The cardiovascular system delivers oxygen and nutrients to all tissues. During exercise the cardiovascular system is also involved in transferring the heat load generated in working skeletal muscles to the skin. Heat is lost by convection (transfer between media of different temperatures), conduction (direct transfer between surfaces in contact), radiation (energy absorbed or emitted at the body surface) and evaporation (conversion of a liquid to vapor and consequent cooling effect at the surface at which the change of state occurs). In equine athletes the most important route of heat transfer during exercise is the evaporation of sweat1 accounting for approximately two-thirds of heat dissipation. Therefore, sweating is an essential mechanism to avoid hyperthermia during exercise. Equine sweat is an isotonic to slightly hypertonic fluid with electrolyte concentrations equal or higher than that of plasma. Consequently, horses performing athletic events of several hours’ duration, in which thermal balance is maintained by evaporation of sweat, will lose considerable amounts of water and electrolytes. When fluid and electrolyte deficits are not partially or totally replaced during and/or after exercise horses may develop clinical signs of dehydration and exhaustion. Clinical disorders associated with fluid, electrolyte and acid-base imbalances are most commonly observed in horses competing in different modalities of endurance rides and three-day events. Fluid and electrolyte balance is not only important from a medical perspective but also because it relates to exercise performance. The reduction in plasma volume associated with dehydration can impair the ability to maintain adequate perfusion to the working skeletal muscle, potentially leading to fatigue, and to the skin, which may lead to excessive hyperthermia and fatigue. The relationship between hydration status and/or fluid and electrolyte supplementation and exercise performance has not been formally investigated in horses. It has been established that horses dehydrated prior to exercise will have compromised thermoregulatory function, and fluid and/or electrolyte supplementation during and immediately after exercise will hasten recovery. However, the effects of dehydration prior to exercise or fluid/electrolyte supplementation upon direct measurements of athletic performance in endurance exercise have not been formally addressed. In human athletes it is well established that exercise performance, assessed as an increase in time to exhaustion at a given exercise intensity, or as a decrease in time to complete a given distance, is enhanced by ingestion of electrolyte-containing solutions during exercise.2 Understanding of some concepts related to fluid composition is necessary for discussion of fluid and electrolyte disturbances, as well as disorders of acid-base balance. Concentrations of electrolytes in plasma or fluids are commonly reported in a variety of forms: weight per volume (mg/dL, g/L or %), or number of mols, osmols or equivalents per volume (mmol/L, mOsm/kg or mEq/L). One mol of any substance is the molecular weight of the substance in grams (Na+ 23 g/mol, K+ 39.1 g/mol, Cl− 35.5 g/mol, Ca2+ 40.1 g/mol, Mg2+ 24.3 g/mol). Electrolytes in body fluids combine based on their charge, the number of positively charged particles (cations) and negatively charged particles (anions) is always equal, and it is often useful to express electrolyte concentrations as mEq/L. One equivalent is defined as the number of mols times its valence or number of ionic charges (valence is 1 for Na+, K+ and Cl−, and 2 for Ca2+ and Mg2+). Osmolality refers to the number of dissociated particles per kg of solvent (e.g. water) and osmolarity refers to the number of dissociated particles per liter of solvent. Those particles in solution that cannot freely diffuse across cellular membranes will exert an osmotic effect, and are referred to as effective osmols. The effective osmolality of a solution is referred to as tonicity. Isotonic fluids are those with a concentration of osmotically active particles similar to that of plasma (osmolality of equine plasma is 270–300 mOsm/kg). Hypotonic and hypertonic fluids are those with an osmolality much lower or much higher than 300 mOsm/kg, respectively. Acid-base balance physiology and its regulation is further discussed in greater detail in Chapter 39. Suffice here to say that plasma pH is determined by strong ion difference (SID), non-volatile weak buffers (Atot) and partial pressure of CO2 (pCO2). Measured strong ion difference (SIDm) is usually calculated as SIDm = (Na + K) − (Cl + lactate), in other words, the difference between cations and anions. An increased SID (due to hypochloremia or hypernatremia) will result in metabolic alkalosis, whereas decreases in SID (due to hyponatremia or hyperlactacidemia) will result in metabolic acidosis. Non-volatile weak buffers (Atot) are those weak acids that are not fully dissociated in blood and have a smaller impact on acid-base balance, mainly albumin, globulins and phosphate. An increased Atot (due to dehydration and increased plasma proteins) will result in mild metabolic acidosis, whereas decreases in Atot (due to protein loss and/or hemodilution) will induce mild metabolic alkalosis. Finally, decreased partial pressure of CO2 (due to hyperventilation) will result in respiratory alkalosis, and increased pCO2 (due to hypoventilation) will result in respiratory acidosis. • Most commonly seen in horses competing in endurance events (e.g. endurance races, competitive trail rides and three-day eventing). • Exhaustion is due to the compound effects of heat accumulation, alterations in electrolyte, fluid and acid-base, and substrate depletion. • Therapeutic strategy: stop exercise, cool the horse, and provide access to water and hay in mild cases. In more severe cases administration of oral electrolyte solutions via nasogastric tube and/or intravenous fluids is a must. • Prevention: acclimatization to environment in the case of extreme heat, humidity, or altitude. Administer electrolytes and offer water to horses during endurance exercise. Avoid competitive endurance exercise with unfit or otherwise unsuitable horses (e.g. overweight Quarter Horses). Provide supplementary electrolytes in the diet to compensate for electrolyte losses in sweating. Clinical signs observed in horses suffering exercise-induced dehydration/exhaustion include depression, anorexia, lack of thirst despite persistent dehydration, persistently elevated rectal temperature, heart rate and respiratory rate.3,4 Other clinical signs related to dehydration and poor cardiovascular function include dryness of mucous membranes (gums, conjunctiva), delayed capillary refill time, decreased pulse pressure, and persistent skin-fold test. Dry feces, minimal urine production, poor gastrointestinal motility, poor anal tone, ileus, and colic may also be observed. Other less common signs associated with dehydration and exhaustion include cardiac arrhythmia, muscle cramps, and/or synchronous diaphragmatic flutter. The severity of dehydration can be subjectively assessed based on the clinical signs observed (Table 40.1). Dehydration below 5% is not detectable clinically, whereas acute dehydration above 10–12% is considered incompatible with life. Table 40.1 Clinical signs observed depending on severity of dehydration In endurance competitions, horses are evaluated at regular intervals in an attempt to assure that only those capable of safely continuing to exercise remain in the competition. At each mandatory rest period (‘vet gates’), fitness to continue is assessed based on examination of general appearance, attitude, gait, pulse and respiratory rates, and evaluation of dehydration, capillary refill time, color of mucous membranes, and gastrointestinal motility. The cardiac recovery index is performed routinely in some countries during the veterinary examination at a mandatory stop. The cardiac recovery index involves taking a resting heart rate, the horse is then trotted 30 m (33 yards) out and back and the heart rate is taken again 1 minute after starting the trot out. Generally, if the resting heart rate is low (50 bpm or less), and the heart rate taken after trotting is equal or lower than the resting heart rate, the horse is considered fit to continue.5,6 Close examination of horses at the mandatory stop and use of the cardiac recovery index may help to identify those horses with significant dehydration/exhaustion, as it has been suggested in a recent study of a 160-km endurance ride in Western Australia.7 Routine hematology reveals alterations related to dehydration and stress, which include increased plasma total protein, packed cell volume, red blood cell count, and hemoglobin; and the total white blood cell count and differential generally show neutrophilia and lymphopenia.8–10 Plasma electrolyte concentrations in dehydrated/exhausted horses are variable. Moderate hypochloremia is most commonly observed after endurance exercise in exhausted and non-affected horses. However, the degree of dehydration and hypochloremia, with consequent metabolic alkalosis due to increased strong ion difference (SID), is generally more pronounced in affected horses. Although the characteristic acid-base abnormality is hypochloremic metabolic alkalosis, it is also reported that the fastest horses in an endurance race may develop mild metabolic acidosis due to lactate production,9,11 which probably results in a mixed acid-base disorder with concurrent lactic acidosis and hypochloremic metabolic acidosis. In a recent study of a 120-km endurance race, most horses had complex acid-base imbalances characterized by mild strong ion alkalosis (due to hypochloremia attenuated by hyperlactacidemia), non-volatile buffer acidosis and compensatory mild respiratory acidosis.12 Other abnormalities can include hypokalemia, hypocalcemia, hypomagnesemia and hypo- or hypernatremia. Substantial decreases in total body electrolyte content due to sweating will be poorly reflected by changes in plasma electrolyte concentrations, because fluid and electrolyte losses are isotonic and of similar composition to plasma. Plasma electrolyte concentrations observed in horses performing endurance rides and three-day events are shown in Table 40.2 and Table 40.3. Table 40.2 Plasma electrolyte concentrations (from jugular venous blood unless specified otherwise) observed in horses competing in endurance rides (average ± SD, except Lindinger 1995,12,17 Schott 2006100 and Viu 201012 average ± SE) iCa2+, ionized calcium; tCa2+ total calcium; tMg2+ total magnesium. Table 40.3 Plasma electrolytes concentrations observed in horses competing in three-day event competitions (average ± SD, except Ecker 199520 average ± SE) iCa2+, ionized calcium; tCa2+ total calcium; tMg2+ total magnesium. Immediate care should include stopping exercise and, if possible, moving the horse into the shade. If available, fans should be placed close to the horse to promote cooling. The horse should be cooled by repeated rinsing/bathing with abundant volume of cold water over the entire body. In studies performed under field and laboratory conditions, repeated application of cold water over the entire body surface decreases rectal temperature more quickly than no bathing or bathing with water at ambient temperature.13,14 These treatments do not cause muscle cramps or myopathies. Wet sheets or towels left in place over the horse’s neck or trunk should be avoided, unless cold water is sprayed repeatedly because wet towels will only serve to provide unneeded insulation in a heat-compromised patient.3 The choice of oral versus intravenous fluid administration or a combination of both should be based mostly on severity of the clinical signs, but cost and manpower may be other considerations. Oral fluid therapy may be considered in mildly affected horses that may not drink sufficiently on their own. It is generally recommended to administer isotonic electrolyte solutions at a rate of 6–8 liters every 30–60 minutes via nasogastric tube. Contraindications for oral fluid therapy are nasogastric reflux and signs of colic. If the patient’s clinical condition does not improve within the following 2–4 hours, intravenous fluids should be administered as soon as possible. Table 40.4 lists some recipes for homemade isotonic solutions. Table 40.4 Composition and tonicity of homemade recipes for electrolyte solutions for nasogastric administration Note: Lite salt: mixture of KCl:NaCl at 50 : 50, Morton Lite salt mixture, Morton International, Rohm and Haas, Philadelphia, PA, USA; tsp, teaspoon (5 mL); tbsp, tablespoon (15 mL), oz, ounce (28.35 g), 1 US gallon = 3.8 L. A balanced polyionic solution that is approximately isotonic is recommended. To that effect, Carlson4 described the following formula, which is similar in composition to electrolytes lost in sweat and is close to isotonic: Mix 1 level tablespoon of table salt (16.6 g NaCl) and 1 level tablespoon of Lite salt (16.9 g of 50 : 50 mixture of NaCl : KCl) in 4 L (~1 gallon) of water (Na+ 107 mEq/L, K+ 28 mEq/L and Cl− 135 mEq/L, 270 mOsm/L).4 The ionic composition, pH and osmolality of commonly used crystalloid fluids are presented in Table 40.5. The composition of polyionic fluids resembles that of extracellular fluid. Note that Ringer’s solution and 0.9% saline solution have a relative excess of sodium, and especially chloride, when compared to extracellular fluid concentrations. Exhausted/dehydrated horses generally have greater deficits of electrolytes relative to water (i.e. hypotonic dehydration) because fluid loss (sweat) is isotonic to slightly hypertonic and fluid intake (fresh water) is hypotonic. Therefore, the relative excess of sodium and chloride in Ringer’s and saline solutions works to our advantage in treating exhausted/dehydrated endurance horses. Table 40.5 Electrolyte composition of selected common sterile intravenous fluids ‡Buffers used: lactate (L), acetate (A). aRinger’s injection, Abbott Laboratories, Abbott Park, IL, USA. bLactated Ringer’s injection, Abbott Laboratories, Abbott Park, IL, USA. cPlasma-Lyte A, Baxter Healthcare Corp, Deerfield, IL, USA. however, a more accurate explanation is that fluids containing lactate and acetate are mildly alkalinizing because of their SID being close to or higher relative to that of plasma, as well as hemodilution and consequent decrease in non-volatile weak acids (Atot, i.e. albumin, globulin and phosphates). In contrast, isotonic saline and Ringer’s solution are acidifying solutions because of their low SID relative to that of plasma (see Table 40.5). Horses engaged in prolonged strenuous exercise events, like endurance competitions of 80 to 160 km or more, are reported to lose 4–8% of body weight (as much as 10% in a few horses) during the competition.10,15–17 Horses undertaking the speed and endurance test of a three-day event or a combined test may lose an average of 2–4% of body weight, which increases to up to 6–9% in a few horses in certain competitions.18–21 However, sweating rates and consequently fluid and electrolyte losses depend upon environmental conditions, and horses exercising in hot or hot and humid conditions develop greater deficits when compared to more temperate conditions.22 If 90% of the body weight loss is fluid loss, then an estimated 20–40 liters of fluids are lost in a 450-kg horse (≈1000 lb) (Table 40.6). A simple estimation of the fluid deficit can be calculated as: Table 40.6 Fluid deficits (L) depending on body weight (kg) and estimation of dehydration (%)
Abnormalities of body fluids and electrolytes in athletic horses
Introduction
Exercise-associated dehydration/exhaustion
Recognition
Physical examination
% OF BODY WEIGHT
VOLUME DEFICIT (L) IN 450 kg HORSE
CLINICAL SIGNS
Mild
5–6
≈20–30
Tacky mucous membranes, decreased skin turgor
Moderate
7–9
≈30–40
Dry mucous membranes, depression, sunken eyes
Severe
>9
>40
Cold extremities, recumbency, moribund

Laboratory examination
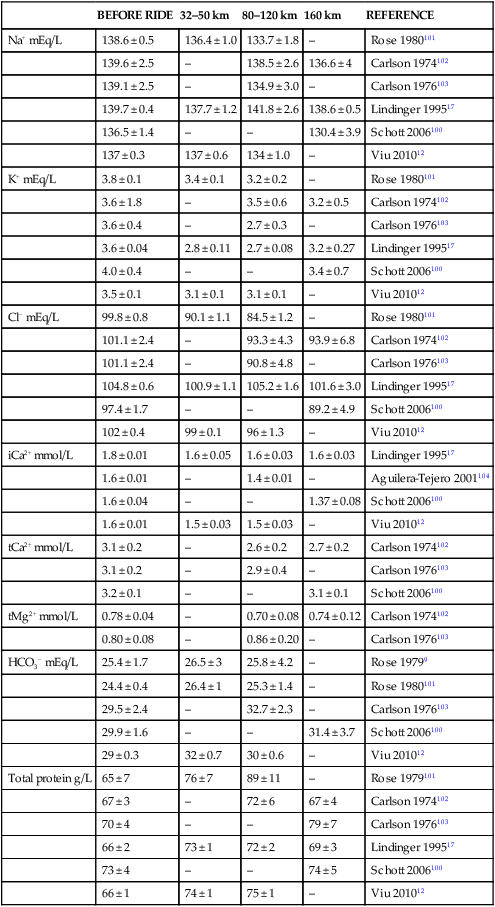
BEFORE RIDE
32–50 km
80–120 km
160 km
REFERENCE
Na+ mEq/L
138.6 ± 0.5
136.4 ± 1.0
133.7 ± 1.8
–
Rose 1980101
139.6 ± 2.5
–
138.5 ± 2.6
136.6 ± 4
Carlson 1974102
139.1 ± 2.5
–
134.9 ± 3.0
–
Carlson 1976103
139.7 ± 0.4
137.7 ± 1.2
141.8 ± 2.6
138.6 ± 0.5
Lindinger 199517
136.5 ± 1.4
–
–
130.4 ± 3.9
Schott 2006100
137 ± 0.3
137 ± 0.6
134 ± 1.0
–
Viu 201012
K+ mEq/L
3.8 ± 0.1
3.4 ± 0.1
3.2 ± 0.2
–
Rose 1980101
3.6 ± 1.8
–
3.5 ± 0.6
3.2 ± 0.5
Carlson 1974102
3.6 ± 0.4
–
2.7 ± 0.3
–
Carlson 1976103
3.6 ± 0.04
2.8 ± 0.11
2.7 ± 0.08
3.2 ± 0.27
Lindinger 199517
4.0 ± 0.4
–
–
3.4 ± 0.7
Schott 2006100
3.5 ± 0.1
3.1 ± 0.1
3.1 ± 0.1
–
Viu 201012
Cl− mEq/L
99.8 ± 0.8
90.1 ± 1.1
84.5 ± 1.2
–
Rose 1980101
101.1 ± 2.4
–
93.3 ± 4.3
93.9 ± 6.8
Carlson 1974102
101.1 ± 2.4
–
90.8 ± 4.8
–
Carlson 1976103
104.8 ± 0.6
100.9 ± 1.1
105.2 ± 1.6
101.6 ± 3.0
Lindinger 199517
97.4 ± 1.7
–
–
89.2 ± 4.9
Schott 2006100
102 ± 0.4
99 ± 0.1
96 ± 1.3
–
Viu 201012
iCa2+ mmol/L
1.8 ± 0.01
1.6 ± 0.05
1.6 ± 0.03
1.6 ± 0.03
Lindinger 199517
1.6 ± 0.01
–
1.4 ± 0.01
–
Aguilera-Tejero 2001104
1.6 ± 0.04
–
–
1.37 ± 0.08
Schott 2006100
1.6 ± 0.01
1.5 ± 0.03
1.5 ± 0.03
–
Viu 201012
tCa2+ mmol/L
3.1 ± 0.2
–
2.6 ± 0.2
2.7 ± 0.2
Carlson 1974102
3.1 ± 0.2
–
2.9 ± 0.4
–
Carlson 1976103
3.2 ± 0.1
–
–
3.1 ± 0.1
Schott 2006100
tMg2+ mmol/L
0.78 ± 0.04
–
0.70 ± 0.08
0.74 ± 0.12
Carlson 1974102
0.80 ± 0.08
–
0.86 ± 0.20
–
Carlson 1976103
HCO3− mEq/L
25.4 ± 1.7
26.5 ± 3
25.8 ± 4.2
–
Rose 19799
24.4 ± 0.4
26.4 ± 1
25.3 ± 1.4
–
Rose 1980101
29.5 ± 2.4
–
32.7 ± 2.3
–
Carlson 1976103
29.9 ± 1.6
–
–
31.4 ± 3.7
Schott 2006100
29 ± 0.3
32 ± 0.7
30 ± 0.6
–
Viu 201012
Total protein g/L
65 ± 7
76 ± 7
89 ± 11
–
Rose 1979101
67 ± 3
–
72 ± 6
67 ± 4
Carlson 1974102
70 ± 4
–
–
79 ± 7
Carlson 1976103
66 ± 2
73 ± 1
72 ± 2
69 ± 3
Lindinger 199517
73 ± 4
–
–
74 ± 5
Schott 2006100
66 ± 1
74 ± 1
75 ± 1
–
Viu 201012
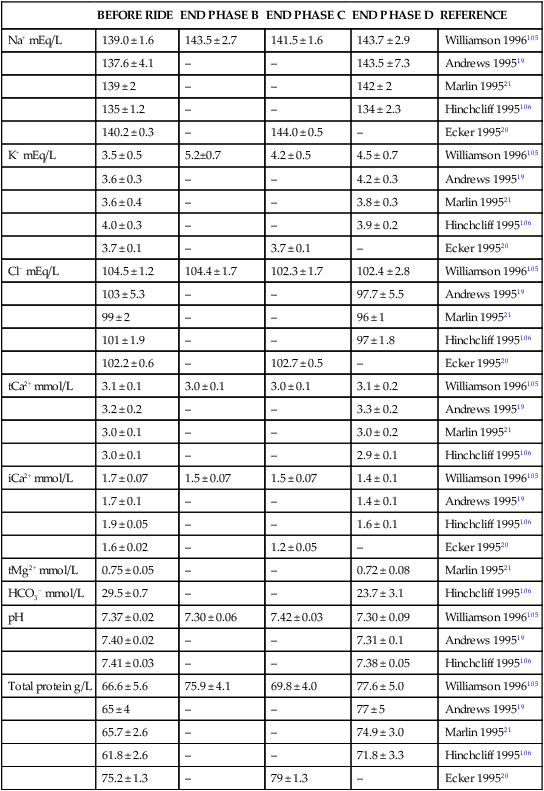
BEFORE RIDE
END PHASE B
END PHASE C
END PHASE D
REFERENCE
Na+ mEq/L
139.0 ± 1.6
143.5 ± 2.7
141.5 ± 1.6
143.7 ± 2.9
Williamson 1996105
137.6 ± 4.1
–
–
143.5 ± 7.3
Andrews 199519
139 ± 2
–
–
142 ± 2
Marlin 199521
135 ± 1.2
–
–
134 ± 2.3
Hinchcliff 1995106
140.2 ± 0.3
–
144.0 ± 0.5
–
Ecker 199520
K+ mEq/L
3.5 ± 0.5
5.2±0.7
4.2 ± 0.5
4.5 ± 0.7
Williamson 1996105
3.6 ± 0.3
–
–
4.2 ± 0.3
Andrews 199519
3.6 ± 0.4
–
–
3.8 ± 0.3
Marlin 199521
4.0 ± 0.3
–
–
3.9 ± 0.2
Hinchcliff 1995106
3.7 ± 0.1
–
3.7 ± 0.1
–
Ecker 199520
Cl− mEq/L
104.5 ± 1.2
104.4 ± 1.7
102.3 ± 1.7
102.4 ± 2.8
Williamson 1996105
103 ± 5.3
–
–
97.7 ± 5.5
Andrews 199519
99 ± 2
–
–
96 ± 1
Marlin 199521
101 ± 1.9
–
–
97 ± 1.8
Hinchcliff 1995106
102.2 ± 0.6
–
102.7 ± 0.5
–
Ecker 199520
tCa2+ mmol/L
3.1 ± 0.1
3.0 ± 0.1
3.0 ± 0.1
3.1 ± 0.2
Williamson 1996105
3.2 ± 0.2
–
–
3.3 ± 0.2
Andrews 199519
3.0 ± 0.1
–
–
3.0 ± 0.2
Marlin 199521
3.0 ± 0.1
–
–
2.9 ± 0.1
Hinchcliff 1995106
iCa2+ mmol/L
1.7 ± 0.07
1.5 ± 0.07
1.5 ± 0.07
1.4 ± 0.1
Williamson 1996105
1.7 ± 0.1
–
–
1.4 ± 0.1
Andrews 199519
1.9 ± 0.05
–
–
1.6 ± 0.1
Hinchcliff 1995106
1.6 ± 0.02
–
1.2 ± 0.05
–
Ecker 199520
tMg2+ mmol/L
0.75 ± 0.05
–
–
0.72 ± 0.08
Marlin 199521
HCO3− mmol/L
29.5 ± 0.7
–
–
23.7 ± 3.1
Hinchcliff 1995106
pH
7.37 ± 0.02
7.30 ± 0.06
7.42 ± 0.03
7.30 ± 0.09
Williamson 1996105
7.40 ± 0.02
–
–
7.31 ± 0.1
Andrews 199519
7.41 ± 0.03
–
–
7.38 ± 0.05
Hinchcliff 1995106
Total protein g/L
66.6 ± 5.6
75.9 ± 4.1
69.8 ± 4.0
77.6 ± 5.0
Williamson 1996105
65 ± 4
–
–
77 ± 5
Andrews 199519
65.7 ± 2.6
–
–
74.9 ± 3.0
Marlin 199521
61.8 ± 2.6
–
–
71.8 ± 3.3
Hinchcliff 1995106
75.2 ± 1.3
–
79 ± 1.3
–
Ecker 199520
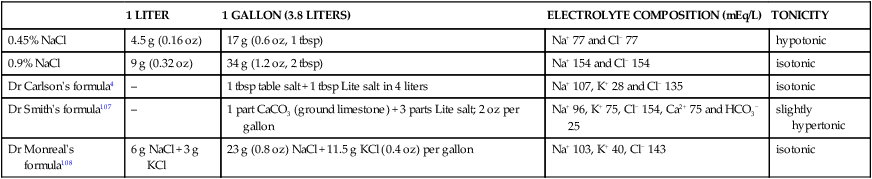
Treatment and prognosis
1 LITER
1 GALLON (3.8 LITERS)
ELECTROLYTE COMPOSITION (mEq/L)
TONICITY
0.45% NaCl
4.5 g (0.16 oz)
17 g (0.6 oz, 1 tbsp)
Na+ 77 and Cl− 77
hypotonic
0.9% NaCl
9 g (0.32 oz)
34 g (1.2 oz, 2 tbsp)
Na+ 154 and Cl− 154
isotonic
Dr Carlson’s formula4
–
1 tbsp table salt + 1 tbsp Lite salt in 4 liters
Na+ 107, K+ 28 and Cl− 135
isotonic
Dr Smith’s formula107
–
1 part CaCO3 (ground limestone) + 3 parts Lite salt; 2 oz per gallon
Na+ 96, K+ 75, Cl− 154, Ca2+ 75 and HCO3− 25
slightly hypertonic
Dr Monreal’s formula108
6 g NaCl + 3 g KCl
23 g (0.8 oz) NaCl + 11.5 g KCl (0.4 oz) per gallon
Na+ 103, K+ 40, Cl− 143
isotonic
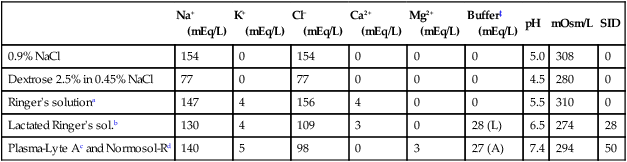
Intravenous fluid therapy
Na+
(mEq/L)
K+
(mEq/L)
Cl−
(mEq/L)
Ca2+
(mEq/L)
Mg2+
(mEq/L)
Buffer‡
(mEq/L)
pH
mOsm/L
SID
0.9% NaCl
154
0
154
0
0
0
5.0
308
0
Dextrose 2.5% in 0.45% NaCl
77
0
77
0
0
0
4.5
280
0
Ringer’s solutiona
147
4
156
4
0
0
5.5
310
0
Lactated Ringer’s sol.b
130
4
109
3
0
28 (L)
6.5
274
28
Plasma-Lyte Ac and Normosol-Rd
140
5
98
0
3
27 (A)
7.4
294
50
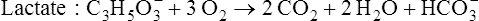
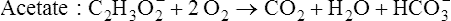
Volume of intravenous fluids and rate of administration
350 kg
400 kg
450 kg
500 kg
550 kg
5%
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
