Adequate oxygen delivery to the tissues is critical for life and is of upmost importance during extreme athletic performance. As hemoglobin is the major determinant of the arterial oxygen content (CaO2), a normal hemoglobin concentration is one of the prerequisites for normal performance.1 There is evidence for a strong positive relationship between blood volume, red blood cell volume, and aerobic capacity in humans, dogs, and horses.2,3 Conversely, a decrease in total red blood cell volume will negatively affect aerobic capacity and performance in athletes. Anemia is defined as a decrease in red cell mass leading to a reduction in measured erythrocyte concentration, hemoglobin concentration, and hematocrit below the reference interval.4,5 Anemia is functionally characterized by a reduced oxygen-carrying capacity of the blood.6–8 Several compensatory mechanisms serve to increase tissue oxygenation in the presence of anemia.4,5,9 One of the initial adaptations to anemia is an increase in 2,3-biphosphoglycerate (2,3-BPG; formerly called 2,3-diphosphoglycerate, 2,3-DPG) content of the red blood cells. It is probably caused by a change in intracellular pH, and leads to a decreased oxygen affinity of hemoglobin; oxygen is therefore more readily released to the tissue. Blood viscosity is markedly decreased at lower erythrocyte concentrations,10 while tissue hypoxia activates mechanisms of local blood flow regulation and causes peripheral vessels to dilate.11 This results in a dramatic fall in peripheral vascular resistance, a baroreceptor-mediated sympathetic activation, an increase in cardiac output, and improvement in oxygen delivery to the tissues.4,5,9,12 Individual changes in vascular tone result in redistribution of blood from non-vital areas (skin, intestine) to highly oxygen-dependent tissues (heart, brain, muscles). Finally, diminished oxygen supply to the kidneys leads to increased synthesis of erythropoietin, which accelerates erythropoiesis in the bone marrow and elicits a regenerative response to anemia.5 • It is not associated with acute hemolysis or blood loss. • There is no loss of blood volume. • No other disease processes or conditions (such as infection, fever, severe injury, exercise, agitation, or advanced pregnancy) are present which increase oxygen demand. Free hemoglobin in solution, as present in intravascular hemolysis, exerts strong vasoconstrictor effects due to binding of nitric oxide,13 and has an increased affinity for oxygen due to a loss of 2,3-BPG.14 The resulting decline of effective oxygen delivery and release in the tissue contributes to the pathophysiologic effects of hemolytic anemia. When tissue oxygen demand is increased, or when anemia becomes severe enough to exceed the compensatory capacity of the organism, the degree of oxygen extraction from the blood cannot be increased, and the oxygen uptake by the tissue decreases as the oxygen supply decreases (supply dependent oxygen uptake; Fig. 44.1). The resulting anaerobic metabolism leads to accumulation of lactate and metabolic acidosis.6,7,15 Ultimately, tissue hypoxia and limited metabolic capacity of the affected tissue can lead to tissue damage and organ failure.5,16 Subtle anemia is difficult to detect and has mild clinical effects. Total red cell volume and hemoglobin concentration are correlated with the exercise capacity of a horse.17–19 During exercise, tissue demand for oxygen is increased. Small decrements in red cell mass lower the level of exercise intensity at which tissue hypoxia occurs, and therefore result in reduced exercise capacity.9 More severe anemia is characterized by depression, weakness, tachypnea, tachycardia, hyperkinetic pulses, and pale or icteric mucous membranes. Systolic, functional heart murmurs are frequently associated with anemia. Hemoglobinuria or bilirubinuria can be seen in horses with hemolytic anemia. Cardiac arrhythmias may indicate myocardial hypoxia. Abdominal pain and signs of functional intestinal ileus are likely related to splanchnic ischemia, which can lead to sloughing of the intestinal mucosa, endotoxemia, and bacteremia.15,16 Anemia is diagnosed when hematocrit, hemoglobin concentration, and erythrocyte concentration on a complete blood count (CBC) are below the reference range for the respective breed. The clinical definition of anemia can be problematic, as hydration status and splenic contraction due to exercise, excitement, or stress can also influence the hematologic values. Changes in hematocrit and hemoglobin concentration might therefore not reflect the true change in oxygen-carrying capacity of the blood.4,20 The traditional approach to determine the etiology and pathogenesis of anemia is to establish whether the anemia is regenerative or non-regenerative.21 In horses, assessment of a regenerative response is difficult based on a routine hemogram, because reticulocyte maturation is mostly completed in the bone marrow and release of reticulocytes into the blood is minimal compared to other species, even in response to severe anemia.20,22,23 However, horses under severe erythropoietic stress may release reticulocytes with unique characteristics into the circulation, which may be detected using sensitive modern hematology analyzers.24 Reticulocytes and nucleated red blood cells can be found in cases with severe hemolysis.25 Young erythrocytes are usually larger than normal erythrocytes. Therefore, a regenerative response in most species is characterized by release of macrocytes into the bloodstream, independent of reticulocytosis.21,22 Macrocytosis and anisocytosis may therefore be used as indicators of regeneration in the peripheral blood of horses.21,24 It can be detected by determination of erythrocyte indices (mean corpuscular volume and red blood cell distribution width), red blood cell cytograms and volume distribution histograms, or blood smear evaluation.21 The mean corpuscular volume (MCV) is not very sensitive for detection of abnormal or variable sizes of erythrocytes. Large numbers of macrocytes are necessary to increase the MCV above the normal range.21,22,26,27 Macrocytosis is more pronounced in hemolytic anemia than in anemia due to acute blood loss.28,29 The mixture of pre-existing normocytes and newly produced macrocytes during the recovery period in regenerative anemias leads to anisocytosis.30 The red blood cell distribution width (RDW) is an index of anisocytosis and is calculated by many automated hematology systems as coefficient of variation of the erythrocyte volume distribution.22,27 Significant increases in MCV and RDW are detectable 14 to 20 days after onset of anemia and can persist for several weeks 22,27 Although the RDW might be somewhat more sensitive than the MCV in detecting a regenerative response in horses, its increase is not a consistent indicator of regeneration, and it does not occur in mildly anemic horses.22 Therefore, absence of these two findings does not exclude regenerative anemia. Red blood cell (RBC) cytograms, RBC volume distribution curves, and hemoglobin concentration histograms are more sensitive in detecting changes in erythrocyte size or hemoglobin content, and allow earlier detection of abnormal erythrocyte subpopulations than the routine erythrocyte indices (MCV, RDW).21,22 26,27,31 Most modern automated hematology analyzers will report this information on request (Fig. 44.2). Single hematologic measurements may be difficult to assess. Series of samples can reveal the severity of anemia and the presence of a regenerative response. The nadir of the hematocrit is reached around 2–7 days after acute blood loss and as late as 13–22 days after onset of hemolysis.22,23,27–29 Reported daily increases in hematocrit following hematocrit nadir in regenerative anemias in horses vary between 0.32 and 0.67%, and are slower than reported in other species.22,23 The rate of erythrocyte and hemoglobin production after hemolysis is higher than after acute blood loss, probably because iron and protein is not lost from the body and is readily available for erythropoiesis.20,27,29,30 Concurrent measurements of total plasma protein concentration may also be useful for determination of the type of anemia. Regenerative anemia, associated with a low, increasing plasma protein concentration, is consistent with blood loss, whereas a normal protein concentration more likely indicates hemolysis.20 The mean cellular hemoblogin concentration can be calculated as: MCHC = [Hb] (g/dL) / PCV (%) × 100, where [Hb] is the whole blood hemoglobin concentration and PCV is the packed cell volume. Normal MCHC varies little and equals 32–38 g/dL.32 Intravascular hemolysis causes the PCV to decrease while [Hb] remains unchanged, leading to an increased MCHC. Conversely, iron deficiency anemia causes a decrease in MCHC. Although not routinely used in horses, haptoglobin may be helpful to screen for and monitor intravascular hemolytic anemia. Haptoglobin is an acute-phase protein that is synthesized in the liver.33 It binds free hemoglobin in the plasma and forms a hemoglobin–haptoglobin complex, which is then quickly removed by the reticuloendothelial system, allowing the recycling of iron and globin. A decrease in haptoglobin concentrations is therefore consistent with intravascular hemolysis.34–37 Conversely, in extravascular hemolysis, hemoglobin is not released in the circulation and haptoglobin concentrations remain normal. Haptoglobin concentration in healthy horses may differ depending on breed, age, and laboratory assay (see Table 44.4). Furthermore, haptoglobin concentrations increase in the presence of inflammatory diseases, which may limit its diagnostic value for detection of intravascular hemolysis in some patients. Table 44.4 Normal values for the evaluation of the iron status in horses n.d. = not determined. *Normal values expressed as mean ± SD, where applicable. Blood smear evaluation is an easy and very important procedure in diagnosing anemia. Autoagglutination, alterations in erythrocyte morphology, presence of blood parasites, or the brown color of methemoglobin cannot be accurately detected by automated hematology analyzers.21,38 The physiologic property of equine erythrocytes to aggregate and form rouleaux on the peripheral blood smear (Fig. 44.3) requires special consideration and should be distinguished from pathologic autoagglutination. The latter leads to formation of irregularly shaped erythrocyte clusters of different sizes, whereas rouleaux formation leads to a more orderly association with the erythrocytes lining up in rows. Mixing of the blood with normal saline (in a ratio of 1 part blood to 4 parts saline) will break up rouleaux, but not agglutinated erythrocytes.20,39,40 Wedge smears of EDTA-anticoagulated whole blood can be made using two clean glass slides. The smears are then air dried and stained with Romanowsky-type stains (Wright, Giemsa, Diff-Quick).38 New methylene blue stain can be used to detect Heinz bodies. Normal equine erythrocytes are approximately 5.4 µm in diameter. Changes in erythrocyte morphology may be useful to determine the underlying mechanism of anemia.38 Table 44.1 summarizes common findings in blood smears of anemic horses. Table 44.1 Common findings on peripheral blood smears in anemic horses20,21,38 Bone marrow evaluation is the most accurate method for assessment of a regenerative response in horses. It is indicated in cases for which the hemogram does not adequately provide evidence of normal bone marrow function.21,30,41 Bone marrow aspirate cytology allows evaluation of individual cellular morphology and the use of special stains and procedures, but might not reflect true cellularity of the bone marrow and might be affected by hemodilution. Core biopsy examination is indicated if repeated aspirates are of marginal cellularity, if hypoplasia, aplasia, inflammation, necrosis, or myelofibrosis is suspected, or when megakaryocyte numbers should be determined.42 In some cases only a combination of both techniques provides the desired information. Both procedures are easy to perform on the standing sedated horse and carry small risk of complications. Standard techniques for bone marrow collection, sample preparation, and staining have been described.42–45 For aspirates, a 13 to 15 gauge two-inch needle with stylet is recommended. For core biopsies, a larger needle (11 to 13 gauge) should be used (Fig. 44.4). Several types of bone marrow collection needles are commercially available. An alternative biopsy technique using an electric drilling machine has been described.46 Possible sites for bone marrow sampling in horses are the cranial sternum, tuber coxae, and the dorsal third of the ribs where hematopoietic activity is maintained throughout life. The sternum is most frequently used. The sampling is performed in the ventral midline on an imaginary line between the two elbows. The site is clipped, aseptically prepared, and 5 to 10 mL of a local anesthetic is injected into the subcutis down to the periosteum. A stab incision is then made at the sampling site and the bone marrow needle with stylet is inserted through the skin to the sternum. To take a bone marrow aspirate, the needle is then advanced approximately 1 to 1.5 centimeters with a rotational movement using firm pressure. The stylet is removed, a 10 mL syringe is attached, and aspiration is performed. The risk of extensive contamination of the sample with peripheral blood can be reduced by forceful, rapid aspiration. The suction should be released when blood becomes evident in the syringe. The marrow specimen should be processed immediately to avoid coagulation and to preserve cellular morphology. The sample is transferred through the needle to several glass slides and direct smears are made before the sample clots. Alternatively, bone marrow aspirates can be transferred onto a Petri dish containing 0.5 to 1 mL 2% to 3% EDTA or a citrate–phosphate–dextrose solution for anticoagulation. The marrow particles adhere to the Petri dish and can be easily detected as small, granular, gray particles, even when some blood contamination is present. They are selected and transferred to glass slides using a Pasteur pipette. Bone marrow squash preparations are made using a second slide. The slides are then air-dried. Multiple smears (4 to 10, depending on volume of material obtained) should be submitted for routine stains and for additional special stains. Cytologic examination of bone marrow aspirates or histologic examination of bone marrow biopsies should be assessed together with a complete blood count and differential taken on the same day and, if available, with results from previous CBCs and bone marrow evaluations.21,42,45 Accurate interpretation of bone marrow samples requires training and considerable expertise. Routine bone marrow aspirate cytology is performed using Romanowsky-type stains (Wright, Giemsa, Diff-Quick). These stains allow assessment of particle cellularity; megakaryocyte numbers and maturity; erythroid and myeloid development, maturation, and morphology; and the presence of lymphoid cells, macrophages, stromal cells, and unusual cell populations.45 Normocellular bone marrow particles contain 50% fat and 50% cells (Table 44.2). Hypocellularity is consistent with decreased hematopoiesis, whereas hypercellularity suggests hematopoietic response, infiltrative disease, or neoplasia.45 Normocellular bone marrow with normal-appearing erythroid cells and increased iron deposits in the marrow is seen in anemia of inflammatory disease.21,47 Erythroid hyperplasia, accompanied by a lack of hemosiderin in macrophages, is seen in iron deficiency.21 Aplastic anemia (aplastic pancytopenia) is characterized by peripheral pancytopenia and bone marrow panhypoplasia. The bone marrow space is replaced by adipose tissue.48 Infiltration and replacement of the bone marrow by inflammatory cells, neoplastic cells, or fibrous tissue (myelofibrosis) is referred to as myelophthisis. The marrow usually appears normocellular or hypercellular.48 Myelodysplasia is characterized by morphologic abnormalities in hemic cells. Excessive blast cell content of the bone marrow is indicative for leukemia.21 Severe suppression of only the erythroid cell line is called pure red cell aplasia.48 Table 44.2 Normal and abnormal bone marrow findings used for assessment of anemia in horses PRBC = polychromatophilic erythrocytes ; HPF = high power field (100x oil immersion); M : E ratio = myeloid-to-erythroid ratio. Although subjective microscopic evaluation is a valuable and important method, it is often inadequate to assess regenerative response to anemia. Differential cell counts should be determined to increase the accuracy of quantification of myeloid and erythroid cells.41 The myeloid-to-erythroid ratio (M : E ratio) is commonly used to assess the erythroid compartment. The ratio is determined by counting 200 to 500 cells and dividing the number of cells of the myeloid series by the number of cells of the erythroid series. The normal M : E ratio in horses is between 0.5 and 1.5. A regenerative response leads to a decrease in M : E ratio, whereas a hypoproliferative anemia is characterized by an increased M : E ratio.41,49–51 The regenerative response after acute loss of 25% of blood volume (20 mL/kg) leads to an expansion of the erythroid compartment within the first three days, and the peak erythroid response is reached after nine days, corresponding with the lowest M : E ratio. The regenerative response in horses is prolonged and may be detected for more than one month.41 Coexisting changes in the myeloid compartment can also influence the M : E ratio and should be taken into consideration. Leukocytosis with left shift, as detected on complete blood count, results from augmented granulopoiesis. In this instance an activation of the myeloid series increasing the M : E ratio is to be expected. In addition to the M : E ratio, determination of the number of young erythrocytes in bone marrow is useful to assess erythropoietic activity.52 Anucleate erythrocytes in Romanowsky-stained smears are referred to as polychromatophilic erythrocytes (PRBC). In new methylene blue-stained smears, these cells appear as reticulocytes.45 The presence of more than 5 PRBCs per high power field (100× oil immersion) and more than 3% reticulocytes (up to 66%) in relation to the total red blood cells confirm a regenerative response.20,41,52–54 Prussian blue stain allows semiquantitation of iron storage in the bone marrow. Normal bone marrow particles contain a moderate to large amount of erythroblasts with hemosiderin granula.50 The amount of stainable iron is decreased in horses with chronic blood loss and iron deficiency.51 Normoblasts and macrophages containing iron-rich granules (siderocytes, sideromonocytes) can be seen in horses with hemolytic anemia.55,56 Anemia of inflammatory (chronic) disease leads to increased iron storage,47,50 whereas iron deficiency anemia leads to decreased iron storage in bone marrow.57,58 Determination of the serum bilirubin concentration can be useful in diagnosis of anemia. Bilirubin is a breakdown product of hemoglobin metabolism. Horses frequently develop mild increases in serum unconjugated (indirect, prehepatic) bilirubin concentrations (up to 6–8 mg/dL [103–137 µmol/L]) as a result of anorexia. Larger increases of unconjugated bilirubin concentrations in anemic horses support the diagnosis of hemolysis. The degree of elevation depends on the rate of red cell destruction and the capacity of the liver to conjugate and excrete the newly formed bilirubin.59 Monitoring of the serum creatinine and serum urea nitrogen concentrations is critical in horses with hemolytic anemia, as hemoglobinuria and bilirubinuria can lead to acute tubular nephrosis (pigment nephropathy). Hypovolemia associated with acute blood loss is characterized by prerenal azotemia. Furthermore, moderate non-regenerative anemia can occur secondary to chronic renal failure.59,60 Serum activities of sorbitol dehydrogenase (SDH), other liver-derived enzymes, and bile acid concentrations can be increased with severe acute anemia due to hypoxic or ischemic liver damage or increased iron storage and iron overload with severe hemolytic anemia.59,61 Increased cardiac troponin I serum concentrations can indicate hypoxic myocardial damage. Blood gas analysis and measurement of blood or plasma lactate concentration can be useful for assessment of tissue oxygenation in horses with severe anemia. Metabolic acidosis, increased anion gap, and elevated blood or plasma lactate concentration (i.e., >4 mmol/L) is consistent with tissue hypoxia or ischemia. A low venous or (preferably) mixed venous oxygen partial pressure (pvO2 < 25–30 mmHg) in association with normal pulmonary function and adequate arterial oxygenation (paO2 > 90 mmHg) indicates increased oxygen extraction from the blood secondary to inadequate oxygen delivery (see Fig. 44.1).4,7,62,63 However, the clinical application of these measurements to assess the severity of anemia, tissue oxygenation, and the need of blood transfusions in horses with anemia has not been well established and requires further investigation. Iron exists in several anatomically, chemically, and functionally distinct compartments in the organism.64,65 The functional pool contains approximately 70% of the total body iron. It consists of hemoglobin, myoglobin, and several iron-containing or iron-dependent enzymes. Iron is stored primarily in bone marrow, spleen, and liver, either in a soluble form (ferritin), or as insoluble, aggregated deposits (hemosiderin). Plasma transferrin is the major iron transport protein. The iron content of several of these compartments can be determined to assess a patient’s iron metabolism. Hemoglobin, the largest iron compartment of the body, is most commonly measured and routinely included in complete blood counts. However, hemoglobin is probably the least sensitive variable for detection of iron deficiencies. Overt anemia occurs only in late stages of severe iron deficiency.64,65 Serum iron, ferritin, iron-binding capacity, and evaluation of the iron content of bone marrow are more accurate indicators of the iron status (Table 44.3 and Table 44.4).57,58,64,65 Total serum iron concentration is low with chronic iron deficiency, but it is also decreased in inflammatory disease, hypoproteinemia, and renal disease.66 Serum ferritin concentration correlates well with body iron stores and is a sensitive indicator of iron deficiency.66,67 It is also an acute-phase reactant protein and can increase secondary to inflammation or liver disease, leading to an overestimation of total body iron content. Serum ferritin should therefore be assessed together with a complete blood count and plasma fibrinogen concentration.68 Plasma transferrin is indirectly measured after complete saturation with iron, with the resulting iron content referred to as total iron-binding capacity (TIBC). The TIBC is typically increased in iron deficiency states, but decreased with acute-phase reactions and inflammatory disease. The amount of stainable iron in bone marrow samples correlates well with iron stores69 and is a sensitive indicator for evaluation of the iron metabolism (see above). The amount of marrow stainable iron is decreased with iron deficiency, and increased with inflammatory disease. Table 44.3 Variables used for assessment of iron status in horses 57,58,64,65,68,70 Copper deficiency might be commonly overdiagnosed in horses because of inappropriately high reference ranges available for horses. Normal serum copper concentrations in healthy horses probably vary between 0.5 and 1.5 mg/L (8–24 µmol/L).76–79 Concentrate ration feeding and copper supplementation increase the serum copper concentrations to 1–1.7 mg/L (17–26.6 µmol/L).80 Serum copper concentrations lower than 0.7 mg/L (11.5 µmol/L) can indicate copper deficiency.81 Liver copper concentration and plasma ceruloplasmin activity can be used in addition to serum copper concentration to assess copper status of an animal.77,81,82 Normal copper concentrations in liver tissue vary between 3.2 and 11 mg/kg (51–176 µmol/kg) fresh weight.81,83,84 Mean plasma ceruloplasmin activity in mature Standardbreds has been determined to be 39.1 ± 0.5 IU/L (mean ± s.d.),78 and serum ceruloplasmin concentration in adult horses varies between 4.07 ± 0.41 and 6.06 ± 0.74 mg/mL.85 Acute-phase reactions can increase copper metabolism and cause the plasma copper concentration and ceruloplasmin activity to rise.76,82,85 Complete blood count and plasma fibrinogen concentration should therefore be assessed together with serum copper concentrations to detect falsely elevated values. Data on normal folate and vitamin B12 serum concentrations for horses are limited, and comparison between different studies is complicated by differences in feeding and management regimen. Serum folate concentrations in non-supplemented stabled horses and intensively training Thoroughbreds and Standardbreds (range: 1.5–15.1 ng/mL) are generally lower than in pastured horses (range: 6.4–21.7 ng/mL) or in racehorses receiving dietary folate supplements (range: 2.0–21.1 ng/mL).86–89 Similar differences were found for red cell folate concentrations, with racehorses in training having lower values (35–372 ng/mL) compared to stabled horses (70–630 ng/mL) or horses on pasture (123–986 ng/mL).89 Measurement of red cell folate concentration could be more accurate for the assessment of the folate metabolism of horses.89,90 Serum vitamin B12 concentrations in non-supplemented horses vary between 1.25 and 5.4 µg/L.88,89 Vitamin B12 levels do not seem to be significantly affected by feeding and exercise. Anemia is caused by hemorrhage (blood loss anemia), red blood cell destruction (hemolytic anemia), or lack of erythrocyte production (hypoproliferative anemia).20,21 Blood loss anemias and hemolytic anemias are referred to as regenerative anemias, whereas hypoproliferative anemias are non-regenerative (see Table 44.5). Table 44.5 Causes of anemia in athletic horses Anemia of inflammatory (chronic) disease Immune-mediated hypoproliferative anemia
Abnormalities of the erythron
Anemia
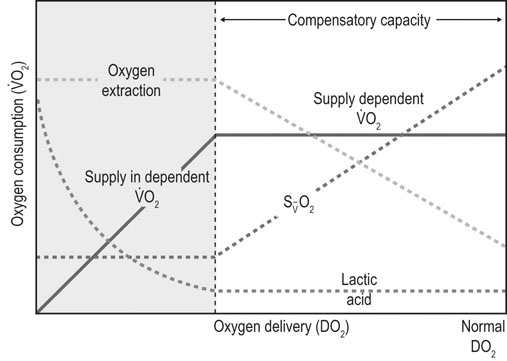
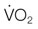 ), and oxygen extraction in the systemic circulation. DO2 can decline either because of a decrease in cardiac output or a decrease in arterial oxygen concentration, which largely depends on hemoglobin concentration and % saturation of hemoglobin with oxygen. As DO2 decreases, a compensatory increase in oxygen extraction in the tissue allows maintaining the
), and oxygen extraction in the systemic circulation. DO2 can decline either because of a decrease in cardiac output or a decrease in arterial oxygen concentration, which largely depends on hemoglobin concentration and % saturation of hemoglobin with oxygen. As DO2 decreases, a compensatory increase in oxygen extraction in the tissue allows maintaining the 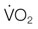 at a constant level (red line). Consequently, the mixed-venous oxygen saturation (
at a constant level (red line). Consequently, the mixed-venous oxygen saturation (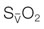 ) decreases, the arterio-venous oxygen difference widens, and the oxygen extraction ratio increases. Once the compensatory capacity is exceeded (shaded area), the level of maximal oxygen extraction is reached and the
) decreases, the arterio-venous oxygen difference widens, and the oxygen extraction ratio increases. Once the compensatory capacity is exceeded (shaded area), the level of maximal oxygen extraction is reached and the 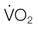 becomes supply dependent, hence decreases parallel to the DO2. Anaerobic metabolism in the tissues then leads to accumulation of lactic acid and metabolic acidosis. Venous (mixed venous) oxygen saturation, blood pH, and blood lactate concentration are clinically useful for assessment of oxygen delivery and oxygen extraction in the tissues.
becomes supply dependent, hence decreases parallel to the DO2. Anaerobic metabolism in the tissues then leads to accumulation of lactic acid and metabolic acidosis. Venous (mixed venous) oxygen saturation, blood pH, and blood lactate concentration are clinically useful for assessment of oxygen delivery and oxygen extraction in the tissues.
Diagnosis of anemia
Recognition
Clinical signs
Hematology
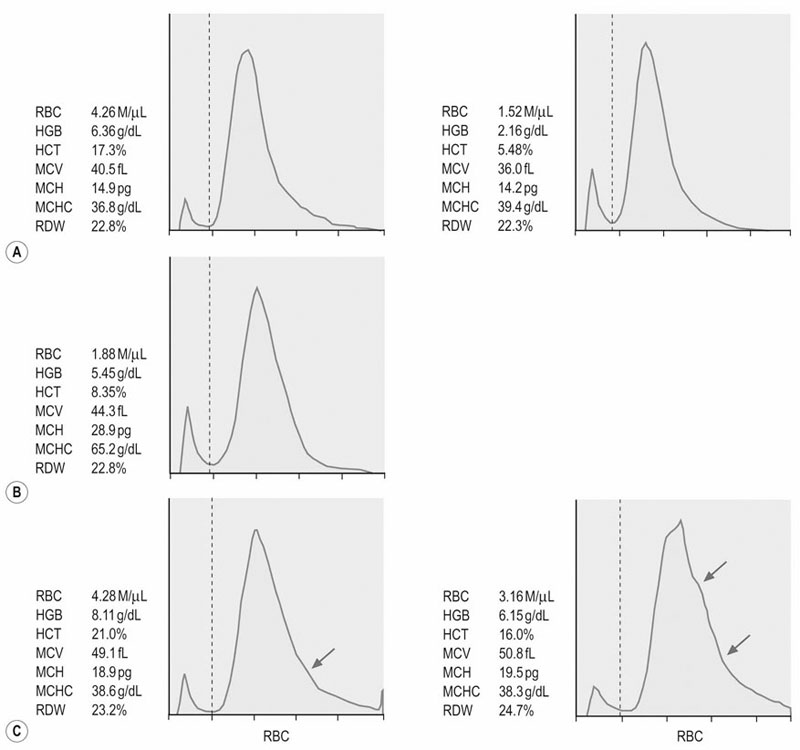
(A) Pure red cell aplasia associated with administration of rhEPO. The RBC indices and erythrocyte volume histograms of two examinations 80 days apart indicate lack of regenerative response despite decreasing hematocrit (PCV).
(B) Heinz body hemolytic anemia due to maple leaf toxicity. RBC indices and erythrocate volume histograms four days after onset of the clinical signs do not indicate regeneration.
(C) Erythrocyte volume histogram of the same horse two days (left) and eight days (right) later. Widening of the right tail of the histograms (arrows) indicate increased numbers of macrocytes consistent with a regenerative response. Note that the MCV and RDW are increasing over time, but do not exceed the upper limit of the normal range. (Normal ranges: MCV 43–55 fL; RDW 19.8–25.4%).
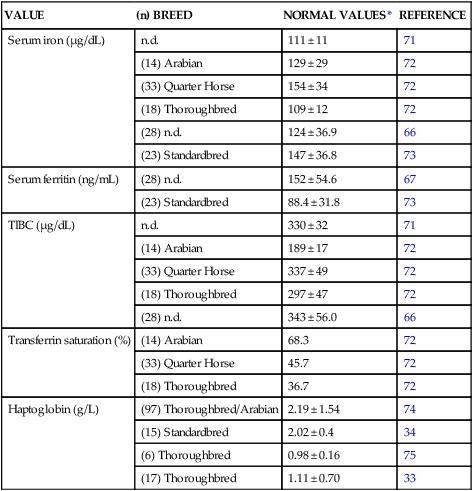
VALUE
(n) BREED
NORMAL VALUES*
REFERENCE
Serum iron (µg/dL)
n.d.
111 ± 11
71
(14) Arabian
129 ± 29
72
(33) Quarter Horse
154 ± 34
72
(18) Thoroughbred
109 ± 12
72
(28) n.d.
124 ± 36.9
66
(23) Standardbred
147 ± 36.8
73
Serum ferritin (ng/mL)
(28) n.d.
152 ± 54.6
67
(23) Standardbred
88.4 ± 31.8
73
TIBC (µg/dL)
n.d.
330 ± 32
71
(14) Arabian
189 ± 17
72
(33) Quarter Horse
337 ± 49
72
(18) Thoroughbred
297 ± 47
72
(28) n.d.
343 ± 56.0
66
Transferrin saturation (%)
(14) Arabian
68.3
72
(33) Quarter Horse
45.7
72
(18) Thoroughbred
36.7
72
Haptoglobin (g/L)
(97) Thoroughbred/Arabian
2.19 ± 1.54
74
(15) Standardbred
2.02 ± 0.4
34
(6) Thoroughbred
0.98 ± 0.16
75
(17) Thoroughbred
1.11 ± 0.70
33
Blood smears
FINDING
DESCRIPTION / APPEARANCE
CAUSE
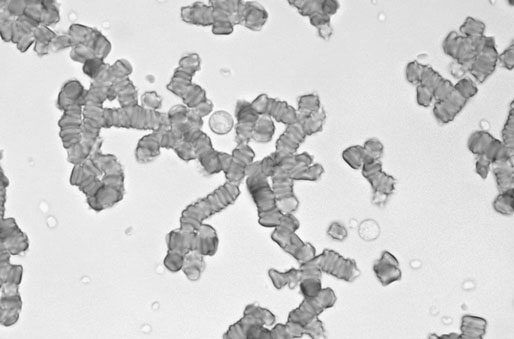
Rouleaux formation
Erythrocytes lining up in rows, very prominent in equine blood
Normal
Howell–Jolly bodies
Round, basophilic inclusion bodies, found near the periphery of the erythrocytes
DNA remnants, found in 0.1% of normal erythrocytes; more often with severe (regenerative) anemia
Heinz bodies
Pale-pink (Romanowsky stain) or dark-blue (new methylene blue stain) round protrusions of the erythrocyte membrane or bodies within the cell
Denaturated aggregates of hemoglobin due to oxidative damage (maple leaf, onion, or phenothiazine toxicity)
Eccentrocytes (‘hemighosts’)
Erythrocytes with small peripheral cytoplasmatic clearing
Alterations in the erythrocyte membrane and condensation of hemoglobin in the remainder of the cell due to severe oxidative damage (maple leaf toxicity)
Schistocytes (erythrocyte fragments)
Small, irregularly shaped erythrocyte fragments with two to four angular or pointed projections
Endothelial damage and alterations in the microcirculation, associated with DIC, neoplasia, or inflammation of very vascular organs (liver, spleen, lung, kidney, bone marrow, placenta)
Autoagglutination
Irregularly shaped erythrocyte clusters of different sizes
Immune-mediated hemolytic anemia, adverse effect of unfractioned heparin, occasionally incidental finding as a result of anti-erythrocyte IgM antibodies present in normal animals
Echinocytes
Uniformly spaced, pointed membrane projections
Crenation artifact, electrolyte depletion
Macrocytosis
Large erythrocytes
Regenerative anemia, prolonged storage of blood
Microcytosis
Small erythrocytes
Iron deficiency, chronic blood loss
Spherocytes
Small round erythrocytes with lack of central pallor (difficult to detect in horses)
Result from partial removal of red cell membrane by macrophages secondary to antibody or complement attachment (immune-mediated) or oxidative damage (maple leaf toxicity)
Parasites
Teardrop-shaped inclusions with two or four (‘maltese cross’) organisms per cell
Babesia caballi or Theileria equi
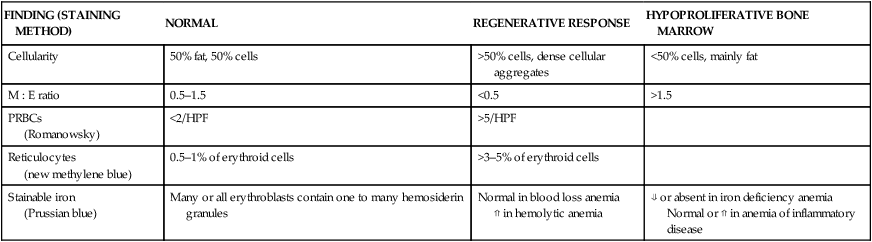
Bone marrow evaluation
FINDING (STAINING METHOD)
NORMAL
REGENERATIVE RESPONSE
HYPOPROLIFERATIVE BONE MARROW
Cellularity
50% fat, 50% cells
>50% cells, dense cellular aggregates
<50% cells, mainly fat
M : E ratio
0.5–1.5
<0.5
>1.5
PRBCs
(Romanowsky)
<2/HPF
>5/HPF
Reticulocytes
(new methylene blue)
0.5–1% of erythroid cells
>3–5% of erythroid cells
Stainable iron
(Prussian blue)
Many or all erythroblasts contain one to many hemosiderin granules
Normal in blood loss anemia
⇑ in hemolytic anemia
⇓ or absent in iron deficiency anemia
Normal or ⇑ in anemia of inflammatory disease
Serum biochemistry
Blood gas analysis and lactate
Assessment of minerals, trace elements, and vitamins
Iron
INCREASED
DECREASED
Serum iron
Hemolytic anemia
Refractory anemia
Iron overload
Liver disease
Iron deficiency
Acute-phase reactions
Chronic inflammation
Hypoproteinemia
Renal disease
Stress response (transport)
Serum ferritin
Iron overload
Hemolysis
Acute-phase reactions
Inflammation
Liver disease
Some neoplasia
Iron deficiency
Total iron-binding capacity
Iron deficiency
Acute-phase reactions
Chronic inflammation Hypoproteinemia
Renal disease
Haptoglobin
Acute-phase reactions
Intravascular hemolysis
Bone marrow stainable iron
Acute-phase reactions
Inflammation
Iron deficiency
Copper
Folic acid and vitamin B12 (cobalamin)
Specific causes of anemia
REGENERATIVE ANEMIA
NON-REGENERATIVE
(HYPOPROLIFERATIVE) ANEMIA
BLOOD LOSS ANEMIA
HEMOLYTIC ANEMIA