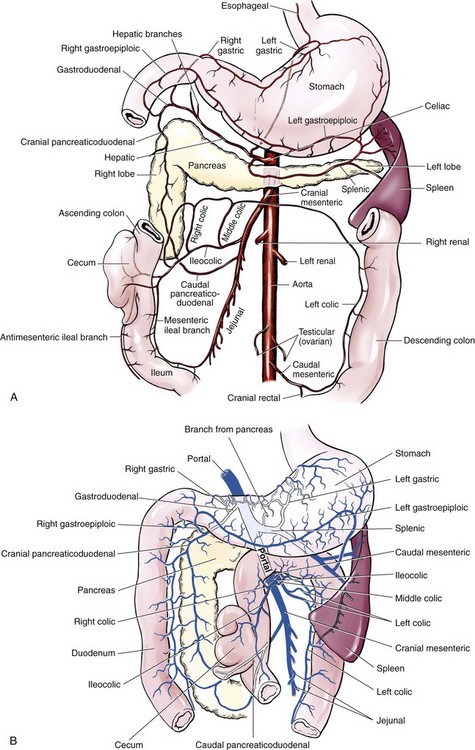
Chapter 91 Grossly, the stomach is divided into the cardia, fundus, body, and pyloric portions (Figure 91-1). The point where the intraabdominal esophagus blends into the stomach on the left side is termed the cardia. The cardiac notch is formed between the cardia and the blind outpouching of the stomach, termed the fundus. On the medial aspect, the esophagus joins the lesser curvature of the stomach without a distinct anatomic landmark of the junction. The incisura angularis (angular notch) produces an intraluminal protrusion of tissue at approximately the midpoint of the lesser curvature that separates the antrum and the cardia. This angular notch is the area in which the papillary process of the liver lies. During endoscopic examination of the stomach, the incisura angularis is an important landmark. The greater and lesser omentum are attached to the stomach at the greater and lesser curvatures, respectively. The greater omentum is divided into three portions known as the bursal, splenic, and veil portions (Figure 91-2). The bursal portion attaches along the greater curvature except on the left, where it runs obliquely across the dorsal surface of the stomach and joins the lesser omentum to close the omental bursa. The splenic portion of the greater omentum forms the gastrosplenic ligament, through which the gastroepiploic vessels course to the stomach. A portion of the lesser omentum forms the hepatogastric ligament that passes from the liver to the stomach. The arterial blood supply of the stomach originates from the celiac artery, a direct branch from the aorta. The celiac artery divides into its component parts, the splenic, hepatic, and left gastric arteries (Figure 91-3, A). Each of these arteries provides blood flow to a portion of the stomach. The splenic artery gives off tributaries to the left limb of the pancreas and the spleen before becoming the left gastroepiploic artery, which supplies the greater curvature of the stomach and anastomoses with the right gastroepiploic artery. The hepatic artery gives off branches to the liver and gallbladder and then continues as the right gastric artery, which supplies blood to the pylorus and pyloric antrum, after which it anastomoses with the left gastric artery along the lesser curvature of the stomach (see Figure 91-3, A). After the right gastric artery branches from it, the hepatic artery continues as the gastroduodenal artery. The gastroduodenal artery travels to the duodenum, where its branch—the cranial pancreaticoduodenal artery supplies the right pancreatic limb; its branch—the right gastroepiploic artery—supplies the greater curvature of the stomach, as described above. The left gastric artery is a direct branch from the celiac artery that supplies the fundus of the stomach and provides small branches to the caudal esophagus before joining the right gastric artery. The short gastric arteries originate from splenic branches of the splenic artery and anastomose with the gastric branches of the left gastric artery to supply the fundic area of the stomach. The lesser curvature is supplied by the left gastric artery and its anastomosis with the right gastric artery. The venous drainage of the stomach to the portal vein is through the gastrosplenic vein on the left and gastroduodenal vein on the right (see Figure 91-3, B). Lymphatic drainage of the stomach is through the gastric and splenic lymph nodes to the hepatic lymph nodes.15 The stomach is innervated by parasympathetic fibers of the vagus nerves and sympathetic fibers of the celiac plexus.47a The ventral vagal trunk passes through the esophageal hiatus and then sends small branches to the pylorus, liver, and lesser curvature of the stomach. The dorsal vagal trunk sends branches to the lesser curvature and ventral wall of the stomach and then continues across the celiac plexus to follow branches of the celiac and cranial mesenteric arteries. Sympathetic fibers arise from the celiacomesenteric plexus and follow gastric branches of the celiac artery. Glands of the stomach include the cardiac, pyloric, and gastric glands proper. Their composition differs with various regions of the stomach. The cardiac glands are located primarily around the cardia but are also present in the antrum; they produce a serous secretion. The pyloric glands may be found in the pylorus and gastric body; they primarily produce mucus. The gastric glands proper are located in the fundus and body and are composed of parietal, chief, mucous neck, and endocrine cells (Figure 91-4 and Table 91-1). The parietal (oxyntic) cells have two primary functions: to maintain gastric acidic pH (pH, 2–3) by pumping hydrogen ions into the gastric lumen and to produce intrinsic factor, a mucoprotein that binds to B12 to permit its absorption in the distal small intestine. The acidic gastric environment allows activation of gastric enzymes necessary for the breakdown of food. Chief cells secrete pepsinogen, which is converted in the acidic environment to pepsin, which in turn functions to break down proteins. Neck mucous cells secrete mucus that protects other glandular cells from the action of the proteases and the hydrochloric acid. Gastric endocrine cells produce gastrin, histamine, and serotonin. Table • 91-1 Location and Function of the Secretory Cells of the Stomach During active digestion, the body of the stomach dilates and motility increases in order to mix food and move it toward the antrum. The pattern of motility in the antrum serves to churn the food by forcing ingesta aborally into the area of the pylorus, and the pylorus closes before completion of the antral contraction. This sequence of events allows only the liquid chyme to pass into the duodenum; the remaining gastric contents are forced retrograde, where additional churning results in breakdown of food into appropriate-sized particles. The gastric emptying rate for solid food is therefore greatly impacted by this coordinated contraction of antrum and pylorus; the emptying rate for liquids is more influenced by fundic motility.94,161 Normal gastric mucosa is undergoing constant renewal. In the stomach, mucosa is protected by normal cytoprotective mechanisms from autodigestion by gastric acid and digestive enzymes. When superficial epithelial injury occurs, it is repaired rapidly by epithelial migration without proliferation. Gastric mucosa erosions heal rapidly by epithelial regeneration.68 Injury that extends into the submucosal layer is considered an ulcer and is associated with a fibrotic repair process. Scar formation with fibrous protein synthesis occurs and predominates over wound contraction.44 If the depth and duration of gastric insult are minimized, the resultant scar is often resorbed. In long-term injury, a profound fibrotic response may result in permanent scar formation. Incisional healing differs from the previously described healing process because, although a full-thickness injury is created, it is of short duration. Standard phases of wound healing, including inflammation, debridement, repair, and maturation, occur in gastric healing. In contrast to other healing tissues in which collagen is produced by only fibroblasts, smooth muscle cells of the gastrointestinal tract contribute to collagen production during gastrointestinal wound healing.68 Healing in the stomach is enhanced by its extensive and redundant blood supply. Fasting before gastric surgery, however, may have negative consequences. Longer fasting times in humans and dogs reportedly decrease gastric pH, are linked to a higher incidence of gastroesophageal reflux, and do not reliably decrease gastric content volumes.58,81,110,118,153 Gastroesophageal reflux is an important complication associated with any anesthetic event and with gastric surgery in particular. Wilson et al.180 documented gastroesophageal reflux in 57% (51 of 90) of dogs undergoing anesthesia for orthopedic procedures, but clinically obvious regurgitation was noted in only 14% of anesthetized patients. These findings suggest that the esophagus is exposed to an acidic environment in more than 50% of anesthetized patients but that this event is clinically evident in only a small percentage of animals. This “silent” exposure of the esophagus to acidic gastric contents may predispose anesthetized animals to esophagitis and esophageal stricture. In a report of 13 cases of postanesthesia esophageal dysfunction, only 46% (6 of 13) of cases were noted to have clinical evidence of regurgitation while under anesthesia.179 Ten of the 13 dogs developed esophageal stricture, and three were ultimately euthanized. Based on these findings and the findings of Savvas et al.153 small amounts of canned food fed 3 hours before surgery may decrease gastric acidity and minimize the occurrence and clinical impact of gastroesophageal reflux while having minimal to no impact on gastric content volume. In rare instances when exposure of only a portion of the stomach is required, a paracostal approach may be used. A paracostal approach is achieved by making a curved incision approximately 2 cm caudal to the last rib. The underlying muscle layers, including the external and internal abdominal oblique and transversus abdominis muscles, are split longitudinally along the direction of the muscle fibers. When ventral extension of the incision is necessary for visualization, the rectus abdominis muscle may require transection. Closure is achieved with a simple continuous pattern in each muscle layer (Figure 91-5). The abdominal cavity should be lavaged with sterile saline or lactated Ringer’s solution and suctioned dry after the gastrointestinal tract is closed. Temperature recommendations for lavage solution vary, with 37° C to 39° C (98.6° F to 102.2° F) most commonly reported. Nawrocki et al.124 confirmed that lavage of the canine abdominal cavity with room temperature saline 21° ± 1° C (70° ± 3° F) significantly decreased body temperature. Additionally, lavage with a saline solution heated to 43° ± 2° C (110° ± 4° F) significantly increased body temperature compared with body temperature before lavage and with dogs undergoing room temperature lavage.124 Increased body temperature may be beneficial to patients undergoing abdominal surgery because core body temperature decreases significantly during these procedures. One concern not addressed in this study, however, was the long-term effect of high temperature lavage solution. Warmer fluids may result in vasodilation and resultant hypotension and an increased risk of adhesion formation.143 The surgeon must balance the need for increasing core body temperature before closure with potential detrimental effects of abdominal lavage with fluids 43° ± 2° C (110° ± 4° F). The choice of appropriate suture material for closure of the stomach is dictated by the need for a material that resists rapid degradation in the acid- and enzyme-rich environment of the gastric lumen for the 14 days necessary to regain gastric wall strength.52,164 Most commonly, monofilament absorbable materials such as polydioxanone, polyglyconate, or poliglecaprone 25 are used. Polydioxanone undergoes a rapid and significant loss of tensile strength in an acidic environment.57,166 In fact, when incubated in a solution with a pH of 1 for 2 weeks, the tensile strength of polydioxanone was no longer measurable.166 In a biodegradation study by Tomihata et al.57 nine common absorbable sutures were immersed in gastric fluid or a solution with a pH of 2. The half-life of polydioxanone was 10 times shorter at that pH than at a pH of 7.4. Additionally, polydioxanone was the only suture in that study to be significantly negatively impacted in tensile strength by gastric fluid.57 In contrast, polyglycolide and poly l-lactide-coglycolide had initial delays in linear absorption, presumably secondary to the coating on each of these materials. In Tomihata’s study, the half-lives in gastric fluid for polyglyconate, poliglecaprone 25, and polydioxanone were 75, 15, and 12 days, respectively, supporting use of polyglyconate or poliglecaprone 25 in gastric surgery.57 Based on rapid degradation of chromic and plain catgut in gastric juices, use of these materials is not recommended.164 Gastric surgery may be performed with thoracoabdominal, gastrointestinal anastomosis, and skin staplers.10,30,31,80,165 Thoracoabdominal and gastrointestinal anastomosis staplers have been used in gastrectomy, pylorectomy, and gastrojejunostomy procedures.2,30 Because of potential necrosis along the staple line and resultant leakage of gastric content, oversewing of the staple line is recommended.28 Gastropexy for prevention of gastric dilatation and volvulus can be performed with gastrointestinal anastomosis or skin staplers.10,31,80 In one study, use of a skin stapling device for gastropexy provided results similar to hand-sewn belt loop gastropexy with regard to tensile strength. A significant difference in the time required to perform the procedure was noted; however, the difference was less than 4 four minutes and is likely not clinically relevant.31 In a more recent study, gastropexy performed with a gastrointestinal anastomosis stapler was successful in creating a permanent adhesion; in this clinical study, however, the time to perform the gastropexy was not compared with hand-sewn gastropexy.10 Closure of the biopsy site is most commonly achieved with a two-layer pattern. The first layer may be a simple continuous appositional pattern or an inverting pattern such as a Connell or Cushing pattern. A second inverting layer follows the first and incorporates at least the serosal and muscularis layers of the stomach. When placing an inverting pattern, care should be taken to ensure that the inverted gastric tissue does not interfere with outflow from the pylorus. Iatrogenic gastric outflow obstruction has been reported after gastric surgery.54 When performing partial gastrectomy, it is critical that all nonviable tissue be excised. Viability of gastric mucosa does not predict overall health of the gastric wall; thus, other criteria must be used to assess gastric wall viability. Subjective criteria include gastric wall thickness, as measured by palpation; serosal surface color; evidence of serosal capillary perfusion; and presence of peristalsis.112 Subjective findings consistent with nonviable gastric wall include a wall that is thinner than normal or serosal surface color of grey-green to black. Although subjective criteria proved to be 85% accurate in an experimental model when performed by an experienced surgeon, incorrect interpretation of subjective findings resulted in subsequent gastric perforation or dehiscence of gastrectomy sites in dogs with naturally occurring gastric dilatation and volvulus.112 When wall viability is in question based on subjective criteria, the seromuscular layer can be incised to evaluate arterial vascular supply. Objective data to predict gastric perfusion have been pursued through several methods, including fluorescein dye injection, scintigraphy, and laser Doppler flowmetry.13,121,176 In dogs with gastric dilatation and volvulus, fluorescein injection and scintigraphy were accurate in 58% and 79% of dogs, respectively.13,176 Results of laser Doppler flowmetry in dogs with gastric dilatation and volvulus were consistent with concurrent subjective findings of the surgeon.121 Further study is required before this technique has application in clinical decision making. Invagination has been proposed as an alternative to partial gastrectomy in cases with areas of questionable viability.106 A simple continuous or inverting suture pattern and a second inverting suture pattern, with bites placed in healthy tissue on each side of the necrotic portion of the stomach, are placed to invaginate the unhealthy gastric wall. The result is apposition of healthy tissue over necrotic tissue without penetration into gastric lumen, thereby decreasing the risk of gastric contents spillage. The devitalized area of stomach wall is then sloughed into the gastric lumen and digested. In an experimental study, gastric compromise was induced through ligation of varying components of the gastric vascular supply and the affected area invaginated.106 Fourteen days after invagination of the compromised areas, gastric ulcers of varying depths and clinical relevance were reported in the majority of dogs. Based on these results, the authors recommended further study of gastric healing after invagination. One dog developed a gastric ulceration 21 days after invagination that required surgical resection.127 Signs of obstruction secondary to the sloughing of large amounts of tissue into the gastric lumen have also been reported.53 A variety of gastropexy techniques have been reported with varying degrees of success. Techniques include incisional, belt-loop, circumcostal, endoscopically assisted, and laparoscopic gastropexy; gastrocolopexy; incisional gastropexy through a grid approach; and incorporating gastropexy, in which the gastric wall is included in the linea alba closure.* The key component to successful gastropexy is incision through the serosal and peritoneal surfaces and into the muscular portion of each anatomic component being joined. For prevention of gastric dilatation and volvulus, the intraabdominal body wall incision of an incisional, belt-loop, laparoscopic, or endoscopic gastropexy is made caudal to the last rib to prevent penetration of the diaphragm and subsequent pneumothorax. The results of biomechanical testing for commonly used open gastropexy techniques are similar (Table 91-2).55,80,145,174,181 Quantitative results of biomechanical testing must be interpreted carefully because the absolute strength of gastropexy required to prevent development of gastric dilatation and volvulus is unknown. There has not been an experimental study of strength of incorporating gastropexy. Incisional gastropexy for prevention of gastric dilatation and volvulus is performed by creating a 4- to 5-cm seromuscular incision in the gastric antrum either parallel or perpendicular to the long axis of the stomach. Care should be taken to avoid penetration of the gastric mucosa. Application of pressure from the dorsal surface of the stomach directly opposite the incision site aids in opening the incision and allows visualization of the layers as they are incised. Alternatively, digital pressure or pinching of gastric wall on the ventral surface at the site of the proposed incision may allow separation of the mucosal surface away from the seromuscular layer. A second incision is made through the peritoneum and the transverse abdominis muscle on the lateral or ventrolateral right abdominal wall approximately 2 to 3 cm caudal to the last rib. Incisions cranial to the last rib will penetrate the diaphragm and result in pneumothorax, particularly if the incision is made in the dorsal half of the abdominal wall. Before incising the body wall, it is important to manually appose the stomach to the body wall to estimate the appropriate anatomic site of the incision. Attention to the position dorsal to ventral on the body wall is essential because it is natural instinct to place the incision where it will be easy to visualize and suture instead of in the appropriate anatomic location. Removal of the Balfour retractor and retraction and eversion of the body wall with towel clamps aids in visualization and appropriate incision placement. Gastric and abdominal wall incisions are apposed using 2-0 monofilament absorbable suture in a simple continuous suture pattern beginning with the craniodorsal edges of the incision (Figure 91-6). Belt-loop gastropexy is a variation on incisional gastropexy. With the belt-loop technique, a seromuscular flap is elevated from the pyloric antrum and passed through a tunnel created between two parallel incisions in the abdominal wall.177 The seromuscular gastric flap is based along the greater curvature of the stomach and incorporates branches of the gastroepiploic artery in its origin (Figure 91-7). The flap is created by making two parallel incisions approximately 4 cm long and 3 cm apart and connecting these incisions at their most cranial aspect. The resultant seromuscular flap is undermined from the mucosal layer below. Next, two 5-cm–long abdominal wall incisions that penetrate the peritoneum and transverse abdominis muscle are made approximately 3 cm apart. Muscle between the two incisions is undermined, creating a tunnel through which the seromuscular gastric flap is passed. The gastric flap is passed through the tunnel by first bringing the stomach close to the body wall to decrease tension applied to the flap as it is passed and then using a stay suture placed in the free end of the flap to direct the flap through the tunnel. The flap is sutured back to the site from which it was elevated using a simple interrupted or continuous pattern of 2-0 or 3-0 absorbable monofilament. The circumcostal gastropexy technique, originally described in 1982,49 has been successfully performed in experimental cases and with clinical cases of gastric dilatation and volvulus. Modifications of the original technique have been described.34,136 A seromuscular flap is raised from the pyloric antrum similar to the belt-loop gastropexy technique (Figure 91-8). The seromuscular flap may be double or single hinged. When a single-hinged flap is used, it is based from the lesser curvature of the stomach and undermined below the level of the muscularis layer, taking care not to penetrate the gastric lumen. A 5- to 6-cm incision is made directly over the eleventh or twelfth rib at the level of the costochondral junction. A plane of blunt dissection is then established circumferentially in close association with the rib. Care must be taken to avoid creation of pneumothorax or fracture of the rib; both are reported complications of this procedure.102,183 The seromuscular gastric flap is then passed cranial to caudal through the tunnel surrounding the rib; stay sutures placed in the leading edge of the flap facilitate passage around the rib. The seromuscular flap is sutured back to its origin using 2-0 or 3-0 absorbable suture material. Creation of a suture line between the greater curvature of the stomach and the transverse colon has been described to prevent recurrence of gastric dilatation and volvulus.27,42 Gastrocolopexy as reported does not include incision into the seromuscular layer of either the stomach or the colon; instead, the surfaces are scarified and then apposed with nonabsorbable sutures. Whether a permanent adhesion is created is unknown. An incorporating gastropexy is performed by including approximately 5 cm of gastric wall near the pyloric antrum with the cranial portion of the linea alba closure during apposition of a ventral midline abdominal incision. Abdominal wall closure and gastric incorporation are performed with absorbable suture material.116 As described originally, no incision is made into the seromuscular layer of the stomach. The strength of adhesion formed using this technique has not been evaluated experimentally. Incorporating gastropexy is easy and quick to perform; if subsequent abdominal exploration is required, however, inadvertent penetration of the stomach while entering the abdominal cavity could occur. Grid Approach.: Prophylactic right-sided gastropexy may be achieved through a minilaparotomy.160 This technique is performed by creating a 6-cm vertical skin incision immediately caudal and ventral to the thirteenth rib. An opening is then created through the external abdominal oblique, internal abdominal oblique, and transversus abdominus muscles by blunt dissection along the direction of the muscle fibers. The peritoneal surface is penetrated bluntly, and the gastric antrum is retracted into the surgical field with Babcock intestinal forceps. Visualization of landmarks specific to the stomach, such as pylorus, omental attachments, and gastric vasculature, is critical to ensure that the appropriate portion of the gastrointestinal tract is being anchored to the body wall. After placing stay sutures in the pyloric antrum, a 3-cm longitudinal incision is made through the gastric serosal and muscular layers. Edges of the gastric incision are apposed to the cut edges of the transversus abdominus fascia and muscle with a continuous suture pattern of absorbable monofilament. The more superficial muscle layers are closed individually over the gastropexy site, again with continuous suture patterns. Endoscopically Assisted Gastropexy.: Endoscopically assisted prophylactic gastropexy has been reported in dogs and is achieved with the dog in left oblique recumbency.40,41 A gastroscope is passed into the stomach, and the stomach is insufflated. While the pyloric antrum is viewed through the gastroscope, the right abdominal wall is palpated to identify the appropriate anatomic location for gastropexy. The stomach is stabilized with stay sutures: number 2 polypropylene suture on a cutting needle (needle length, 76 mm) is passed from the right external body wall into the gastric lumen immediately caudal to the thirteenth rib and back out through the skin, incorporating a minimum of 2 cm of gastric tissue. The needle is viewed endoscopically as it passes in and out of the stomach lumen to ensure that it is engaging the pyloric antrum, and the resulting stay suture is secured with a hemostat. A second stay suture is similarly placed 5 cm aborally from the first suture. An incision is then made through the body wall between the two stay sutures to the level of the stomach. Unlike the grid dissection, muscle layers are transected, not bluntly dissected. Visualization is assisted through placement of Gelpi retractors, and a longitudinal incision is made through the gastric serosa and muscular layers. The edges of the gastric incision are apposed to cut edges of the transversus abdominus muscles, as described previously for the grid gastropexy procedure. The muscle layers, subcutaneous tissues, and skin are closed routinely, and the stay sutures are removed. Laparoscopic Gastropexy.: Laparoscopic and laparoscopic-assisted gastropexy have been reported for prevention of gastric dilatation and volvulus in dogs.80,113,144–146 Laparoscopic visualization ensures appropriate stomach positioning. Mayhew et al.113 reported two techniques for total laparoscopic gastropexy. Three portals are used to achieve either technique, with all ports placed on the ventral midline. The first port is placed approximately 1 cm caudal to the umbilicus. An instrument port is placed 3 to 4 cm caudal to the xiphoid, and the final port is positioned midway between the first two portals and directly medial to the proposed gastropexy site. After all portals have been established, the camera is introduced through the middle portal. A stay suture is introduced through the body wall, grasped intracorporally, inserted through the pyloric antrum, and returned through the body wall adjacent to the entry site of the needle. This stay suture is used to hold the antrum in apposition with the body wall. An incision is made into the transversus abdominus muscle with endoscopic Metzenbaum scissors at the site of the proposed gastropexy. The serosal and muscular layers of the stomach are then incised in the same orientation as the incision in the body wall. The gastric wall and body wall incisions are opposed with either a suture–assist device and laparoscopic needle holders or via intracorporeal hand suturing. An important technical consideration in both of these intracorporeal procedures is the need to initially introduce adequate lengths of suture to complete the suture lines and the need for the surgeon to have training and experience in either procedure. Stapled gastropexy can also be performed laparoscopically.80 Using laparoscopic instruments, Hardie et al.80 created subserosal tunnels in the pyloric antrum and right body wall. A laparoscopic stapler was inserted in the tunnels and used to appose, staple together, and divide the pyloric and body wall tissues, creating a permanent gastropexy with two staple lines. With the laparoscopic-assisted gastropexy technique, the laparoscope is used to identify the portion of the stomach to be incorporated into the pexy and to bring it to a body wall incision, permitting a smaller exposure than the grid technique.145,146 A camera port is established on the midline 2 to 3 cm caudal to the umbilicus. A second working port is established lateral to the right margin of the rectus abdominus muscle and 3 cm caudal to the last rib (Figure 91-9). Laparoscopic Babcock forceps are introduced through the right port and used to grasp the pyloric antrum and retract it to a position adjacent to the body wall. Forceps and associated cannula are withdrawn while maintaining traction on the pyloric antrum, exteriorizing the antrum through the body wall opening created for the port. The incision in the abdominal musculature is extended to approximately 4 cm in length, and stay sutures are placed in the pyloric antrum. Gastric serosal and muscular layers are incised longitudinally, and cut edges are apposed to the cut edges of the transversus abdominus muscle. The stay sutures are removed, and the muscle layers, subcutaneous tissue, and skin are closed routinely. Fredet-Ramstedt pyloromyotomy is performed by making a longitudinal incision through serosa and muscularis of the ventral pylorus. The incision should be centered over the pylorus and extend 1 to 2 cm orad and aborad. When performed correctly, the gastric mucosa is not penetrated; however, its submucosal surface should protrude through the incision. This partial-thickness incision is left open, permitting enlargement of the pylorus in cases in which restriction is limited to the serosa or muscularis (Figure 91-10). Because pyloromyotomy does not allow visualization of the gastric mucosa, relieve restrictions associated with mucosal or submucosal disease, or provide a means for full-thickness biopsy of the stomach wall, its use is limited. Heineke-Mikulicz pyloroplasty is similar to pyloromyotomy in that a longitudinal incision is made in the ventral surface of the pylorus; however, the incision is created full thickness and then closed transversely (Figure 91-11). Stay sutures can be placed in the middle of either side of the longitudinal incision and retracted to provide apposition for the transverse closure; alternatively, stay sutures can be placed at each end of the incision and pulled together to provide the same effect. Closure is achieved with 2-0 or 3-0 absorbable monofilament suture material in an appositional pattern. Heineke-Mikulicz pyloroplasty permits full-thickness gastric wall biopsy and resection of small masses or thickened tissues along the incision. Conventional and laparoscopic pyloromyotomy and pyloroplasty have been described.11,126,151 In one study, gastric emptying of solids was significantly enhanced in dogs undergoing pyloroplasty, but pyloromyotomy did not significantly change gastric emptying relative to preoperative values.151 Other studies have reported no change to slowed gastric emptying after these pyloric procedures.126 Y–U advancement pyloroplasty increases pyloric outflow tract diameter by advancing a portion of the pyloric antrum into the region of the pyloric sphincter. A Y-shaped full-thickness incision is centered over the pylorus. The incision for the body (or main stem) of the Y is made through the antimesenteric border of the duodenum, and the incisions for the arms of the Y extend into the pyloric antrum (Figure 91-12). The arms of the Y should curve slightly to form a U-shaped flap instead of a sharp V to maximize vascular supply to the enclosed tissue. Visualization of the outflow tract, mucosal resection, and full-thickness biopsy may be performed before closure. The U-shaped flap is then advanced forward using stay sutures at its tip and sutured into the most aboral portion of the incision at the duodenum using 2-0 absorbable monofilament suture material in an appositional pattern. Pyloric resection is technically more challenging than other techniques involving the pylorus and requires detailed knowledge of regional anatomy. The bile duct, pancreatic ducts, and vascular supply to the stomach and duodenum must be identified before any surgical excision (see Figure 91-1). The bile duct is best identified by manual expression of the gallbladder: bile can be detected as it descends through and dilates the bile duct as it approaches the duodenum. Stay sutures are placed in the duodenum and stomach to facilitate retraction and minimize potential leakage. Atraumatic tissue forceps are used proximal and distal to the site of resection to minimize leakage of gastrointestinal contents. The hepatogastric ligament can be transected to facilitate caudoventral retraction of the pylorus; however, care should be taken to avoid bile duct transection deep and lateral to this area. Inadvertent transection of the bile duct has been reported during this procedure.172 A minimum of 5 to 10 mm of healthy tissue should be maintained between the duodenal level of excision and the opening of the bile duct to prevent inadvertent damage or obstruction.172 Branches of the right gastric and right gastroepiploic blood vessels supplying the area to be resected are ligated and transected. Omental and mesenteric attachments are ligated and divided. After making sure the area is packed off from the rest of the abdomen with laparotomy sponges, the pylorus is removed with Metzenbaum scissors or a scalpel. If a significant discrepancy in lumen diameter is created by this resection, the surgeon may choose to incise the antimesenteric border of the duodenum to increase its diameter or close a portion of the gastric antrum to narrow gastric diameter to facilitate anastomosis (Figure 91-13). End-to-end anastomosis of the stomach and duodenum is performed with a one- or two-layer appositional pattern using a simple interrupted or simple continuous pattern of 2-0 or 3-0 absorbable monofilament suture. No difference in leakage was reported when one- and two-layer closures were compared.172 The prognosis after pylorectomy and gastroduodenostomy depends on the underlying disease. In one study, 18 of 24 dogs that underwent this procedure survived 14 days; however, 10 of the dogs died by 3 months after surgery.43a Postoperative complications included hypoalbuminemia (62.5%) and anemia (58.3%). Decreased survival time was correlated with preoperative weight loss and malignant neoplasia.16a
Stomach
Anatomy
Gross Anatomy of the Stomach
Omentum
Vasculature, Lymphatics, and Innervation of the Stomach
Gastric Morphology and Glandular Organization
Glands
CELL
LOCATION
PRODUCT(S)
Parietal
Body
Acids and intrinsic factor
Mucus
Body, antrum
Mucus
Chief
Body
Pepsinogen
Surface epithelium
Diffuse
Mucus, bicarbonate
Endocrine cells
Body
Histamine
Gastrin
Serotonin
Physiology
Healing Characteristics
Presurgical Preparation
Fasting
General Surgical Principles
Approach
Lavage
Gastric Closure
Suture Material
Staples
General Surgical Techniques
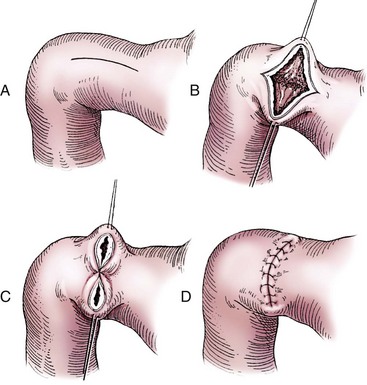
Partial Gastrectomy
Determining Tissue Viability
Gastric Wall Invagination
Gastropexy
Incisional Gastropexy
Belt-Loop Gastropexy
Circumcostal Gastropexy
Gastrocolopexy
Incorporating Gastropexy
Minimally Invasive Prophylactic Gastropexy Techniques
Pyloromyotomy and Pyloroplasty
Fredet-Ramstedt Pyloromyotomy
Heineke-Mikulicz Pyloroplasty
Y–U Advancement Pyloroplasty
Gastroduodenal Anastomosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree