal organisms, especially anaerobes, from the oral cavity or intestinal tract. It is difficult to reproduce actinomycosis through inoculation of pure cultures of Actinomyces spp. alone. Actinomyces spp. that possess fimbriae bind to specific cell surface receptors on other bacteria, especially streptococci. This interspecies bacterial coaggregation markedly inhibits neutrophil phagocytosis of and bactericidal activity against the bacterial complex.132 Rarely, co-infections with Mycobacterium spp. and Actinomyces spp. may occur in humans145,174 and also in dogs.170 Underlying neoplasia may also create an anaerobic environment that favors growth of Actinomyces spp. The organisms initially incite an acute inflammatory response through induction of neutrophil chemotaxis, activation of macrophages, and stimulation of B-lymphocyte hyperplasia. These bacterial-cellular interactions produce the characteristic actinomycosis lesion—a dense mat of Actinomyces spp. and associated organisms surrounded by neutrophils, macrophages, and plasma cells. Proteolytic enzymes from the associated bacteria, macrophages, and degranulated neutrophils destroy connective tissue, facilitating extension of the disease through normal tissue planes.190 Organisms conglomerate to form “sulfur granules,” tan to yellow colonies of actinomycetes, which may be microscopic or visible grossly. In some cases, the inflammatory reaction is accompanied by extensive fibrosis. Grossly, nodular lesions or masses may be seen, which can be mistaken for neoplasia. The center of the lesions may eventually suppurate and soften, or draining tracts may develop. The tracts may close and reappear over weeks to months.148 Cervicofacial actinomycosis produces acute to chronic subcutaneous soft tissue swellings, abscesses, or mass like lesions in the head or neck region.46,52,130,168 The mandibular, submandibular, and ventral or lateral cervical areas are most frequently affected, but infections involving the face, retrobulbar space, and temporal area have been reported. The lesion can be fluctuant or firm, may be indurated, can have draining sinuses, or rarely be ulcerated. Involvement of the muscles of mastication may occur. Pain and fever are variable. Radiographically, adjacent bone can have periosteal new bone formation, and a chronic infection may be characterized by osteomyelitis. Ultrasonography and magnetic resonance imaging can be useful to identify linear grass awn foreign bodies.60,161 Material aspirated from fluctuant masses or discharged from sinuses appears serosanguineous to purulent and may contain “sulfur granules.” Despite their name, the color of these granules can vary from white to tan to gray (Fig. 47-1). Aspiration of firm lesions is often unrewarding. Ocular infections, including keratitis and endophthalmitis, have also been reported in dogs.12,98 Cutaneous-subcutaneous actinomycosis is characterized by one or more soft to firm masses, which may have a draining sinus.46,61,93,130,168 These infections may involve the lateral thoracic wall, flank region, and occasionally the limbs.25,74 Cutaneous lesions involving the thoracic or abdominal walls may represent extensions of thoracic, abdominal, or retroperitoneal actinomycosis. Lameness, mass lesions with draining sinuses, and periosteal new bone formation are characteristic of limb involvement. Actinomycosis involving the thoracic or abdominal cavity is characteristically chronic and progressive, but occasionally may be acute. Weight loss, sometimes severe, and fever may occur. Thoracic actinomycosis may be limited to the lung parenchyma but can involve multiple structures within the thorax, including the mediastinum, pleura, pericardium, and chest wall.* Clinical signs include coughing and less commonly hemoptysis, tachypnea, decreased lung sounds (as a result of pyothorax or the presence of a mass lesion), and subcutaneous soft tissue masses on the lateral thoracic wall. Sometimes there is a history of neck pain, gagging, or hypersalivation before the onset of respiratory signs, which likely relates to the presence of a penetrating foreign body. Thoracic wall masses may develop a draining sinus. Radiographically, pulmonary involvement may manifest as interstitial or alveolar infiltrates, sometimes with consolidation. Air bronchograms may be seen within mass lesions, suggesting the presence of a nonneoplastic process. Occasionally, pneumothorax is documented.170 Variable findings include pleural thickening, pleural effusion (often loculated), widening of the mediastinum, mass lesions, enlargement of the cardiac silhouette (with pericardial involvement), and periosteal new bone formation or osteomyelitis involving adjacent ribs, vertebral bodies, or sternebrae. Pericardial mass lesions may be mistaken for peritoneopericardial diaphragmatic hernias on echocardiography. Clinical features of abdominal actinomycosis include palpable intra-abdominal masses and abdominal distention due to ascites.35,55,63,74 Radiographic and sonographic abnormalities include variable amounts of peritoneal effusion and mass lesions that incorporate or displace adjacent structures. The ascites often appears flocculent on abdominal sonography. Retroperitoneal actinomycosis may be manifested by spinal pain, sometimes with rear leg paresis or paralysis.46,61,84,96 A subcutaneous mass with a draining sinus involving the caudal thorax or flank area is often present. Radiographic findings include periosteal new bone formation involving the ventral aspects of two or three adjacent vertebral bodies (usually T-13 through L-3); involvement of disk spaces is uncommon (Fig. 47-2). Sometimes this new bone formation may be detected during abdominal ultrasound. Ultimately, osteomyelitis and compression fractures of the vertebral bodies may develop. Actinomycosis of the brain and meninges in dogs can also occur, although it is uncommon.6,43,43 Clinical findings associated with brain infections are nonlocalizing and include altered behavior, decreased consciousness, cervical pain, vision loss, ataxia, tetraparesis, abnormal reflexes, and seizures. The results of magnetic resonance imaging may reveal meningeal thickening, contrast-enhancement, and intraparenchymal contrast-enhancing mass lesions.170 Cystitis in dogs has been associated with Arcanobacterium pyogenes and a Nocardia-like organism, which most likely represents A. turicensis, a common isolate from urogenital tract infections.18,83,86,149 Actinomycotic endocarditis has been reported in dogs.85,170 In human patients, actinomycotic endocarditis is frequently associated with thromboembolic complications.118 Pyothorax is perhaps the most common disorder in cats from which Actinomyces spp. are isolated, followed by subcutaneous bite wound abscesses, peritonitis, and meningitis.110,111 Abscesses may have a malodorous, yellow to sanguineous exudate, and Actinomyces spp. are generally mixed with one to five other pathogens. Intra-abdominal masses have been reported in cats.90,155 Involvement of the cervicofacial region,36,104,104 thoracic cavity,77 and subcutaneous tissue with extension to the spinal canal17,164 has been reported. Otitis externa associated with Arcanobacterium pyogenes infection has been reported in a cat.18 Underlying retroviral infection is uncommonly reported and does not appear to predispose to disease. Hematologic test results in animals with actinomycosis vary according to the location, extent, and duration of disease. Animals with extensive, chronic disease have mild to moderate nonregenerative anemia, leukocytosis with a mild to moderate left shift and monocytosis, hypoalbuminemia, and hyperglobulinemia, which may be marked. Dogs with body cavity effusions may be hypoglycemic. Aspirates of abscesses or effusions transtracheal or bronchoalveolar lavages, and samples of cerebrospinal fluid (CSF) often reveal a suppurative to pyogranulomatous inflammatory response (greater than 75% neutrophils). Total protein in pleural fluid is generally above 3.0 g/dL, with erythrocyte and nucleated cell counts often exceeding 70,000 cells/µL. The protein and cell content of abdominal fluid tends to be slightly lower. The result of CSF analysis in dogs and cats with cerebral actinomycosis typically yields elevated protein concentrations and highly cellular fluid that may resemble pus grossly. Aspirates of firm masses may yield only blood. In some specimens, especially those from effusions, sulfur granules are visible macroscopically or microscopically (see Fig. 47-1). Organisms are not always identified within pleural, peritoneal, or CSF samples. When present, the filamentous rods suggestive of Actinomyces spp. may be present, or other “companion” microorganisms may be visualized. Sometimes, mixed bacterial populations containing rods and cocci may be seen. The actinomycetes are gram-positive, non-acid-fast filamentous organisms that are occasionally branched (Fig. 47-3). The filaments are less than 1 µm wide, vary considerably in length, and can stain irregularly, producing a beaded appearance.23,35,58,74,93 Nocardia spp., Corynebacterium spp., and Filifactor villosus can be confused with Actinomyces species.111,180 Actinomyces spp. either are facultative (A. canis, A. catuli, A. coleocanis, A. bowdenii, A. denticolens, A. hordeovulneris, A. naeslundii, A. odontolyticus, A. viscosus, Arcanobacterium pyogenes) or obligate (A. bovis, A. israelii, A. meyeri) anaerobes.21,80–82,134 Specimens should be collected and processed anaerobically and cultured on blood agar or enriched thioglycolate media in the presence of 5% to 10% carbon dioxide. Species that are facultatively anaerobic are variably aerotolerant and can grow aerobically. A. viscosus grows best in aerobic conditions. All species cultured aerobically require carbon dioxide, except A. bowdenii, A. naeslundii, and A. odontolyticus.78 Submission of samples for both aerobic and anaerobic culture in patients suspected to have actinomycosis is recommended. Tissue, pus, or sulfur granules represent ideal specimens for anaerobic culture, and swabs should be avoided if possible.148 The laboratory should be alerted if actinomycosis is suspected. Growth of Actinomyces spp. can be observed within 48 hours but usually requires 5 to 7 days. It may be necessary to hold plates 2 to 4 weeks. Colonies on blood agar are flat to convex, circular with entire or irregular margins, and translucent to opaque and white; surfaces are smooth and moist or rough (bread crumb or molar tooth surface). Some strains of A. israelii produce aerial filaments, resulting in a powdery or cotton-ball appearance. Actinomyces spp. are heterogeneous, and species identification using traditional biochemical tests is difficult.152 Sequencing of the 16S rRNA gene may be necessary for precise species identification. Results of 16S rRNA gene sequence analysis of previously identified species indicates that several genera in addition to Arcanobacterium and Actinobaculum will emerge from the species now classified in the genus Actinomyces.37,71,71 Variants of A. hordeovulneris that are cell wall deficient have been produced in culture, suggesting that L-forms of Actinomyces spp. may be associated with clinical disease; however, because of special culture requirements, these variants would be isolated infrequently.29 In addition to actinomycetes, one to five other associated bacteria are often recovered from properly handled specimens. The most commonly isolated organisms are resident flora of the oral cavity or intestinal tract and include Fusobacterium spp., Peptostreptococcus spp., Prevotella spp., Bacteroides spp., Pasteurella multocida, Escherichia coli, and Streptococcus spp.. Most of the associated bacteria are facultative or obligate anaerobes; therefore, isolation requires appropriate specimen handling. Unfortunately, the growth of a mixed microflora can impair the isolation of Actinomyces.61 Occasionally pure cultures of Actinomyces spp. are isolated. Actinomycosis is characterized by a poorly defined, often indurated mass that incorporates adjacent structures.* The mass may contain one or more pockets of a reddish-brown exudate. Fistulas and sulfur granules may be found. Thoracic and abdominal infections produce a diffuse, red, velvety to granular thickening of the parietal pleura and peritoneum and omentum. The visceral pleura and peritoneum may be less affected. A variable amount of a reddish-brown exudate that may contain sulfur granules is present. Sulfur granules may be identified when pus is poured down the side of a glass tube, to which the granules tend to adhere.148 Lung involvement is usually localized and may appear as a consolidation or mass; infrequently, multiple pulmonary nodules are present. Masses can affect multiple internal structures (e.g., pericardium, mediastinum, lung, diaphragm, and chest wall) and produce an external subcutaneous swelling that may have a draining sinus. With abdominal disease, only one organ may be affected (e.g., liver), but typically a mass or masses involve multiple adjacent structures.35,55,55 Subcutaneous masses in dogs are frequently an extension of cervicofacial, thoracic, or retroperitoneal disease.46,61,61 The histologic reaction to Actinomyces infection is characterized by a core of neutrophils encapsulated by fibrosing granulation tissue. The granulation tissue contains macrophages, plasma cells, and lymphocytes in a dense, fibrous tissue matrix. Demonstration of sulfur granules in tissues is most helpful for diagnosis of actinomycosis. Granules are generally centrally located but can be very difficult to find, so multiple tissue sections may be needed to confirm the diagnosis. When associated with appropriate clinical signs, identification of true actinomycotic granules is diagnostic of actinomycosis. In tissue sections stained with hematoxylin and eosin (H&E), the granules appear as round, oval, or scalloped amphophilic solid masses with an outer basophilic band (Fig. 47-4, A). The granules vary in size (from 30 to 3000 µm in diameter) and often are rimmed by partially confluent radiate, eosinophilic serrate, or club-shaped structures known as the Splendore-Hoeppli phenomenon. This phenomenon is not specific for actinomycosis, and has also been reported with phycomycosis, sporotrichosis, parasitic infections, foreign body reactions, and hypereosinophilic syndrome.141,172 Antigen-antibody complexes, complement, and major basic protein of eosinophils have been detected within the Splendore-Hoeppli phenomenon. Neutrophils frequently contact or appear enmeshed in the material. Individual actinomycete filaments are not delineated by H&E stain, whereas special stains, such as Gram stains (i.e., the Brown-Brenn procedure), Giemsa stains, and silver stains reveal clumps of tangled, intermittently branched, thin (less than 1 µm in diameter) filaments that are gram-positive and slightly beaded at the periphery of the granule (Fig. 47-4, B). Gram-positive or gram-negative nonfilamentous bacteria can be mixed with the Actinomyces spp. Actinomyces are non–acid-fast when stained by the Fite-Faraco modification of the Ziehl-Neelsen technique, which uses a weaker decolorizing agent of 1% sulfuric or 1% hydrochloric acid. With the rare exception of some Nocardia spp., other fungi and bacteria that produce tissue granules can be reliably distinguished from Actinomyces by tinctorial and morphologic properties.33 Features of nocardiosis that distinguish it from actinomycosis are listed in Table 47-1. TABLE 47-1 Comparison of Actinomycosis Versus Nocardiosis Successful treatment of actinomycosis involves prolonged administration of antibacterials; the role of surgery varies with the form of the disease. High doses of penicillin given for prolonged periods (weeks to months) is the treatment of choice (Table 47-2).101 No strains of Actinomyces spp. have shown in vitro resistance to easily attainable serum concentrations of penicillin, and acquired resistance in vivo has not been confirmed. Poor drug penetration of the dense granulomatous tissue reaction necessitates the prolonged, high-dose therapy. A minimal dose of penicillin G (benzylpenicillin) or penicillin V (phenoxymethylpenicillin) of 40 mg/kg every 8 hours is recommended.55 Units of penicillin equivalency per milligram depend on the formulation (see the Drug Formulary in the Appendix). In human patients with actinomycosis, high doses of penicillin are given parenterally for 2 to 6 weeks, followed by oral therapy with amoxicillin for 6 to 12 months. If the animal is stable clinically, oral therapy can be tried from the outset.55,129 Because food reduces the absorption of most penicillins, medication should be given 1 hour before or 2 hours after feeding. Therapy must be extended significantly (weeks to months) beyond resolution of measurable disease to prevent relapse; in some cases, treatment can exceed 1 year.55,74 For animals that develop adverse drug reactions to penicillins, other appropriate choices include erythromycin, clindamycin, doxycycline, chloramphenicol, and ceftriaxone.101,156,156 Anecdotal success with ciprofloxacin was reported in a human patient with recalcitrant actinomycosis of 20 years’ duration115; however, Arcanobacterium bernardiae and Actinomyces neuii are resistant to ciprofloxacin, and in vitro resistance to fluorinated quinolones was reported for an Actinomyces isolate from a dog with thoracic disease.21,51 Oxacillin, dicloxacillin, cephalexin, metronidazole, and aminoglycosides have poor in vitro activity against most Actinomyces spp.; however, Arcanobacterium pyogenes is sensitive to aminoglycosides (other than streptomycin) but resistant to tetracycline, minocycline, and doxycycline.69 A poor or incomplete response to penicillin may be attributable to inadequate doses, or poor surgical drainage and failure to eliminate associated bacteria.101 Infections with these organisms usually resolve with penicillin, but on occasion they require broader spectrum antibacterials during the initial treatment period followed by long-term administration of penicillin. Cats with pyothorax or subcutaneous abscess that have not developed a granulomatous tissue reaction often can be cured with drainage and a shorter duration of antibacterial treatment. TABLE 47-2 Drugs Used to Treat Actinomycosis in Dogs and Cats B, Both dog and cat; C, cat; D, dog; IM, intramuscular; IV, intravenous; PO, by mouth; SC, subcutaneous. aSee the Drug Formulary in the Appendix for more information on these drugs. bDose per administration at specified interval. For duration, see text. cGive at least 1 hr before or 2 hr after feeding to facilitate GI absorption. dMinimum recommended dose (see text); 1 mg = 1600 U (see the Drug Formulary in the Appendix). Surgery has a controversial role in the treatment of actinomycosis. In human medicine, an initial attempt to control disease with medical therapy alone has been suggested, with surgical therapy if there is an inadequate response to therapy.148 Draining of abscesses and effusions (thoracic, abdominal, and pericardial) should always be used as an adjunct to antibacterial treatments.74,77,77 Continuous suction and intermittent drainage techniques have been used for thoracic effusions in dogs.59,178 Drain tubes are removed when the purulent exudate changes to a serosanguineous transudate, usually within 4 to 10 days. Complications of drainage include pneumothorax and subcutaneous abscess formation at the drain tube insertion site. Animals not responding to drainage and appropriate antibacterial therapy warrant exploratory surgery.59,146,146 In animals with pulmonary abscesses, diseased lung lobes may require removal. With diffuse disease involving body cavities, tissue resection should be restricted to decrease the chance of death. The characteristic invasive fibrotic lesions obliterate tissue planes, preventing conservative dissection, and the tissue is well vascularized; therefore, moderate to severe bleeding is common. In dogs with solitary masses involving the thoracic and abdominal walls, radical surgical excision has a high cure rate, although repeat surgeries may be needed.59 The masses often can be reduced and better defined by an initial period of antibacterial therapy. Frequently, grass florets or awns are found in these lesions and during surgical exploration of diseased retroperitoneal regions.59,60,84,161 Surgery should never be performed in lieu of and should always be followed by appropriate antibacterial therapy. Appropriate treatment of dogs with actinomycosis, which can involve extremely prolonged use of antibacterials and surgery, results in a cure rate of greater than 90%.55,59,59 Cutaneous and thoracic disease in cats has a reasonable chance of cure depending on the financial resources of the owner and ability to medicate the cat. Cure rates with meningitis/meningoencephalitis and advanced thoracic or peritoneal disease with extensive mass lesions may be lower. No reports exist of actinomycosis being transmitted from clinically infected animals to humans or other animals; however, humans bitten by dogs, cats, or other people can develop actinomycosis (see Chapter 51).142 Animal care workers handling infected tissues or discharge should wear protective gloves to avoid inadvertent contact by inoculation or through damaged skin. Nocardiosis is a suppurative to granulomatous, localized or disseminated bacterial infection caused by aerobic actinomycetes that are members of the family Nocardiaceae.14,15 In contrast to Actinomyces spp., which are commensals of mucous membranes, Nocardia spp. are ubiquitous soil saprophytes that degrade organic matter and are also found in fresh and salt water, in dust, and on decaying plants and fecal matter.14,26,26 They may be carried mechanically on the claws or skin. Infections are considered opportunistic and are acquired via inhalation of organisms or inoculation through puncture wounds. Transfer of infection between animals does not occur. The species belong to the family Nocardiaceae, which in turn belong to the order Actinomycetales. In contrast to mycobacteria, they possess short-chain mycolic acids and exhibit characteristic branching on Gram staining. Traditional laboratory methods for identification of Nocardia spp. that rely on biochemical reactions and hydrolysis tests do not adequately distinguish between Nocardia spp. Historically, differentiation of species has also been based on antimicrobial susceptibility testing. The application of 16S rRNA gene sequencing to Nocardia species has expanded the number of species to more than 50, approximately half of which are human and animal pathogens, although the validity of some species is debated.26 The advent of molecular methods has led to extensive revision of the taxonomy of Nocardia spp. Many isolates previously identified as belonging to the Nocardia asteroides complex were misclassified before the advent of molecular testing, and in fact the name N. asteroides may ultimately disappear from the literature. Species belonging to the former N. asteroides complex are now considered distinct species and include Nocardia cyriacigeorgica (formerly N. asteroides sensu stricto), Nocardia abscessus, Nocardia nova, Nocardia farcinica, and Nocardia otitidiscaviarum.26 Different Nocardia spp. differ with respect to their antimicrobial susceptibility patterns, epidemiology, and pathogenicity. Nocardiosis is less frequently reported in dogs and cats than actinomycosis, although cases are increasingly recognized in both human medicine and companion animals in association with an increasing number of immunocompromised hosts, including those receiving immunosuppressive drug therapy.160 In one veterinary teaching hospital, the prevalence of nocardiosis in dogs was The prevalence of nocardiosis and the infecting species may also vary geographically. In the United States, nocardiosis has been most commonly reported in humans from the southwestern part of the country. It was hypothesized that the dry, dusty, and windy conditions in these areas may facilitate aerosolization and dispersal of nocardiae.166 Nocardia brasiliensis is the most common species infecting humans in tropical locations such as southwestern United States, Central and South America, and Australia. N. otitidiscaviarum was the most common species isolated in dogs from Brazil144 and has also been isolated from a cat from Spain.113 Dogs and cats from Australia and the western United States are most commonly infected with Nocardia nova.49,116,116 Nocardia africana and Nocardia elegans have been isolated from cats in Japan,73,76 N. brasiliensis infections have been reported in both dogs and cats.3 N. cyriacigeorgica has been isolated from a cat, and N. farcinica from both dogs and cats in Australia.* An organism resembling Nocardia tenerifensis was detected in a cat in Indiana.140 More species are likely to be documented in dogs and cats in the future. In a survey of 53 dogs diagnosed with nocardiosis from California before 1983, males were infected three times more frequently than females; 65.4% of the dogs were younger than 1 year and 82.7% were younger than 2 years; 26.9% had an underlying condition, most often canine distemper.15 Canine distemper was diagnosed in 5 of 9 dogs with nocardiosis from Brazil.144 Apparently in dogs, as in humans, predisposing factors (i.e., diseases) increase susceptibility to nocardiosis. Approximately 60% of people with nocardiosis have underlying immunosuppressive disorders.160 These include human immunodeficiency virus infection, chronic obstructive pulmonary disease, autoimmune diseases requiring immunosuppressive drug therapy, solid organ transplantation, metabolic disorders (e.g., diabetes mellitus), and neoplasia (especially lymphosarcoma and leukemia).14,122,122 Clusters of infection have been documented in human oncology and transplant patients visiting health care facilities relating to common exposure to a source of organisms in dust or on contaminated fomites.26,160 Nocardiosis has been documented in dogs receiving ciclosporin, multidrug immunosuppressive therapy, and a dog with lymphoma.115a,135a,170 In reported cases of feline nocardiosis, there is a striking male predisposition, with more than 75% to 80% of cases in males.* Ages have ranged from 5 months to 16 years, with a median age of 10 years in one study.116 A breed predilection has not been identified. A majority of infected cats have draining wounds or abscesses that are associated with scratches or bite wounds,54,116 which may explain the strong male predisposition for this disease. Some cats have underlying disorders that may predispose them to nocardiosis, including a history of renal transplantation, underlying retroviral infection, or glucocorticoid administration. Others have no obvious underlying immunosuppressive disease. The pathogenesis of pulmonary nocardiosis is similar to that of the deep mycoses. Branching nocardial filaments can fragment late in the growth cycle, resulting in the formation of small, unicellular particles that can be easily aerosolized and inhaled, possibly in dust. Nocardial lesions develop in alveolar spaces and frequently erode into blood vessels, resulting in systemic spread of the disease. Secondary lesions from systemic spread can develop in any tissue, but especially brain, eye, liver, spleen, lymph nodes, and kidney. Involvement of contiguous structures within the thorax (e.g., pleura, mediastinum, pericardium) may occur. Other solitary extrapulmonary sites of infection are likely caused by localization from a transient bacteremia. The primary source may be an inapparent pulmonary infection.14,122 The pathogenicity of Nocardia spp. is influenced by the strain and growth phase of the organism and host susceptibility. Virulent strains of Nocardia are facultative intracellular pathogens that inhibit phagosome-lysosome fusion, neutralize phagosomal acidification, resist oxidative burst, secrete superoxide dismutase, and alter lysosomal enzymes within neutrophils and macrophages.16,160 These effects are partly related to the content and structure of mycolic acids within the bacterial cell wall, which vary among strains and during the growth phase. The filamentous log phase cells found in the environment can be highly resistant to phagocytosis. Some strains exhibit organ-specific tropism, especially relating to invasion of the brain.14,122 Nocardia spp. are also able to invade blood vessels, with associated vascular necrosis and ischemia.160
Actinomycosis and Nocardiosis
Actinomycosis
Etiology and Epidemiology
Pathogenesis
Clinical Findings
Dog
Cat
Diagnosis
Clinical Laboratory Findings
Bacterial Isolation and Identification
Pathologic Findings
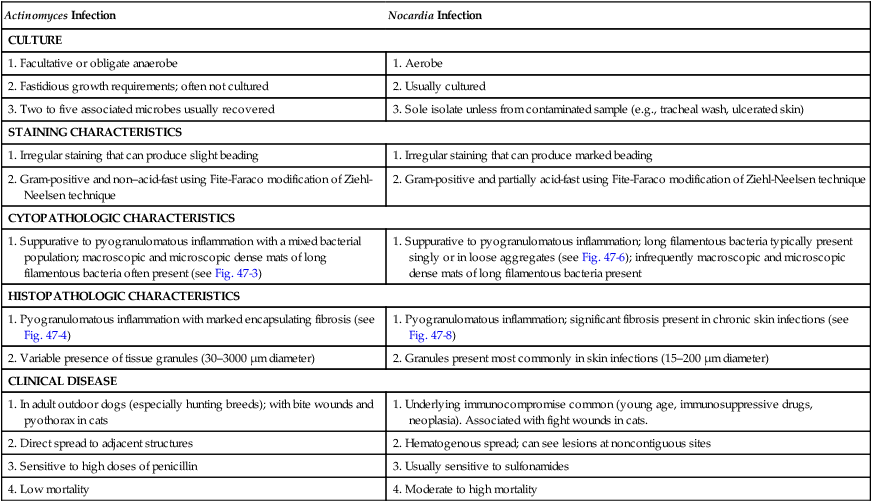
Actinomyces Infection
Nocardia Infection
CULTURE
1. Facultative or obligate anaerobe
1. Aerobe
2. Fastidious growth requirements; often not cultured
2. Usually cultured
3. Two to five associated microbes usually recovered
3. Sole isolate unless from contaminated sample (e.g., tracheal wash, ulcerated skin)
STAINING CHARACTERISTICS
1. Irregular staining that can produce slight beading
1. Irregular staining that can produce marked beading
2. Gram-positive and non–acid-fast using Fite-Faraco modification of Ziehl-Neelsen technique
2. Gram-positive and partially acid-fast using Fite-Faraco modification of Ziehl-Neelsen technique
CYTOPATHOLOGIC CHARACTERISTICS
1. Suppurative to pyogranulomatous inflammation with a mixed bacterial population; macroscopic and microscopic dense mats of long filamentous bacteria often present (see Fig. 47-3)
1. Suppurative to pyogranulomatous inflammation; long filamentous bacteria typically present singly or in loose aggregates (see Fig. 47-6); infrequently macroscopic and microscopic dense mats of long filamentous bacteria present
HISTOPATHOLOGIC CHARACTERISTICS
1. Pyogranulomatous inflammation with marked encapsulating fibrosis (see Fig. 47-4)
1. Pyogranulomatous inflammation; significant fibrosis present in chronic skin infections (see Fig. 47-8)
2. Variable presence of tissue granules (30–3000 µm diameter)
2. Granules present most commonly in skin infections (15–200 µm diameter)
CLINICAL DISEASE
1. In adult outdoor dogs (especially hunting breeds); with bite wounds and pyothorax in cats
1. Underlying immunocompromise common (young age, immunosuppressive drugs, neoplasia). Associated with fight wounds in cats.
2. Direct spread to adjacent structures
2. Hematogenous spread; can see lesions at noncontiguous sites
3. Sensitive to high doses of penicillin
3. Usually sensitive to sulfonamides
4. Low mortality
4. Moderate to high mortality
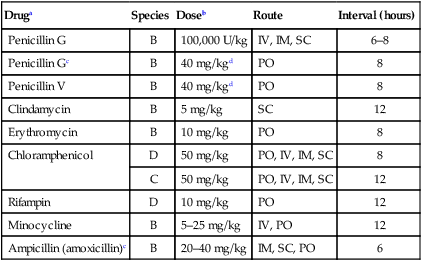
Therapy
Druga
Species
Doseb
Route
Interval (hours)
Penicillin G
B
100,000 U/kg
IV, IM, SC
6–8
Penicillin Gc
B
40 mg/kgd
PO
8
Penicillin V
B
40 mg/kgd
PO
8
Clindamycin
B
5 mg/kg
SC
12
Erythromycin
B
10 mg/kg
PO
8
Chloramphenicol
D
50 mg/kg
PO, IV, IM, SC
8
C
50 mg/kg
PO, IV, IM, SC
12
Rifampin
D
10 mg/kg
PO
12
Minocycline
B
5–25 mg/kg
IV, PO
12
Ampicillin (amoxicillin)c
B
20–40 mg/kg
IM, SC, PO
6
Public Health Considerations
Nocardiosis
Etiology and Epidemiology
 that of actinomycosis.1 In one study, nocardiosis was considerably more prevalent in cats than in dogs,116 but canine infections may be increasingly recognized in association with multidrug immunosuppressive therapy for immune-mediated disease. Where vaccination for canine distemper is inadequate or not widely instituted, nocardiosis in dogs may be associated with immunosuppression as a result of concurrent canine distemper virus infection.144
that of actinomycosis.1 In one study, nocardiosis was considerably more prevalent in cats than in dogs,116 but canine infections may be increasingly recognized in association with multidrug immunosuppressive therapy for immune-mediated disease. Where vaccination for canine distemper is inadequate or not widely instituted, nocardiosis in dogs may be associated with immunosuppression as a result of concurrent canine distemper virus infection.144
Pathogenesis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine